Abstract
Using nuclear DNA sequences we studied the phylogeny and genetic structure of trout in Morocco, and estimated the impact of stocking upon natural populations. We supplemented the sample set from a previous study with new sample localities and with specimens from the main hatchery in Morocco. Phylogeny was performed using Bayesian inference, identification of genetic clusters and the genetic structure of populations were assessed from microsatellite polymorphism using discriminant analysis of principal components, and genealogical relationships among mitochondrial haplotypes were re-estimated with an unrooted phylogenetic network using statistical parsimony. Nuclear DNA sequences demonstrated the existence of three major clades in north-west Africa: (1) an Afro-Atlantic clade in Moroccan Mediterranean coastal rivers and the Sous drainage, stemming from a colonisation wave of the Atlantic lineage from Iberia, (2) a relict and native Draa clade (Dades trout), which has maintained an incipient nuclear DNA and mitochondrial DNA signature, and (3) a native Atlas clade, sister to Draa, in which only Atlantic mitochondrial DNA was found, suggesting invasion by Atlantic trout that introgressed into local populations and resulted in a reticulate evolutionary event. Microsatellite analysis and the distribution of the haplotypes revealed a large impact of hatchery trout stocking on wild populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
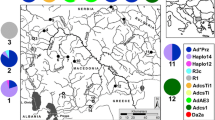
In north-west Africa (Morocco and Algeria), most rivers are too warm to support populations of trout. Such populations that do exist are limited to high-altitude streams and lakes and close-to-source reaches of short Mediterranean rivers at low altitudes with permanent springs. In Morocco, trout (Salmo sp.) are found mainly in the river systems fed from the High Atlas: the drainages of the rivers Tensift, Sous, Draa and Ziz, and the left tributaries of the Oum Er-Rbia and right tributaries of the Moulouya. Most of these rivers flow toward the Atlantic Ocean, apart from the Ziz, which flows into the Sahara Desert, and the Moulouya with its outflow into the Mediterranean Sea. Trout exist also in the Middle Atlas, which supplies the water to the left tributaries of the Moulouya, the upper Oum Er-Rbia river system and the Sebou drainage, with the last two flowing into the Atlantic Ocean, and in the coastal rivers flowing from the Rif Mountains into the Mediterranean Sea (Fig. 1).
Map of major Moroccan drainages with sampling sites. Pie charts show the fraction of different mt DNA clades (see Fig. 4) detected at each site (see legend in the lower right corner). The colour of the rim of the pie charts refers to nc data and corresponds to the colours in Figs. 2 and 3, while the grey colour indicates missing data. The asterisk locates the region of Lake Sidi Ali. In the small-scale map, the sampling area is roughly denoted
The deep gorges and canyons delineating the adjacent upper tributaries of the major river systems in the High Atlas make it implausible that trout populations have ever been in contact with each other here, or indeed have crossed into adjacent river systems. However, such contact appears to have taken place, in at least the endorheic Ziz drainage, which used to be a part of a peri-Saharan ancient lake system ca. 10,000 years ago (Clavero et al., 2017), and was thus probably colonised by trout via a river capture event rather than from the sea. Recent research into trout from Morocco (Snoj et al., 2011; Doadrio et al., 2015; Sanz, 2018; Tougard et al., 2018) included all the above-mentioned river drainages except the Sebou, while in the Sous only Lake Ifni was sampled. This lake drains via underground connections into the Tifnoute River, a tributary to the Sous River. An interesting question concerns the genetic similarity between the trout of the lake and those from Tifnoute, and whether the trout populated the lake naturally or were introduced.
Molecular genetic studies on trout in north-west Africa have so far been performed using mainly mitochondrial (mt) DNA sequences. Only a single investigation into trout from Morocco was designed to study the phylogeography of that trout (Snoj et al., 2011), while other recent molecular studies that have referred to the molecular phylogeny of African trout were primarily focused on verifying the taxonomic status of Salmo macrostigma (Duméril, 1858) (Tougard et al., 2018) or on the phylogeography of trout in Sicily (Fruciano et al., 2014). Nevertheless, through those surveys, conducted on the mt DNA control region (CR) or on concatenated sequences of CR and the cytochrome b gene, all the haplotypes found in north-west Africa have been found to belong to or tightly cluster with the Atlantic mt lineage, with the exception of a highly divergent haplotype restricted to the upper Dades river system in the Draa drainage. Occurrence of trout in the Dades River was first mentioned by Mouslih (1987), while Snoj et al. (2011) first reported on the molecular divergence of the Dades haplotype, suggesting coalescence with the nascent stage of the major mitochondrial clades of brown trout (Salmo trutta Linnaeus, 1758), which were based on the origin of the first haplotypes identified named Atlantic (AT), Danubian (DA), Mediterranean (ME), marmoratus (MA), and Adriatic (AD) lineages (Bernatchez et al., 1992). On the basis of its molecular and phenotypic distinctiveness, there have been attempts to describe Dades trout as a separate species (e.g., Doadrio et al., 2015).
There are reports of trout being bred in hatchery facilities in the Azrou area prior to 1948 with the intention of propagating native ‘S. macrostigma’, (Pellegrin, 1924a; Joleaud, 1938; see Tougard et al., 2018 for taxonomic description) by means of artificial restocking or improvement of watercourses in suitable areas (Vivier, 1948). As a curiosity, hybrids between ‘Salmo pallaryi Pellegrin, 1924’ from Lake Aguelmame Sidi Ali in the Middle Atlas (Pellegrin, 1924b) and ‘S. macrostigma’ were bred in this, probably the first, hatchery in Morocco (Vivier, 1948). The existence of two small trout-rearing facilities in Midelt and the upper Issil river system in the Tensift drainage near Oukaimeden was also reported (Vivier, 1948). Furthermore, imports and introduction of ‘Salmo trutta fario’ originating from France were reportedly performed in the rivers of the Middle Atlas in 1952 (Mouslih, 1987). In 1957, the hatchery Ras el Ma was established, located close to the source of the Tigrigra River (Beht river system in Sebou drainage) with the intention of producing trout for restocking needs. In addition, in 1981, a government authority—the National Centre of Hydrobiology and Fish Farming (CNHP)—located in nearby Azrou, was founded, becoming a reference centre for trout production and responsible for managing Ras el Ma.
