Abstract
Even after more than a century of research, the processes underlying species abundance distribution patterns are controversial. Here, we gathered abundance and size (standard length) data of fish species in 54 streams in the Midwest of Brazil to test whether subordinate species abundances (i.e., any species that is not dominant in a community) in each stream are correlated with the absolute size difference between dominant and subordinate species. A negative relationship between these variables would suggest a predominant role of environmental filtering because those species that differ more from the dominant species (the one with the optimum trait value) would become progressively less abundant. On the other hand, a positive relationship would suggest a limit to the similarity as the abundances of subordinate species that differ more from the dominant species would increase. Our results clearly indicated that subordinate species were those that most differed from the dominant species in terms of size. In addition, we found that the subordinate species were larger than the dominant species. Taken together, we infer that environmental filters favoring small body sizes (e.g., shallow water depth and scarcity of large shelters) are the main processes determining species abundance distributions in the streams we studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely known that local communities are composed of a few dominant species and many rare species (McGill et al., 2007). In the search for mechanisms that would explain variations in species abundance distribution (SAD) patterns, several models (e.g., geometric series, log series, log normal, broken stick, zero-sum multinomial distribution) have been developed (MacArthur, 1957; Tokeshi, 1993; Hubbell, 2001; McGill et al., 2007; Ferreira and Petrere-Jr., 2008; Matthews & Whittaker, 2015). However, several studies have shown that the simple fit of a model is insufficient to indicate a particular mechanism because different mechanisms can generate the same SAD pattern (see reviews of McGill, 2003; Magurran, 2005; McGill et al., 2007).
In the last decades, the resurgence of interest in the mechanisms underlying SAD patterns in local communities occurred with the use of species traits (McGill et al., 2007; Umaña et al., 2015; Klipel et al., 2021). Instead of simple correlating species mean trait values with species abundances, the relationship between species differences in traits and abundances can help discriminating among competing mechanisms (Sugihara et al., 2003; Mouillot et al., 2007; Mason et al., 2008; Hidasi-Neto et al., 2020). In this context, Mouillot et al. (2007) made three predictions based on environmental filtering processes, the limiting similarity theory (MacArthur & Levins, 1967), and the neutral theory (Hubbell, 2001). First, under the environmental filtering theory, abundant (or common) species would be similar in trait values “because environmental conditions act as a filter allowing only a narrow spectrum of traits to persist” (Mouchet et al., 2010)—in other words, deviations from optimum trait values would imply lower abundances. In this case, one would expect a negative relationship between subordinate species abundances (SSA) and the absolute differences in, for example, body size (between the dominant species and all other subordinate species—DIFS; Fig. 1A, B). Second, under the limiting similarity theory, owing to resource limitation and interspecific competition, a positive relationship between DIFS and SSA would emerge (Fig. 1C,D): the larger the difference in body size, the lower the niche overlap and, therefore, the higher the abundance of the subordinate species. Third, under neutral theory, there would be no relationship between niche similarity and abundance (Fig. 1E, F; see also Mouillot et al. 2007 and their Fig. 2). This would be so because, under neutrality, abundance distribution in a local community would be determined by drift only (McGill et al., 2006). Recently, Hidasi-Neto et al. (2020) tested these predictions and found negative relationships between relative abundance (of subordinate species) and difference in body size (dominant—subordinates) for 88 local communities of small mammals distributed around the world. These results are consistent with the hypothesis of environmental filtering. On the other hand, using the general idea of testing the relationship between niche similarity and fish abundance in six lakes in France, Mason et al. (2008) found that pairs of abundant species had low niche overlap and, in addition, rare species tended to have high niche overlap with abundant species. According to the authors, these results suggest that competition limited the relative abundance of pairs of species with similar niches by reducing the abundance of the two species or allowing only one to achieve high abundance.
Hypothetical communities (A and C) with six species each. Different colors and sizes of the silhouettes represent different species and different body sizes, respectively. The arrows indicate the rank in abundance. B Assuming a preponderant role of environmental filtering, subordinate species (e.g., blue species) that are similar (in terms of body size; i.e., low DIFS) to the dominant species (red) are also abundant and as the difference in body size increases, the abundance of subordinate species decreases (e.g., the black species is the most different from the red species and are also the least abundant). Thus, we are assuming that the environmental selects for an “optimal” size. D Assuming a preponderant role of competition, subordinate species (e.g., blue species) that are similar to the dominant species would have the lowest abundances and as the difference in body size (DIFS) increases, the abundance of the species increases. Thus, we are assuming that, due to competition, if a species (e.g., blue) is too similar (in terms of body size) to the dominant species, this species “is very likely to have a low abundance under the niche limitation theory” (Mouillot et al., 2007; see also their Fig. 2). The absence of relationship between subordinate species abundance (SSA) and DIFS (E and F) would indicate that none of the processes can be inferred. For further explanations on how the variable DIFS was calculated, see Fig. S1
The focus on body size in studies concerned with different ecological patterns can be justified in different ways (Peters, 1983). An emblematic example is the importance of body size in the development of the metabolic theory of ecology (e.g., Brown et al., 2004). Also, several key traits correlate with body size, including abundance (White et al., 2007), especially when data from different sources are used to analyze the relationship between these variables (Damuth, 1981; Blackburn & Gaston, 1997). However, at local scales, the power of body size in explaining and abundance is substantially weaker (e.g., White et al., 2004), hindering further inferences on the mechanisms underlying species abundance distribution patterns.
Here, using fish abundance data from 54 streams, we followed a different approach (as introduced above) to infer the processes (environmental filtering, niche limitation, or neutral processes) behind species abundance distribution patterns. To do so, we tested whether the absolute difference in body size between the dominant species and the subordinate species was correlated with the abundances of the latter. As discussed above, negative and positive relationships between these variables (absolute difference in body size between the dominant species and abundance of subordinate species), would support the mechanisms of environmental filtering and competition (or limiting similarity), respectively (Fig. 1). A lack of relationship would be consistent with the neutral theory (Mouillot et al., 2007). There is a large amount of evidence suggesting the preponderant role of environmental filtering in structuring stream fish communities (e.g., Erős et al., 2017). However, the processes that regulate community structure in stream networks may depend on the geographical position (Brown & Swan, 2010). In this context, as explained by Henriques-Silva et al. (2019), one of the predictions of the network position hypothesis (NPH), formalized by Schmera et al. (2017), states that the role of environmental filtering would be stronger in more isolated than in less isolated sites. Thus, because we surveyed small streams (first to fourth order), we first hypothesized that a negative relationship between SSA and DIFS would emerge more often across the local communities (but see Mason et al., 2008), a pattern that is more consistent with the environmental filtering hypothesis. Second, according to the NPH, deviation from this pattern would be more frequent in higher‐order streams and, therefore, a positive relationship between our measure of effect size (measuring the relationship between SSA and DIFS) and stream order would emerge.
Methods
Study area
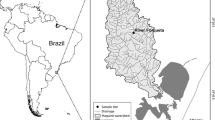
We collected data on fish communities in 54 streams in the Upper Araguaia River and Middle Rio das Mortes basins (State of Mato Grosso, Brazil) (Fig. 2). The streams are located in the Cerrado biome. During the sampling period (2014–2017), the average annual precipitation was 1286 mm and the average annual temperature was 26 °C (INMET network data, www.inmet.gov.br). The sampled streams (first to fourth order according to Strahler classification) are distributed between altitudes of 263 and 427 m above sea level.
Fish sampling
We surveyed a reach of ca. 50 m in each stream. During the field work conducted in 2014 and 2015, we used trawls hand, sieves, and dip net (with a mesh size of 3.0 mm) (Ueida & Castro, 1999). The sampling was carried out by four people during one hour in each reach. In 2016 and 2017, we used electrofishing with a single pass. The characteristics of the electrofishing equipment that we used are described elsewhere (Oliveira et al., 2020). For both sampling methods, the upstream and downstream ends of the reaches were blocked with seines (3.0 mm between adjacent knots). We emphasize that the difference in the sampling methods is unlikely to affect our results given that our analyses (see below) were carried out for each stream. The fishes caught were anesthetized in a solution with benzocaine and then fixed in formaldehyde (2014 and 2015) or alcohol (2016 and 2017). In the laboratory, we identified the individuals to the lowest possible taxonomic level (usually species) using specialized literature and online databases (Reis et al., 2003; Venere & Garutti, 2011; Fricke et al., 2019; Froese & Pauly, 2019). We weighed (g) and measured (standard length; cm) all individuals caught using a precision scale (minimum 0.005 g) and a digital caliper (accuracy of 0.001 cm), respectively. The results of our analyses were independent of the measure of size and, for the sake of brevity, only the results obtained with standard length are shown (Table S1). Sampling permits were issued by the Chico Mendes Institute for Conservation and Biodiversity (ICMBio; SISBio—Nº 45,316–1), and the Animal Use Ethics Committee at the Federal University of Mato Grosso (CEUA/UFMT—Nº 23,108.152116/2016–04).