Molecular analysis has indicated that domestic North Atlantic brown trout strains, used widely for commercial purposes all over the world, have not been used for stocking in Moroccan rivers (Snoj et al., 2011). Nevertheless, the possibility of anthropogenic transfers between drainages of hatchery-reared local trout must be considered. Although haplotype endemism and haplotype fixation in many sampling locations imply that the distribution of trout in Morocco is natural, possible traces of stocking and transfer between river systems is suggested (Snoj et al., 2011). Meanwhile, microsatellite analysis shows a certain degree of overlap among some geographically isolated populations, though, because no reference from the trout hatchery was included in the sample set, the method did not resolve the stocking question (Snoj et al., 2011).
The objective of the present research was to provide a more reliable and detailed phylogeographic structure of trout from north-west Africa and to study the impact of stocking. Given that mt phylogenetic analysis does not always match the phylogeny of a species or the nuclear (nc) DNA (e.g., Sušnik et al., 2007; Gissi et al., 2008; Pustovrh et al., 2011; Wallis et al., 2017), we re-assessed the phylogeny of trout in Morocco with nc markers that we had previously developed and used in defining taxonomic units of Salmo in the north-west Balkans (Pustovrh et al., 2014a). The impact of stocking was studied using microsatellites.
Materials and methods
Samples
Forty-one individuals were collected in 2018 from the following sampling sites: Tifnoute River (N = 14; the intermittent outlet of Lake Ifni); Lake Tamda (N = 10), which belongs to the Draa drainage and could thus be an unidentified part of the Dades trout natural range; and Tessaout River (N = 17; left tributary of Oum Er-Rbia River). A sample from Ras el Ma hatchery comprised seven individuals: four from 2009, provided by P. Berrebi, and three from 2018, provided by H. Benaisa (Table 1; for sampling site details, see Online Appendix 1). In the present study, CR sequences and microsatellite data were obtained from this sample set (N = 48). Nuclear sequences were obtained both from this sample set and from the earlier study of Snoj et al. (2011) (Tables 1 and 2), whereby two or three individuals per sampling site were sequenced, giving a total of 34 individuals (Tables 1 and 2).
The nc sequences of Salmo ohridanus Steindachner, 1892, all main evolutionary lineages of the S. trutta complex, and trout from Sicily (see Pustovrh et al., 2014a, b) were also included as references, while Salmo salar Linnaeus, 1758 was used as an outgroup.
Each of the collected individuals (from the present study and Snoj et al., 2011) was screened for their CR sequence and microsatellite analysis (N = 107 and 108, respectively; Tables 1 and 2).
Three CR sequences of trout specimens from the ichthyology collection of the Natural History Museum, Paris, one from Morocco, collected from the Tigrigra River (Sebou drainage) in 1923 (MNHN-IC-1977-0272), and two from Algeria, one collected from Oued-el-Abaïch in 1858 (MNHN-IC-A-7585) and another before 1909 (unknown location; MNHN-IC-0000-1909) were also included in the analysis, along with eight new CR sequences, detected in extant trout in Morocco by Doadrio et al. (2015) (Table 1; see Online Appendix 2 for GenBank accession numbers).
A series of trout CR sequences previously detected in the Iberian Peninsula and covering three main clades within the southern Atlantic lineage of S. trutta (Cortey et al., 2009), along with the one detected in trout in Sicily (Schöffmann et al., 2007), were used as a reference in haplotype network analysis (see Online Appendix 2 for GenBank accession numbers).
Phylogeny based on nc sequences
DNA polymorphism in the samples was studied using 21 nc sequences containing multiple SNPs per locus. These markers were described in Pustovrh et al. (2011) as polymorphic among Salmo marmoratus Cuvier, 1829, Salmo obtusirostris (Heckel, 1851) and Danubian, Atlantic and Adriatic evolutionary lineages of S. trutta and later used by Pustovrh et al. (2014a, b) to study Salmo sp. phylogeny.
Polymerase chain reaction (PCR) primers and conditions are described in Pustovrh et al. (2011), while PCR mixtures were prepared as in Pustovrh et al. (2012). For DNA sequencing, 100 ng of PCR product was purified using ExoSAP and used in cycle sequencing reactions following BigDye Terminator v3.1 Cycle Sequencing protocols (Applied Biosystems). The amplified, fluorescently labelled and terminated DNA was salt-precipitated and analysed with an ABI 3130 XL Genetic Analyser. All newly determined sequences were submitted to GenBank (MW143095–MW143239).
Sequences were aligned with MEGA 6 software (Tamura et al., 2013) using CLUSTAL W (Thompson et al., 1994).
A phylogenetic tree was constructed using Bayesian inference implemented in MrBayes 3.2.5 (Ronquist et al., 2012), with 25 million generations sampled using eight chains with 0.3 temperature difference in two runs. The best-fit substitution model and partitioning strategy of loci were selected using PartitionFinder v1.1.1 (Lanfear et al., 2017), which, based on Bayesian Information Criterion (BIC) selection, proposed an HKY + G + I nucleotide substitution model with all loci concatenated in one partition. For any missing loci in each individual, n-characters were incorporated into concatenated alignment. Indels were treated as missing data.
Heterozygosity sites were observed on chromatograms as double peaks and coded as ambiguous using the IUPAC code.
Microsatellite amplification and data analysis
Twelve microsatellite loci were chosen for analysis using primers for PCR amplification and multiplex PCR conditions as described in Lerceteau-Köhler and Weiss (2006) and Snoj et al. (2011). Microsatellites were genotyped on the ABI 3130 XL GeneticAnalyzer (Applied Biosystems, USA) using GeneMapper Software v4.0 (Applied Biosystems).