Datasets
Our dataset consisted of a matrix with S species in each stream (in rows), mean standard length, and abundance (in columns). Following Hidasi-Neto et al. (2020) and the ideas described in Mouillot et al. (2007), our first variable, the abundance of the subordinate species (SSA), consisted of a vector with the abundance of the S-1 subordinate species, for each stream. For example, consider a local community with six species (e.g., species A with 100 individuals, B with 50 individuals, C with 10 individuals, D with 5 individuals, E with 3 individuals and F with 2 individuals); thus, A is the dominant species (i.e., the one with the highest abundance) and SSA, for that community, is represented by the values 50, 10, 5, 3, and 2. Subsequently, for each stream, we calculated the absolute difference in body size (log-transformed) between the dominant and the different subordinate species (DIFS). Therefore, after this procedure, each stream was represented by S-1 lines. For example, supposing that the mean standard lengths (in cm) of the species above were 10.31 (species A), 10.43 (B), 12.51 (C), 10.76 (D), 10.70 (E), and 10.99 (F), then the values of DIFS associated to the subordinate species would be |10.31–10.43|= 0.12, |10.31–12.51|= 2.20, |10.31–10.76|= 0.45, |10.31–10.70|= 0.39, and |10.31–10.99|= 0.68, respectively (for a diagrammatic representation of the procedures, see Fig. S1).
Data analysis
We use two approaches to test the relationship between DIFS (based on mean standard length) and subordinate species abundance (SSA). In the first approach, we calculated the Pearson's correlation coefficient between DIFS and the square root of SSA for each stream. After, we transformed each correlation coefficient into Fisher’s z statistic and used a meta-analytical approach, following the methods described in Borenstein et al. (2009), to estimate a weighted average effect size (see Koricheva & Gurevitch, 2014 for the advantages of using a meta-analysis on data from multisite studies). The weight (Wi) for each estimate (zi statistic for each stream) was calculated according to a random effects model [Wi = 1/Vi + T2, where Viis the variance of the statistic z (Vi = 1/S-1–3), in an given stream i and T2 is the variance between streams; see Eq. 12.2 in Borenstein et al., 2009]. The weighted mean effect size (z++) was then calculated using the following equation: \({z}_{++}=\sum_{i=1}^{k}{W}_{i}{z}_{i}/\sum_{i=1}^{k}{W}_{i}\). We used a permutation test (with 1000 iterations) to test the significance of the weighted mean effect size (z++) and a forest plot to show the Fisher’s z statistic estimated for each stream. The higher the value of the Fisher’s z statistic, the higher the correlation (in modulus) between SSA and DIFS. A negative z value indicates that, for a given stream, the abundances of subordinate species decrease as they become more different (in terms of body size) from the dominant species (i.e., with the increase of DIFS), whereas a positive z value indicates the opposite. For this analysis, it was not possible to estimate the variance of the z statistic for two streams because the richness of subordinate species was equal to 3. Thus, the weighted mean effect size (z++), evaluating the correlation between SSA and DIFS, was estimated with a sample of 52 streams. We used the “metafor” package (Viechtbauer, 2010) in R environment (R Core Team, 2021) for this analysis.
In the second approach to test the relationship between SSA and DIFS, we used a generalized linear mixed-effect model (GLMM; Zuur et al., 2009). Stream reach was included in the model (i.e., SSA ~ DIFS) as a random effect. We assumed random variations in the coefficients (intercepts and slopes) and a negative binomial distribution. For this analysis, we used the “lme4” package (Bates et al., 2015) in the R environment.
To test our second hypothesis, related with the network position hypothesis, we (meta)regressed (see Borenstein et al., 2009) our measure of effect size (Fisher’s z statistic, as described above) on stream order.
The relationships between SSA and DIFS cannot inform which body size values (whether small or large animals) are related to dominance. Thus, we used two approaches to test whether the groups of species (dominant and subordinate) differed in terms of size and mainly to know the direction of the difference. First, we calculated the means and standard deviations of the size (standard length) of the individuals of dominant and subordinate species for each stream. Second, we used Hedges’ g (and their variance; see Eqs. 4.23 and 4.24 in Borenstein et al., 2009) to calculate, in each stream, the difference in size between the subordinate and the dominant species. Third, we used the same procedures described above to calculate the weighted mean effect size using the g values. This analysis was also carried out using the “metafor” package (Viechtbauer, 2010) in the R environment. Second, we used linear mixed-effect models (Zuur et al., 2009) to test whether groups of species (dominant and subordinate) differed in term of size. Again, stream reach was included in the model [i.e., log (length) ~ abundance group] as a random effect and we assume random variations in the intercepts and slopes (Eq. 5.11 in Zuur et al., 2009). For this analysis, we used the “nlme” package (Pinheiro et al., 2018) in R.
Results
Local species richness ranged from 4 to 48, whereas the total abundance ranged from 25 to 1234 individuals. Knodus breviceps (Eigenmann, 1908), Astyanax goyacensis Eigenmann, 1908, and Characidium zebra Eigenmann, 1909 were the most abundant species in the study area (total number of individuals = 883, 537, and 450, respectively). In general, dominant species belonged to the family Characidae (e.g., K. breviceps, Hemigrammus cf. rodwayi Durbin, 1909, Odontostilbe sp., and Serrapinus sp.).
We found negative and positive relationships between SSA and DIFS in 40 and 12 streams, respectively (Fig. 3). The weighted mean effect size, although low (z++ = − 0.1823; standard error = 0.0357; CI95% = −0.2523, − 0.1123; number of streams = 52), was highly significant according to the permutation test (Z = − 5.10; P = 0.001). However, we found a high heterogeneity of effect sizes among streams (Q = 51.73, P = 0.4452; where Q is the weighted sum of squares). The results of the generalized linear mixed-effect model also indicated a negative and significant relationship between SSA and DIFS (SSA = 2.18 – 1.46DIFS, standard errors of the intercept and slope = 0.12 and 0.28, respectively; Z-values = 18.28 and − 5.18; P < 0.0001 for both coefficients). Thus, according to both analytical approaches, as the difference in size increases, there is a decrease in the abundance of subordinate species.
Fisher’s z statistics (± 95% confidence interval) showing the correlation between absolute difference in size (dominant-subordinate; DIFS) and abundance of subordinate species (SSA). The results (one for each stream) are ordered according to the magnitude of the effect size (in descending order). The results were ordered by the effect size in descending order. Average effect sizes (z++) are shown as diamonds
The effect sizes (Fisher’s z statistic) were unrelated to stream order (slope = 0.0139; standard error = 0.0424; P = 0.7429). Thus, there is no evidence that the strength of relationship between SSA and DIFS changes with stream order, as one would expect under the network position hypothesis (NPH).
The dominant fish species were significantly smaller than the subordinate species (g++ = − 0.5689; standard error = 0.1274; CI95% =—0.8186, − 0.3192; Z = -4.4652; P = 0.001, Figs. 4 and 5). However, there was a high heterogeneity between the effect sizes (Q = 1903.09; P = 0.0001; number of streams = 54). The results of the mixed linear model were consistent with those of the meta-analysis as they also indicated that dominant species were significantly smaller-bodied than subordinate species [log(length) = 0.55 + 0.09Groups(subordinate), standard errors of the coefficients = 0.04 and 0.02, respectively; t-values = 21.7 and 3.84; P < 0.0001 for both coefficients].
Hedges’ g statistics (± 95% confidence interval) showing the size difference between dominant and subordinate species (one result per stream). The results (one for each stream) are ordered according to the magnitude of the effect size (in descending order). Average effect sizes (g++) are presented as diamonds. A negative g value indicates that the dominant species are smaller-bodied than the subordinate species, whereas a positive value indicates the opposite
Discussion
We show that (i) the abundances of the subordinate fish species decreased consistently with the increase in the difference in body size in relation to the local dominant species. This pattern was independent of stream order. Also, we found that (ii), in most local communities, subordinate species were larger-bodied than the dominant species. The first result is expected under the environmental hypothesis, whereas the second result supports the Dumuth's rule (Damuth 1981; White et al., 2007), which basically states that “big things are rare” (Isaac et al., 2011).