Basic microsatellite statistics (estimated heterozygosity (He), allelic richness (AR), FIS and pairwise FST values) were calculated in FSTAT (Goudet, 2001).
DAPC analysis using microsatellite data
To assess the genetic structure among the samples and identify clusters of genetically related individuals we used discriminant analysis of principal components (DAPC; Jombart et al., 2010) implemented in the adegenet 2.2.1 (Jombart et al., 2018) package within R (R Core Team, 2017).
This two-step multivariate ordination approach firstly transforms raw genetic data using principal component analysis (PCA) and secondly maximises genetic differentiation between groups, while overlooking within-group variation (Jombart et al., 2010). This is achieved without the need to meet commonly required assumptions (e.g., Hardy–Weinberg equilibrium, linkage disequilibrium, random mating) on underlying genetic data (Dufresne et al., 2014), in stark contrast to Bayesian model-based clustering methods, which are also sensitive to uneven sample sizes among populations (Puechmaille, 2016).
The number of genetic clusters was examined using the find.clusters function, which implements successive K-means clustering. Selection of the optimal K values was achieved using BIC.
In order to find a trade-off between the power of discrimination and avoidance of overfitting we determined the optimal number of PCs to retain for analysis. We calculated the a-score using the optim.a.score function in addition to cross-validation using xvalDapc, both in the adegenet 2.2.1 package.
The relationship between genetic clusters was visualised in a scatterplot, while assignment of probability of individuals to a particular genetic cluster was visualised in a STRUCTURE-like graph from DAPC obtained through probabilistic group assignments.
To resolve differentiation of clusters that overlapped on the scatterplot, the clusters were re-analysed independently (hierarchical approach) in subsequent DAPC analysis.
Mitochondrial DNA
All methodological procedures for PCR amplification of mt CR are the same as those reported in Snoj et al. (2011).
Genealogical relationships among haplotypes were estimated with an unrooted phylogenetic network using statistical parsimony, implemented in TCS v1.21 software (Clement et al., 2000).
Results
Molecular phylogeny based on nc sequences
The sequences of all the loci were successfully amplified in two individuals, while for the others missing sequences were observed at 1–3 loci, with the exception of the two individuals from Lake Isli, for which eight and eleven missing sequences appeared (Online resource 1—Specimen list), likely due to poor quality DNA causing PCR amplification difficulties. However, neither the presence nor absence of these two individuals in the nc-sequence-based phylogenetic analysis affected the topology and branching support of the tree. Thus, despite the missing data, these two samples were retained in the analysis (cf. Jiang et al., 2014; Xi et al., 2016).
The sequences of trout from the Ras el Ma hatchery and Lake Tamda, representatives of artificially created trout stocks (see “Discussion” section), were omitted from the data set.
Final alignment consisted of 21 concatenated nc sequences originating from 100 individuals (34 from Morocco and the remaining from the reference sample set, including two S. salar and two S. ohridanus individuals) totalling 7082 bp in length and revealing 317 variable sites with 286 of them being parsimony-informative (Online resource 2—Sequence alignment).
A phylogenetic tree based on nc sequences revealed two statistically highly supported clusters within trout from the genus Salmo (pp = 100; Fig. 2). According to mt DNA lineage affiliation the first cluster was shared among Atlantic, Duero and marmoratus individuals, and the second among those from the Danubian, Mediterranean and Adriatic mt DNA lineages. Morocco individuals were placed in both clusters.
Trout from the coastal Mediterranean rivers of the Rif Mountains and Sous drainage (Lake Ifni and headstreams of the Tifnoute River) formed a distinct clade we term the Afro-Atlantic clade. Individuals from Sicily formed a sister clade with the Afro-Atlantic clade, and a tight relationship among Afro-Atlantic, Sicily, Duero and Atlantic individuals was evident (Fig. 2). All of the clades within this cluster, as well as the relationships among them, were highly supported.
The other trout from Morocco form part of the second cluster, which was also well supported, and grouped into two sister clades: one from the Dades river system (Draa clade) and one from the other locations in the High Atlas (Tensift, Oum Er-Rbia and Moulouya drainages, and also upper Ziz), the so-called Atlas clade. In the latter, a close relationship between one specimen from Aït Mizane and two from Ourika (both from Tensift) was clearly visible; the two Ourika concatenated nc sequences stemmed from a common ancestor, whilst the other Aït Mizane specimen grouped in the subclade with trout from the Oum Er-Rbia drainage, Berrem and Ziz. The relationship among the Atlas, Adriatic and Danubian clusters, although less supported than the others, suggested adaptive radiation or lack of a phylogenetic signal to identify the evolution of these lineages.
Genetic diversity (He and AR) and F-statistics estimated from microsatellites.
Values for He and AR (Table 2) were considered only for samples comprising more than five individuals. These values ranged from 0.39 to 0.62 for He and 1.8 to 2.6 for AR. The largest values were observed in the Ras el Ma sample (0.62 and 2.6, respectively), followed by the Tamda, Ifni and Tassaout samples. The lowest values (0.39 and 1.8, respectively) were recorded in the Tifnoute sample.
In none of the samples did the FIS value deviate statistically significantly from zero.
The range of genetic differentiation between sample pairs was large, with FST values ranging from 0.100 to 0.609 (Table 3). The lowest FST values (0.100–0.188) were found between the sample from Lake Tamda and those from Ziz, Melloul and Berrem, and between the sample from Ras el Ma and those from Aït Mizane, Melloul and Ziz (the last two comparisons, non-significant). The majority of the remaining sample pairs were characterized by very large FST values (0.359–0.609), with the Dades sample exhibiting the largest level of genetic differentiation from all other samples. Statistically significant FST values (P < 0.05) were found in one-third of the pair comparisons, while samples with a small number of individuals (e.g. Talembote, Adelma, M’Goun) showed non-significant levels of differentiation (Table 3).