Using an analytical approach similar to that developed in previous studies (Sugihara et al., 2003; Mouillot et al., 2007; Hidasi-Neto et al., 2020), our results (i.e., negative relationship between SSA and DIFT) are more consistent with the environmental filtering hypothesis than with the limiting similarity hypothesis (which would be favored in case of a positive relationship between SSA and DIFS). Thus, our inferences differ, for example, from those made by Mason et al. (2008), who found support for the limiting similarity hypothesis in lacustrine fish communities. They also argued that the scarcity of evidence (supporting limiting similarity) may be more related to methodological aspects (e.g., use of co-occurrence analyzes only) than to the lack of effect. However, the predominance of negative relationships between SSA and DIFS suggests that the effects of niche complementarity and competitive interactions, at least considering our sample of streams and the grain of our study (50 m), are unlikely to explain our results. If this was the case, then species with similar body sizes and, theoretically, with similar niches, would compete more and, therefore, subordinate species more similar to dominant species would be the least abundant (Mouillot et al., 2007). Our results are not sufficient to exclude the hypothesis that competitive interactions can determine the distribution of abundance among species, but suggest, as discussed above, that the effect of environmental filtering tends to be stronger. Recent studies in five regions (Belize, Benin, Brazil, Cambodia, and the USA) also support the dominant role of environmental filters in structuring stream fish communities (Bower and Winemiller 2019a,b).
Despite the preponderance of negative over positive relationships between SSA and DIFS and a significant weighted mean effect size, we found a large heterogeneity in effect sizes across sites. To account for this heterogeneity, we tested the relationship between effect size (Fisher’s z) and stream order. The reasoning behind this test is that a growing body of literature indicates that different ecological patterns and mechanisms may depend on the spatial positioning of local communities within stream networks (Hitt & Angermeier, 2008; Brown & Swan, 2010; Thornbrugh & Gido, 2010; Larsen et al., 2021). For example, according to the network position hypothesis, environmental filtering is the strongest driver of more isolated communities (Brown & Swan, 2010; Schmera et al., 2017; Henriques-Silva et al., 2019). A positive relationship between Fisher’s z and stream order would indicate that the effects of environmental drivers constraining body size to vary around an optimum body size would be reduced with increasing order. However, we did not find evidence for a relationship between the strength of relationship between SSF and DIFS (as measured by Fisher’s z) and stream order. We cannot rule out that the range of variation in stream order (1 to 4) may have been too small to yield a significant relationship. In addition, the power of network position in mediating community structuring processes may be contingent on different contexts, including taxonomic group (Schmera et al., 2017) and connectivity variation among local communities (Henriques-Silva et al., 2019).
Our complementary analyses, comparing the average size of subordinate and dominant species in each local community, clearly revealed that smaller body sizes are related to the dominance in streams. In general, this pattern is consistent with the well-known Damuth’s rule, whose explanation considers that larger-bodied individuals require more resources than smaller-bodied ones (e.g., Damuth, 1981; Brown et al., 2004; White et al., 2007; Hechinger et al., 2011). The adaptability of small sizes in streams (for a particular species) may be related to the efficiency of preying on small individuals and to the use of specific habitats (Wilson et al., 2003). In addition, some studies have shown that in salmonid populations (in temperate streams) the smallest individuals tend to have higher survival rates (Carlson et al., 2004, 2008). On the other hand, studies on body size-dependent differential survival are rare in tropical environments, but there is much evidence indicating that fish communities are mainly composed of small-bodied individuals. These results can be explained by several factors that limit the occurrence of large individuals in local communities, such as low water depth, shortage of large refuges, and high rates of predation on large individuals in shallow environments, especially by birds and mammals (Power, 1984; Harvey & Stewart, 1991; see review in Matthews, 1998). However, other processes that operate on larger spatial scales may also explain our results. For example, in a recent study, Ilha et al. (2018) demonstrated that fish in deforested Amazonian streams have smaller body sizes than fish in preserved streams. The authors related this result to the increase in water temperature in streams impacted by deforestation. Thus, the reduction in the body size of fish populations with the increase in water temperature, after deforestation of the riparian vegetation, is also a process that could explain our results. Independently of the main mechanism, our results complement the "bigger fish—deeper habitat" pattern (Harvey & Stewart, 1991) with the “smaller fish-shallower habitat” pattern.