Discriminant analysis of principal components (DAPC) using microsatellite data
Examination of the number of clusters with BIC indicated the presence of an optimum of 5–12 clusters. Results obtained with K = 12 were largely consistent, with clustering of individuals into locality-predefined populations and with the lowest BIC values (Online resource 3—DAPC). In retaining eleven principal components and two discriminant functions the results described 58 percent of the genetic variance.
For alternative results obtained with K = 5 see Online resource 3—Fig. S5.
A scatterplot showed 47.2 percent of the genetic variation located along the first discriminant axis (DA 1, vertical axis in Fig. 3), distinguishing samples from the Dades and M’Goun cluster (1 in Fig. 3, the Draa group) from the other samples (Fig. 3A). The second axis (DA 2, horizontal axis in Fig. 3) described 21.6 percent of variation, with the more scattered genetic clusters (A2–A5) constituting the samples from the rivers Tifnoute (A2), Adelma (A3), Lake Ifni (A4) and Talembote (A5) forming the so-called Afro-Atlantic group. While clusters A2 and A3 showed clear differentiation from the other clusters, A4 and A5 flowed towards and contacted the so-called Atlas group (Fig. 3A). This latter group comprised clusters A6–A12.
Microsatellite DAPC scatterplot. A clustering of individuals, colour-coded by genetic cluster obtained by K-means clustering (compare with localities in Fig. 1); B sub-sampling of Atlas group, where rim colour in clusters B6 and B7 correspond to colours in C denoting sampling sites Ziz, Ras el Ma, Tamda and Melloul. Indistinguishable clusters of ‘Heavily managed populations’ in B discriminated along single axis (C); D probabilistic group assignment of datasets used in A (DA) and B (DB), with colours indicating reciprocal clusters and vertical lines representing individuals from a specific locality, indicated with horizontal colour coded bands
After re-analysis of the Atlas group using a hierarchical approach (Fig. 3B, DB), seven clusters emerged, revealing a clear genetic distinction of samples from the rivers Isli (B1), Tessaout (B2), Aït Mizane (B3), Ourika (B4) and partially also Berrem (B5), whilst retaining 64 percent of the genetic variance (PC = 10) in a given subset. The four samples Tamda, Ras el Ma, Ziz and Melloul formed a single group of two clusters (B6 and B7) with very similar genetic structuring. After an additional hierarchical step (Fig. 3C), no genetic clustering was observed (K = 1). To further visualise the structure of this group, DAPC on a single DA axis was performed, with individuals classified to sample rather than genetic cluster. Clear inter-sample overlap was visible (Fig. 3C) indicating a shared origin of some individuals from these samples.
Haplotype network and geographic distribution based on CR sequences
The mt DNA haplotype network, comprising 44 haplotypes of trout from Morocco, Algeria, Sicily, north and north-west Iberia and northern Europe (see Cortey et al., 2009 for details including haplotype group nomenclature, and Online Appendix 2 for GenBank accession numbers), revealed the Dades haplotype with a very divergent position from four distinctive groups, of which one was unique to Morocco (M) and two others (AT3-2 and AT3-3) with radiation centres in Europe and covering NW Africa with only some of their haplotypes, with a fourth (AT3-1) containing only haplotypes from north-western Europe (Fig. 4).
Unrooted mt DNA haplotype network. Haplotypes found in Morocco are colour-coded (as in other figures except for Algeria, black; Miaami Lagoon, dark grey; Lakhdar, grey; Tigrigra, light grey) and visualized with frequency pie charts. White circles represent reference haplotypes of the Atlantic evolutionary lineage of brown trout taken from Cortey et al. (2009); circle-sizes do not correspond to haplotype frequency. The names of putative hatchery haplotypes are italicised. Each line between two haplotypes represents one mutation; black dots represent undetected or hypothetical ancestral haplotypes
Group M was found in Aït Mizaine and in the drainageof Oum Er-Rbia (including in the newly sampled Tessaout River) but also in Draa (Lake Tamda) and Ziz, as well as in Ras el Ma hatchery. ATM1 was the most commonly observed haplotype of this group, found in many sampling sites (Table 1). Four out of five haplotypes found in Melloul, belong to this group.
Group M interconnected with the Dades haplotype by twelve mutation steps from ATM1 (Fig. 4).
Group AT3-3, reported by Snoj et al. (2011) to comprise haplotypes from north-western Iberia, Lake Ifni, and Mediterranean coastal rivers in Morocco and Sicily, was supplemented in the present study with haplotype ATM16, which was found in Lake Ifni (see Doadrio et al., 2015), and two haplotypes, ATA1 and ATA2, revealed from museum specimens collected in Algeria (Table 1; Fig. 4; Tougard et al., 2018). In the Tifnoute River, sampled in the present study, the ATM3 haplotype was found, as well as in Lake Ifni. Haplotype ATM4, detected in the Mediterranean river Moulouya, was found also in the Tensift drainage, in the Beht river system (Tigrigra River) and also in Lake Tamda (Table 1).
The group AT3-2 contained many haplotypes with a geographically diverse origin across Europe, including a sub-cluster of ‘Moroccan haplotypes’, found mainly in the Oum Er-Rbia and Moulouya drainages, and also in the Ziz, Lake Tamda and Ras el Ma hatchery. This sub-cluster interconnected with the remaining haplotypes of AT3-2 group via ATcs25 (Fig. 4).
In many places (Lake Isli, Ourika, Dades, M’Goun, Talembote, Adelma, Tifnoute and Tessaout) only one haplotype was found in a single sampled location. However, we also found haplotypes (ATM1, ATM4, ATM7 and ATcs25) that appeared in more than one population. In Lake Tamda, Ras el Ma, Aït Mizane, Ziz, Melloul and Miaami Lagoon genetically distant haplotypes were found.
Discussion
Phylogeny and phylogeography
The phylogeny of African trout based on nc DNA sequences shows only partial consistency with the mt DNA results. Mitochondrial data have revealed two main evolutionarily distinct groups in NW Africa: Dades trout, a very divergent, relict lineage with an unclear phylogenetic position in the Salmo trout complex (Snoj et al., 2011; Sanz, 2018), and other African trout populations, identified by all phylogenetic studies to date as either part of a large Atlantic clade (Bernatchez, 2001; Snoj et al., 2011) or closely related to it (Tougard et al., 2018).
Nuclear DNA-based phylogeny has shown that the trout of the coastal Mediterranean rivers of NW Africa are closely related to the trout of north-west Iberia and Sicily. This has also been suggested using mt CR (Schöffmann et al., 2007; Cortey et al., 2009; Fruciano et al., 2014) on the basis of which trout from Algeria are also included in this group (Tougard et al., 2018; present study, Fig. 4). Thus, it appears that this clade, which we term Afro-Atlantic, is of Iberian origin and not only colonised the Mediterranean rivers in Morocco but also expanded along the African coast to Algeria and further up to Sicily (reviewed in Sanz, 2018). In the present study, we confirmed this hypothesis by using a different type of genetic marker and by expanding the sample set. Interestingly, trout from Lake Ifni, along with its underground outflow Tifnoute River (Sous drainage), which flows into the Atlantic Ocean, also grouped within this clade (Snoj et al., 2011; Tougard et al., 2018). We identified a common origin for these populations with their very close relationship inferred from nc sequences and shared haplotype, whilst a genetic difference revealed by microsatellites suggests a lasting interrupted communication between them. Indeed, the lake and Tifnoute River became further isolated (Philip Hughes, personal communication) following a landslide (Panouse, 1963) in the Holocene prior to 4500 years ago, cutting off any gene flow between neighbouring populations. Microsatellite analysis showed a distinct genetic signature of Tifnoute within the Afro-Atlantic sample (Fig. 3 and Online resource 3—Fig. S5), while some allele sharing is evident between the Ifni and the coastal Mediterranean river Talembote (Fig. 3). Therefore, the dissimilarity between Ifni and Tifnoute populations might not be assigned only to drift but also to differences in their recent histories. The history of colonisation of the Sous drainage, which is geographically discontinuous and remote from the coastal Mediterranean rivers of Morocco, remains unclear, but is probably explained in Pleistocene palaeo-geographic events that generated a mixed pattern of trout colonisations. We have been able to recognise this clade, including the Sous population, also on the basis of its observable phenotypic features: large black and red spots, elongated head with pointed snout and long maxillary, dorsal and anal fins with white leading edges, and never four dark bars on the sides of the body. These traits were observed also in trout from Sicily and in museum specimens from Algeria, stored in the Natural History Museum in Paris. All this information suggests that the Afro-Atlantic clade is a descendant of an Atlantic evolutionary lineage. This lineage has either experienced no gene flow with any incipient lineage in north-west Africa, or it has so out-competed any such lineage that no genetic signature of its existence remains.
On the basis of nc sequences, Dades trout holds a distinct evolutionary position, revealed previously with mt DNA, albeit with a less pronounced ancestral position within the Salmo trout complex and increased affinity with other Atlas specimens. A common ancestor of the Draa (Dades and M’Goun populations) and the Atlas clade forms a sister relation with the Adriatic lineage, consistent with the hypothesis (Sanz, 2018) proposing pre-Pleistocene colonisation of ancestral Adriatic trout spreading from the east (from Anatolia) to the west, to the rest of the Mediterranean, to the Iberian Peninsula and down to North Africa. From this perspective, the findings from a previous study (Snoj et al., 2011), and the results from the nc sequences analysed in the present study, would support the idea that the Dades trout could be a relict element of the pre-Pleistocene colonisation wave that survived warm Pleistocene interglacials in the High Atlas (Sanz, 2018) but which probably occupied a much larger area than it does currently.
The remaining specimens from Morocco grouped, on the basis of nc sequences, into a clade, here named Atlas, also showing no affinity with the Atlantic evolutionary lineage but rather with the Adriatic–Danubian branch. Following the Sanz (2018) hypothesis discussed above, one might conclude that the Atlas and Draa clades split from an ancestral Adriatic lineage that colonised the rivers of Morocco in the pre-or early Pleistocene. As already revealed, the Draa clade has remained independent, possibly as the Draa is the southernmost, and warmest, river, so that later colonisation may not have been possible.
In contrast to the trout of the coastal Mediterranean rivers of NW Africa and Dades trout, ‘Atlas trout’ is characterised by differences in the history of mt DNA and nc DNA markers. Bearing only Atlantic haplotypes, these populations were probably affected by the invasion of a strain—characterised by Atlantic mt DNA—that introgressed with indigenous trout creating a newly admixed lineage, resulting in a reticulate evolution event. Because no haplotypes other than Atlantic have so far been found there it may be hypothesised that the incipient mt DNA was completely replaced. Similar mt DNA capture events have previously been observed in Salmo, for example, in geographically isolated soft-mouth trout (S. obtusirostris) populations with an incipient nc DNA profile, but with brown trout mt DNA (Sušnik et al., 2007). However, in the present study, due to the small sample sizes and limited number of nc sequences analysed, the mt DNA capture is difficult to ascertain.
The Atlantic haplotypes that correspond to the nc DNA Atlas clade can be divided into at least two phylogenetically rather divergent haplogroups: M and AT3-2 (Fig. 4). This distinction indicates that the invasion of Atlantic strains may have taken place even in more than one successive wave.
The haplogroup M was characterised in Snoj et al. (2011) by haplotype ATM1, with early divergence from the common ancestor of the entire Atlantic lineage (see also Sanz, 2018). However, in the present study, which includes additional samples, a larger group of related haplotypes clustered around ATM1, indicating a past abundant distribution of this haplogroup. The origin of this haplogroup is unclear because it has not been found outside of Morocco.