The negative relationship between subordinate species abundance and the absolute difference in size, although highly significant, was not high. This result occurred because, in the different local communities, the rarest species frequently showed similar abundances (a fact commonly observed in an abundance-rank curves, e.g., Murray et al., 1999). Thus, for the same subordinated species abundance value, in general, the rarest, a large variation in DIFS was observed. However, the effect size was much greater when (i) we adopted an unequivocal criterion to separate the species according to their abundance in each local community (i.e., dominant species versus other species) and compared the body size between these groups. These results indicate that our ability to predict SSA in function of DIFS may be limited, however, we can predict with high confidence that the dominant species, within local communities, will be smaller than the subordinate (rarer) species.
In conclusion, our results are more consistent with the environmental filtering hypothesis than with the limiting similarity hypothesis because species with lower abundances tended to differ more from the dominant species in terms of a key trait in community ecology, that is, body size. They also demonstrate that the dominant species in streams tend to have smaller body sizes than the subordinate species. Different environmental characteristics of streams (e.g., reduced depth, shortage of large refuges, higher predation rates) and life-history aspects (e.g., populations of small-bodied species tend to recover faster than larger-bodied species after disturbance events in streams) may explain the lower abundance of species with larger body sizes. In general, our results demonstrate, as in several other studies (e.g., Bower & Winemiller, 2019b, 2019a; Ford & Roberts, 2020), the importance of using traits to reveal drivers of species rarity and commonness in local communities.
Data availability
The datasets of this study are available upon request.
Code availability
Not applicable.
References
Bates, B., M. Maechler, B. M. Bolker & S. C. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48.
Blackburn, T. M. & K. J. Gaston, 1997. A critical assessment of the form of the interspecific relationship between abundance and body size in animals. The Journal of Animal Ecology 66: 233.
Borenstein, M., L. V. Hedges, J. P. T. Higgins & H. R. Rothstein, 2009. Introduction to Meta-Analysis, Wiley, Chichester:
Bower, L. M. & K. O. Winemiller, 2019a. Fish assemblage convergence along stream environmental gradients: an intercontinental analysis. Ecography 42: 1691–1702.
Bower, L. M. & K. O. Winemiller, 2019b. Intercontinental trends in functional and phylogenetic structure of stream fish assemblages. Ecology and Evolution 9: 13862–13876.
Brown, B. L. & C. M. Swan, 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580.
Brown, J. H., J. F. Gillooly, A. P. Allen, V. M. Savage & G. B. West, 2004. Toward a metabolic theory of ecology. Ecology 85: 1771–1789.
Carlson, S. M., A. P. Hendry & B. H. Letcher, 2004. Natural selection acting on body size, growth rate and compensatory growth: an empirical test in a wild trout population. Evolutionary Ecology Research 6: 955–973.
Carlson, S. M., E. M. Olsen & L. A. Vøllestad, 2008. Seasonal mortality and the effect of body size: a review and an empirical test using individual data on brown trout. Functional Ecology 22: 663–673.
Damuth, J., 1981. Population density and body size in mammals. Nature 290: 699–700.
Erős, T., P. Takács, A. Specziár, D. Schmera & P. Sály, 2017. Effect of landscape context on fish metacommunity structuring in stream networks. Freshwater Biology 62: 215–228.
Ferreira, F. & M. Petrere-Jr, 2008. Comments about some species abundance patterns: classic, neutral, and niche partitioning models. Brazilian Journal of Biology 68: 1003–1012.
Ford, B. M. & J. D. Roberts, 2020. Functional traits reveal the presence and nature of multiple processes in the assembly of marine fish communities. Oecologia 192: 143–154.
Fricke, R., W. N. Eschmeyer, & R. Van der Laan, 2019. Eschmeyer Catalog of Fishes: genera, species, references.
Froese, R., & D. Pauly, 2019. FishBase. version (04/2019). www.fishbase.org.
Harvey, B. C. & A. J. Stewart, 1991. Fish size and habitat depth relationships in headwater streams. Oecologia 87: 336–342.
Hechinger, R. F., K. D. Lafferty, A. P. Dobson, J. H. Brown & A. M. Kuris, 2011. A common scaling rule for abundance, energetics, and production of parasitic and free-living species. Science 333: 445–448.
Henriques-Silva, R., M. Logez, N. Reynaud, P. A. Tedesco, S. Brosse, S. R. Januchowski-Hartley, T. Oberdorff & C. Argillier, 2019. A comprehensive examination of the network position hypothesis across multiple river metacommunities. Ecography 42: 284–294.