The haplogroup AT3-2 comprises both Moroccan and Iberian haplotypes with ATcs25 in common (Fig. 4). The haplotypes from this cluster present in Morocco appear in the left side of the Oum Er-Rbia drainage including Lake Isli, and also in the upper tributaries of the Moulouya and Ziz drainages. Apart from in Morocco, Atcs25, along with its closely related haplotypes, was found mainly in the coastal rivers of the Cantabrian Sea (Cortey et al., 2009). Perhaps this area is one of the expansion centres from where trout might have colonised Morocco along the Atlantic coast, possibly via the Oum Er-Rbia drainage. Further expansion of trout between the Oum Er-Rbia, Moulouya and also Sebou (Beht) drainages possibly took place through river capture events, suggested by haplotype sharing (of ATM4). This is most likely to have happened in the Lake Sidi Ali region (Fig. 1), where these three drainages come close together and elevation differences between adjacent upper reaches are the least (ca. 100 m). Certainly, human-mediated fish transfers, which can significantly influence haplotype distributions in affected areas, should not be neglected.
Impact of stocking on the genetic structure of trout in Morocco
In our previous study (Snoj et al., 2011), we found some molecular-based support suggesting that trout are managed in Morocco. There were divergent haplotypes in a small and isolated population in Aït Mizane, and haplotype sharing between geographically remote and disconnected river systems, indicated by the presence of ATM 4 in the Ourika River (Tensift drainage) and in the Berrem River (Moulouya drainage) (Snoj et al., 2011; Fig. 4). However, with this new expanded data set, this effect is more apparent (Figs. 3, 4, and Online resource 3—Fig. S5). Some haplotype sharing exists between the sampling sites of Lake Tamda, Aït Mizane, Berrem, Melloul and Ziz, and the Ras el Ma hatchery sample. Firstly, haplotypes ATM1 and ATM7, found in the sample set obtained from Ras el Ma, may be an indicator of stocking in wild populations, especially where mutually divergent haplotypes co-occur; it is unlikely that these haplotypes would be shared among populations as a result of separate colonisation events. In addition, owing to genetic drift in small populations leading to haplotype fixation (Frankham et al., 2017), a diverse haplotype mixture is unexpected. According to local inhabitants, African trout were brought to Lake Tamda in the middle of the 20th century and used as genitors for artificial reproduction in Ras el Ma hatchery. This information seems to be accurate given the genetic similarity of these heavily managed populations and the lowest pairwise FST values within the entire data set. Furthermore, as Lake Tamda belongs to the Draa drainage, which hosts Dades trout in its headstreams, some genetic similarity was expected, though none was found. Moreover, the genetic composition of the Lake Tamda and Ras el Ma hatchery samples is almost identical (Fig. 3C). Also, marked phenotypic heterogeneity was observed in Tamda trout. The haplotype ATM4, which was considered by Snoj et al. (2011) as indicative of stocking, was found also in Lake Tamda; its presence there thus further confirms its association with stocking. The lack of ATM4 in Ras el Ma may be attributed to the small sample from size.
We therefore conclude that the trout in Lake Tamda is not indigenous but rather an admixed artificial assemblage of various Moroccan populations.
A large number of haplotypes, including those of hatchery origin and genetically distinct, was also found in the Melloul and Ziz samples, while DAPC analysis cannot distinguish those from the Ras el Ma and Tamda samples, indicating a shared origin of individuals from these four sampling sites. Such observations indicate that the geographic origin and genetic signature of these populations are uncorrelated, and confirm the finding from the mt DNA analysis that they have been heavily stocked with hatchery-reared trout of various sources.
As our samples are mostly represented by a relatively small number of individuals, it is difficult to identify from the haplotypes the wild populations (donor populations) used for establishment of the trout hatchery population, or which of those populations acquired ‘hatchery genetic signatures’ via stocking, or both. For example, four out of five haplotypes found in Melloul were private to this sampling site, which clustered the M group on haplotype network (one of those haplotypes (ATM1) was found also in Tamda and Ras el Ma). This observation could suggest Melloul be considered a donor population though, based upon microsatellite data, recognised as a highly stocked one. The Ziz population, where haplotype ATM7 is abundant, could be a putative origin of some of the fish released into Lake Tamda, where this haplotype is also numerous. But DAPC recognized Ziz as a heavily stocked population. In addition, with the absence of a unique genetic signature in the Ziz sample, it is impossible to determine whether the endorheic Ziz drainage was naturally colonised with trout via river capture or by means of introduction. Furthermore, the introduction of non-native trout into Aït Mizane, first suspected using mt DNA (Snoj et al., 2011), was supported from analysis of the nc sequences, which classified these individuals into different monophyletic groups, one sample clustering trout from neighbouring Ourika, and the other clustering geographically very distant samples from Lake Isli and the Ziz. The former Aït Mizane sample bore haplotype ATM1, also found in Ras el Ma and Lake Tamda, whilst the latter bore haplotype Atsc25, which was otherwise found only in the very distant and heavily stocked Ziz. It can therefore be assumed that the Aït Mizane population is a combination of donor and stocked trout. Its donor character is also suggested by the DAPC graph (Online resource 3—Fig. S5), where in most individuals a distinct genetic profile was strongly prevalent and, in addition to this sample, found also in hatchery trout and all allegedly stocked populations (Berrem, Melloul, Ziz and Tamda). Aït Mizane is easily accessible, and therefore activities in trout management in this location are plausible. Also the origin of Ourika trout is not straightforward: the only haplotype (ATM4) detected in Ourika is shared with Berrem, which comprises another ‘hatchery haplotype’ ATM7, suggesting the impact of stocking in both locations. On the other hand, as inferred from nc DNA phylogeny and DAPC, Ourika would appear as a distinct genetic entity (Fig. 3B and DB).
From all these examples it is evident that the population structure within the Atlas group is so complicated that it is hard to claim whether a population represents a distinct lineage, has been affected by stocking, or is another example of reticulate evolution.
A fully preserved indigenous genetic signature was observed only in Dades trout, whose genetic characteristics were not found in any other sample, implying no stocking is carried out here. A similar conclusion could be made for the Afro-Atlantic group, where no hatchery alleles could be traced (Fig. 3 and Online resource 3—Fig. S5) with the exception of Talembote, which showed slight introgression with the Moroccan hatchery trout and where a heterogeneous external phenotype was observed (authors’ observation). Similarly, most of the remaining samples that are considered distinct from DAPC contain, in addition to genetic markers of the dominant genetic cluster, alleles of other clusters, indicating introgression and mixing with Moroccan local hatchery strains. Alternatively, this pattern could be the result of the incomplete genetic differentiation observed in the analysis. Further investigation using a broader genomic survey is required to identify clearer relationships among these highly related populations living on the limits of the natural range of Salmo trout.
A comment on S. marmoratus phylogeny inferred from nc sequences
In the present study, the phylogeny based on nc sequences reveals a sister relationship of S. marmoratus with the Atlantic (AT) evolutionary lineage of S. trutta and deep divergence from the other main lineages (see Bernatchez, 2001 for details) including the Adriatic (AD)–Mediterranean (ME) one (Fig. 2). This finding is in marked contrast to the mt DNA-based phylogeny, where the marmoratus (MA) mt lineage is only minimally divergent from mt AD–ME (e.g. Tougard et al., 2018). This contrasting pattern of mt and nc evolution of S. marmoratus was previously reported by Gratton et al. (2014), who—based on sequence variation at eight nuclear loci—identified a large divergence of S. marmoratus from AD with an ancestral origin dating before the formation of the main mt S. trutta lineages. Thus, it appears the incipient marmoratus mt DNA was lost and replaced by exogenous mitogenomes of the AD–ME lineage.
On the basis of mt DNA, AT is the lineage that apparently diverged initially from the common ancestor of Salmo trutta lineages (e.g. Bernatchez, 2001).
This observation is consistent with the nc-based phylogeny of the present study, which revealed no discordance between mt and nc in the position of AT with regard to the other main lineages; the exception is S. marmoratus, which does not group with AD–ME but with AT. Given that the Atlantic lineage and S. marmoratus evolved prior to the formation of the remaining lineages, the sister relationship inferred from nc sequences is not unexpected.
Conclusions
The complicated and entangled genetic structure of trout in Morocco is impossible to interpret if only one marker system—whether mt DNA or nc DNA—is considered.
Genetic analysis of the colonisation wave that affected the coastal Mediterranean rivers of Morocco, and also Algeria and Sicily, reveals a ‘pure’ Atlantic strain, indicated by mt DNA and nc DNA, with no interaction detected with a hypothetical local lineage in this area of Morocco.
The results inferred from the two marker systems together indicate an early Pleistocene colonisation by a lineage that probably came to Morocco across the Mediterranean from the east. The relict remnant of this lineage is Dades trout.
For the other trout in Morocco, the two-marker approach revealed reticulate evolution, with the presence of nc and mt DNA (Atlantic haplotypes) originating from different ancestors, suggesting the possibility of an ancient invasion of the Atlantic strain and its introgression with incipient African populations, creating newly admixed lineages of hybrid origin.
In addition to the diverse demographic events that disturbed the genetic structure of trout in Morocco, the phylogeographic picture has been further blurred by anthropogenic transfers of local hatchery trout used for stocking, which has apparently been carried out for a long time in the sizeable distribution range of trout in Morocco.
Data availability
The data that support the findings of this study are available in the Online resource of the article and from the corresponding author upon reasonable request.
References
Bernatchez, L., 2001. The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution 55: 351–379.
Bernatchez, L., R. Guyomard & F. Bonhomme, 1992. DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout Salmo trutta populations. Molecular Ecology 1: 161–173.
Clavero, M., A. Qninba, M. Riesco, J. Esquivias, J. Calzada & M. Delibes, 2017. Moroccan desert rivers: fish on the arid extreme of Mediterranean streams. FiSHMED 003: 21p.
Clement, M., D. Posada & K. A. Crandall, 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1659.
Cortey, M., M. Vera, C. Pla & J. L. Garcia-Marin, 2009. Northern and Southern expansions of Atlantic brown trout (Salmo trutta) populations during the Pleistocene. Biological Journal of the Linnean Society 97: 904–917.
Doadrio, I., S. Perea & A. Yahyaoui, 2015. Two new species of Atlantic trout (Actinopterygii, Salmonidae) from Morocco. Graellsia 71: e031.
Dufresne, F., M. Stift, R. Vergilino & B. K. Mable, 2014. Recent progress and challenges in population genetics of polyploid organisms: an overview of current state-of-the-art molecular and statistical tools. Molecular Ecology 23: 40–69.
Frankham, R., J. D. Ballou, K. Ralls, M. Eldridge, M. R. Dudash, C. B. Fenster, R. C. Lacy & P. Sunnucks, 2017. Genetic Management of Fragmented Animal and Plant Populations. Oxford University Press, Oxford.
Fruciano, C., A. M. Pappalardo, C. Tigano & V. Ferrito, 2014. Phylogeographical relationships of Sicilian brown trout and the effects of genetic introgression on morphospace occupation. Biological Journal of the Linnean Society 112: 387–398.
Gissi, C., F. Iannelli & G. Pesole, 2008. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity 101: 301–320.
Goudet, J., 2001. FSTAT, version 2.9.3: a program to estimate and test gene diversities and fixation indices. http://www.unil.ch/izea/softwares/fstat.html/.
Gratton, P., G. Allegrucci, V. Sbordoni & A. Gandolfi, 2014. The evolutionary jigsaw puzzle of the surviving trout (Salmo trutta L. complex) diversity in the Italian region. A multilocus Bayesian approach. Molecular Phylogenetics and Evolution 79: 292–304.
Jiang, W., S. Y. Chen, H. Wang, D. Z. Li & J. J. Wiens, 2014. Should genes with missing data be excluded from phylogenetic analyses? Molecular Phylogenetics and Evolution 80: 308–318.