Hidasi-Neto, J., L. M. Bini, T. Siqueira & M. V. Cianciaruso, 2020. Ecological similarity explains species abundance distribution of small mammal communities. Acta Oecol 102: 103502.
Hitt, N. P. & P. L. Angermeier, 2008. River-stream connectivity affects fish bioassessment performance. Environmental Management 42: 132–150.
Hubbell, S. P., 2001. The unified neutral theory of biodiversity and biogeography, Princeton University Press, New Jersey:
Ilha, P., L. Schiesari, F. I. Yanagawa, K. Jankowski & C. A. Navas, 2018. Deforestation and stream warming affect body size of Amazonian fishes. PLoS ONE 13: e0196560.
Isaac, N. J. B., D. Storch & C. Carbone, 2011. Taxonomic variation in size–density relationships challenges the notion of energy equivalence. Biology Letters 7: 615–618.
Klipel, J., R. S. Bergamin, G. D. D. S. Seger, M. B. Carlucci & S. C. Müller, 2021. Plant functional traits explain species abundance patterns and strategies shifts among saplings and adult trees in Araucaria forests. Austral Ecology 46: 1084–1096.
Koricheva, J. & J. Gurevitch, 2014. Uses and misuses of meta-analysis in plant ecology. Journal of Ecology 102: 828–844.
Larsen, S., L. Comte, A. Filipa Filipe, M. Fortin, C. Jacquet, R. Ryser, P. A. Tedesco, U. Brose, T. Erős, X. Giam, K. Irving, A. Ruhi, S. Sharma & J. D. Olden, 2021. The geography of metapopulation synchrony in dendritic river networks. Ecology Letters 24: 791–801.
MacArthur, R. H., 1957. On the relative abundance of bird species. Proceedings of the National Academy of Sciences 43: 293–295.
MacArthur, R. H. & R. Levins, 1967. The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101: 377–385.
Magurran, A. E., 2005. Species abundance distributions: pattern or process? Functional Ecology 19: 177–181.
Mason, N. W. H., C. Lanoiselée, D. Mouillot, J. B. Wilson & C. Argillier, 2008. Does niche overlap control relative abundance in French lacustrine fish communities? A new method incorporating functional traits. Journal of Animal Ecology 77: 661–669.
Matthews, T. J. & R. J. Whittaker, 2015. On the species abundance distribution in applied ecology and biodiversity management. Journal of Applied Ecology 52: 443–454.
Matthews, W. J., 1998. Patterns in Freshwater Fish Ecology, Springer, Boston:
McGill, B. J., 2003. Does mother nature really prefer rare species or are log-left-skewed SADs a sampling artefact? Ecology Letters 6: 766–773.
McGill, B. J., B. A. Maurer & M. D. Weiser, 2006. Empirical evaluation of neutral theory. Ecology 87: 1411–1423.
McGill, B. J., R. S. Etienne, J. S. Gray, D. Alonso, M. J. Anderson, H. K. Benecha, M. Dornelas, B. J. Enquist, J. L. Green, F. He, A. H. Hurlbert, A. E. Magurran, P. A. Marquet, B. A. Maurer, A. Ostling, C. U. Soykan, K. I. Ugland & E. P. White, 2007. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecology Letters 10: 995–1015.
Mouchet, M. A., S. Villéger, N. W. H. Mason & D. Mouillot, 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology 24: 867–876.
Mouillot, D., N. W. H. Mason & J. B. Wilson, 2007. Is the abundance of species determined by their functional traits? A new method with a test using plant communities. Oecologia 152: 729–737.
Murray, B. R., B. L. Rice, D. A. Keith, P. J. Myerscough, J. Howell, A. G. Floyd, K. Mills & M. Westoby, 1999. Species in the tail of rank-abundance curves. Ecology 80: 1806.
Oliveira, F. J. M., D. P. Lima-Junior & L. M. Bini, 2020. Current environmental conditions are weak predictors of fish community structure compared to community structure of the previous year. Aquatic Ecology 54: 729–740.
Peters, R. H., 1983. The ecological implications of body size, Vol. 2. Cambridge University Press, New York:
Pinheiro, J., D. Bates, S. DebRoy, D. Sakar, & R. C. Team, 2018. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.
Power, M. E., 1984. Depth distributions of armored catfish: predator-induced resource avoidance? Ecology 65: 523–528.