Joleaud, L., 1938. Études de géographie zoologique sur la Berbérie: Les truites. Hespéris. Archives berbères et Bulletin de l’ Institut des Hautes-Études marocaines 25: 247–249.
Jombart, T., Z. N. Kamvar, C. Collins, R. Lustrik, M. P. Beugin, B. J. Knaus & M. T. Jombart, 2018. Adegenet: exploratory analysis of genetic and genomic data. R package version 2.
Jombart, T., S. Devillard & F. Balloux, 2010. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics 11: 1–5.
Lanfear, R., P. B. Frandsen, A. M. Wright, T. Senfeld & B. Calcott, 2017. Partitionfinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773.
Lerceteau-Köhler, E. & S. Weiss, 2006. Development of a multiplex PCR microsatellite assay in brown trout Salmo trutta, and its potential application for the genus. Aquaculture 258: 641–645.
Mouslih, M., 1987. Introductions de poissons et d’écrevisses au Maroc. Revue d’Hydrobiologie Tropicale 20: 65–72.
Panouse, J. B., 1963. Le Lac d’Ifni (Haut Atlas marocain). Bulletin de la Société des Sciences Naturelles et physiques du Maroc 43: 7–24.
Pellegrin, J., 1924a. Les Salmonidés du Maroc. Comptes rendus de l’Académie des Sciences Paris 178: 970–972.
Pellegrin, J., 1924b. Le Salmo pallaryi Pellegrin, poisson du Moyen Atlas marocain. Bulletin du Museum national d’histoire naturelle Paris 30: 181–184.
Puechmaille, S. J., 2016. The program structure does not reliably recover the correct population structure when sampling is uneven: subsampling and new estimators alleviate the problem. Molecular Ecology Resources 16: 608–627.
Pustovrh, G., S. Sušnik Bajec & A. Snoj, 2011. Evolutionary relationship between marble trout of the northern and the southern Adriatic basin. Molecular Phylogenetics and Evolution 59: 761–766.
Pustovrh, G., S. Sušnik Bajec & A. Snoj, 2012. A set of SNPs for Salmo trutta and its application in supplementary breeding programs. Aquaculture 370: 102–108.
Pustovrh, G., A. Snoj & S. Sušnik Bajec, 2014a. Molecular phylogeny of Salmo of the western Balkans, based upon multiple nuclear loci. Genetics Selection Evolution 46: 7.
Pustovrh, G., A. Snoj & S. Sušnik Bajec, 2014b. Erratum to: molecular phylogeny of Salmo of the western Balkans, based upon multiple nuclear loci. Genetics Selection Evolution 46: 21.
Ronquist, F., M. Teslenko, P. van der Mark, D. L. Ayres, A. Darling, S. Höhna, B. Larget, L. Liu, M. A. Suchard & J. P. Huelsenbeck, 2012. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542.
Sanz, N., 2018. Phylogeographic history of brown trout: a review. In Lobón Cerviá, J. & N. Sanz (eds), Brown Trout: Biology, Ecology and Management. Wiley, Hoboken: 17–63.
Schöffmann, J., S. Sušnik & A. Snoj, 2007. Phylogenetic origin of Salmo trutta L. 1758 from Sicily, based on mitochondrial and nuclear DNA analyses. Hydrobiologia 575: 51–55.
Snoj, A., S. Marić, S. Sušnik Bajec, P. Berrebi, S. Janjani & J. Schöffmann, 2011. Phylogeographic structure and demographic patterns of brown trout in North-West Africa. Molecular Phylogenetics and Evolution 61: 203–211.
Sušnik, S., S. Weiss, T. Odak, B. Delling, T. Treer & A. Snoj, 2007. Reticulate evolution: ancient introgression of the Adriatic brown trout mtDNA in softmouth trout Salmo obtusirostris (Teleostei: Salmonidae). Biological Journal of the Linnean Society 90: 139–152.
Tamura, K., G. Stecher, D. Peterson, A. Filipski & S. Kumar, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729.
R Core Team, 2017. R: A Language and Environment for Statistical Computing. https://www.R-project.org/. accessed 9 May 2020.
Thompson, J. D., D. G. Higgins & T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680.
Tougard, C., F. Justy, B. Guinand, E. J. P. Douzery & P. Berrebi, 2018. Salmo macrostigma (Teleostei, Salmonidae): nothing more than a brown trout (S. trutta) lineage? Journal of Fish Biology 93: 302–310.
Vivier, P., 1948. Note sur les eaux douces du Maroc et sur leur mise en valeur. Bulltein Franҫais de Pisciculture 150: 5–27.
Wallis, G. P., S. R. Cameron-Christie, H. L. Kennedy, G. Palmer, T. R. Sanders & D. J. Winter, 2017. Interspecific hybridization causes long-term phylogenetic discordance between nuclear and mitochondrial genomes in freshwater fishes. Molecular Ecology 26: 3116–3127.
Xi, Z., L. Liu & C. C. Davis, 2016. The impact of missing data on species tree estimation. Molecular Biology and Evolution 33: 838–860.
Acknowledgments
We thank Mohamed Ghamizi for all his support and assistance in the realisation of this project. We also thank the local people from Tighza Village for their assistance with sampling on Lake Tamda. Special thanks go to Iain Wilson for editing and proof-reading the manuscript. The authors acknowledge the financial support of the Slovenian Research Agency (Research core funding No. P4-0220) and the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-9/2021-14/20017).
Funding
The study was partially financed by the Slovenian Research Agency (Research core funding No. P4-0220) and the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-9/2021-14/20017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Handling editor: Christian Sturmbauer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Snoj, A., Bravničar, J., Marić, S. et al. Nuclear DNA reveals multiple waves of colonisation, reticulate evolution and a large impact of stocking on trout in north-west Africa. Hydrobiologia 848, 3389–3405 (2021). https://doi.org/10.1007/s10750-021-04567-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04567-0