R Core Team, 2021. R: a Language and environment for statistical computing. Vienna, Austria, http://www.r-project.org/.
Reis, R. E., S. O. Kullander, & C. J. Ferraris, 2003. Check List of the freshwater fishes of South and Central America. EDIPUCRS. Porto Alegre, RS.
Schmera, D., D. Árva, P. Boda, E. Bódis, Á. Bolgovics, G. Borics, A. Csercsa, C. Deák, E. Á. Krasznai, B. A. Lukács, P. Mauchart, A. Móra, P. Sály, A. Specziár, K. Süveges, I. Szivák, P. Takács, M. Tóth, G. Várbíró, A. E. Vojtkó & T. Erős, 2017. Does isolation influence the relative role of environmental and dispersal-related processes in stream networks? An empirical test of the network position hypothesis using multiple taxa. Freshwater Biology 63: 74–85.
Sugihara, G., L.-F. Bersier, T. R. E. Southwood, S. L. Pimm & R. M. May, 2003. Predicted correspondence between species abundances and dendrograms of niche similarities. Proceedings of the National Academy of Sciences 100: 5246–5251.
Thornbrugh, D. J. & K. B. Gido, 2010. Influence of spatial positioning within stream networks on fish assemblage structure in the Kansas River basin, USA. Canadian Journal of Fisheries and Aquatic Sciences 67: 596–596.
Tokeshi, M., 1993. Species abundance patterns and community structure. Advances in Ecological Research 24: 111–186.
Ueida, V. S. & R. M. C. Castro, 1999. Coleta e fixação de peixes de riacho. In Caramaschi, E. P., R. Mazzoni & P. R. Peres-Neto (eds), Ecologia de peixes de riachos Oecologia Brasiliensis, Rio de Janeiro: 1–22.
Umaña, M. N., C. Zhang, M. Cao, L. Lin & N. G. Swenson, 2015. Commonness, rarity, and intraspecific variation in traits and performance in tropical tree seedlings. Ecology Letters 18: 1329–1337.
Venere, P. C. & V. Garutti, 2011. Peixes do Cerrado: Parque Estadual da Serra Azul, Rio Araguaia, MT, RiMa, São Carlos:
Viechtbauer, W., 2010. Conducting meta-analyses in R with the metafor Package. Journal of Statistical Software 36: 1–48.
White, E. P., S. K. M. Ernest, A. J. Kerkhoff & B. J. Enquist, 2007. Relationships between body size and abundance in ecology. Trends in Ecology & Evolution 22: 323–330.
Wilson, A. J., J. A. Hutchings & M. M. Ferguson, 2003. Selective and genetic constraints on the evolution of body size in a stream-dwelling salmonid fish. Journal of Evolutionary Biology 16: 584–594.
Winemiller, K. O., 1991. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecological Monographs 61: 343–365.
Zuur, A. F., E. N. Ieno, N. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R, Springer, New York:
Acknowledgements
We thank two anonymous reviewers for their valuable comments. FJMO was supported by a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are grateful to the Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT) for the financial support (227925/2015). DPLJ and LMB are supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (448823/2014-4 and 308974/2020-4, respectively). This paper was developed in the context of National Institutes for Science and Technology (INCT) in Ecology, Evolution, and Biodiversity Conservation supported by MCTIC/CNPq (465610/2014‐5) and FAPEG (201810267000023).
Funding
Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT Notice 002/2015, Process 155509/2015) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance Code 001). This work was also developed in the context of the National Institutes for Science and Technology (INCT) in Ecology, Evolution and Biodiversity Conservation, supported by MCTIC/CNPq (proc. 465610/20145) and FAPEG.
Author information
Authors and Affiliations
Contributions
LMB and FJMO conceived and designed the study. FJMO and DPLJr performed data collection in the field. FJMO and LMB performed the analyses and wrote the manuscript with the support of DPLJr. FJMO, DPLJr, and LMB reviewed the manuscript and approved the publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Animal Use Ethics Committee of the Federal University of Mato Grosso (CEUA/UFMT – Nº 23108.152116).
Additional information
Handling Editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oliveira, F.J.M., Lima Junior, D.P. & Bini, L.M. Body size explains patterns of fish dominance in streams. Hydrobiologia 849, 2241–2251 (2022). https://doi.org/10.1007/s10750-022-04860-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04860-6