Abstract
The freshwater ichthyofauna of Greece is characterized by high diversity and endemism and faces various conservation challenges. Despite significant efforts, mainly with traditional assessment methods, the precise taxonomic status and geographic range of some of the currently acknowledged fish species remain insufficiently explored. Here, the results of the first comprehensive DNA barcoding survey of the genus Pelasgus are presented, covering its entire known range in Greece. Two hundred and forty COI sequences (retrieved from collected samples, museum specimens and the GenBank) were analysed to determine the number of putative species and delineate their current distribution. Data support the existence of seven haplogroups, corresponding to the currently valid Pelasgus species. Analysis of museum samples of the critically endangered species P. epiroticus from its type locality (now extinct there) uncovers its presence in contemporary samples from a neighbouring aquatic system (Zaravina Lake) and from aquatic systems well outside its native range (possibly as a result of past translocations) for the first time. A subgroup of P. epiroticus haplotypes was also identified in North-western Greece in regions previously believed to host P. thesproticus or P. stymphalicus. Based on the above, hotspots for further Pelasgus taxonomic investigations are identified and conservation actions are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The freshwater ichthyofauna of Greece is characterized by one of the highest levels of diversity and endemism in Europe (Economou et al., 2007; Barbieri et al., 2015). However, according to the available conservation status assessments, 53 species are currently considered as threatened on a global scale, corresponding to 39% of all native freshwater fish species (Barbieri et al., 2015). Thus, both precise taxonomic delineation of species and detailed knowledge of their geographic distribution are prerequisites for the accuracy of their conservation status assessment, which is crucial for conservation planning and management. Despite the significant efforts undertaken to survey and describe the Greek freshwater ichthyofauna, mainly using data deriving from traditional taxonomic approaches, a large part of the species diversity still remains fairly unexplored (Barbieri et al., 2015), as suggested by recent research conducted with genetic methods (e.g. Apostolidis et al., 2011; Geiger et al., 2014; Buj et al., 2019).
One such genus is the recently renamed Pelasgus which currently consists of seven species (Kottelat & Freyhof, 2007a): the Epirus minnow Pelasgus epiroticus (Steindachner, 1896), the Evrotas minnow Pelasgus laconicus (Kottelat & Barbieri, 2004), the Marathon minnow Pelasgus marathonicus (Vinciguerra, 1921), the Prespa minnow Pelasgus prespensis (Karaman, 1924), the Stymphalia minnow Pelasgus stymphalicus (Valenciennes, 1844), the Thesprotian minnow Pelasgus thesproticus (Stephanidis, 1939) and the Ohrid minnow Pelasgus minutus (Karaman, 1924) (common names according to Barbieri et al., 2015). Four of the Pelasgus species are confined to Greek territory (P. laconicus, P. epiroticus, P. marathonicus and P. stymphalicus), one is endemic in western Greece and southern Albania (P. thesproticus), while the remaining two are restricted to three trans-boundary Balkan lakes, Prespa Lakes (P. prespensis) and Lake Ohrid (P. minutus) (Kottelat & Freyhof, 2007a; Barbieri et al., 2015). The taxonomy of these seven species was mainly based on morphological traits (Kottelat & Barbieri, 2004; Kottelat & Freyhof, 2007a, b), and in some cases was further supported by molecular data (Zardoya et al., 1999; Geiger et al., 2014). Geiger et al. (2014) sequenced 45 specimens of Pelasgus from 10 drainages, which resulted in a successful species’ delineation and matches for most of the provided barcodes with 6 recognized Pelasgus species (P. minutus, P. prespensis, P. laconicus, P. stymphalicus, P. thesproticus, P. marathonicus). In addition, the authors have suggested a taxonomic update and highlighted the existence of two potential candidate species assigned to specific groups of divergent barcodes (Pelasgus sp. in Kalamas River and Pelasgus sp. in Acheron River). However, the collection of Pelasgus samples undertaken by Geiger (2014) was restricted in up to two drainages per putative species, while material from P. epiroticus was not included in their analysis, since it was considered to be extinct.
Many ecological and biological aspects of the Pelasgus species have not yet been sufficiently studied, though some information on their ecology and biology has been provided mainly from empirical observations and ad hoc research efforts. All species of the genus Pelasgus have a small body size (maximum Total Length 6–10 cm), short life span, rapid growth rate and are spring spawners, with some species having a protracted spawning period (Kottelat & Freyhof, 2007a). They can be characterized as limnophilic, indicating preference for lentic habitats, with dense aquatic vegetation that provides shelter from predators (Barbieri et al., 2015; Vardakas et al., 2017). Their diet consists mainly of invertebrates, algae and detritus (Kottelat & Freyhof, 2007a).
The restricted and fragmented geographic distribution of the Pelasgus species, combined with the degradation of their habitats, mainly due to pollution, water abstraction and hydro-morphological alterations, has resulted in the decline of their populations (IUCN, 2019). Thereby, three of the Pelasgus species have been assigned to the endangered category status in the IUCN Red List (P. epiroticus critically endangered; P. laconicus critically endangered and P. prespensis endangered) (IUCN, 2019). The rarest and most endangered of these is P. epiroticus (Crivelli, 2006), which is referred as endemic to Lake Pamvotis (Northwestern Greece) and the surrounding area (Barbieri et al., 2015). This species is included in Annex II of the EU Habitats Directive 92/43/EEC as a priority species for protection; however in recent years it was considered to be extinct (Perdikaris et al., 2005). In the past, it used to form large populations in Lake Pamvotis and had been traditionally consumed by the residents of the City of Ioannina (Leonardos et al., 2008). A declining tendency had already been observed in the 1980s, followed by a gradual population collapse in the mid-2000s (Leonardos et al., 2005; Perdikaris et al., 2005). Similarly, P. laconicus is assigned to the critically endangered status, due to the small area of occupancy, habitat fragmentation and decreasing population trend, while P. prespensis is assigned to an endangered status, due to its restriction to a single location and an ongoing population decline in recent decades (Kottelat & Freyhof, 2007a).
In the present study, 193 Pelasgus specimens were collected, representing the entire known range of the genus in Greece. In addition, 33 museum specimens from the collections of the Natural History Museum of Vienna (Austria) were also analysed. It is worth noting that 18 museum specimens included in the analysis originated from the type locality of P. epiroticus (Pamvotis Lake). Newly generated and published barcodes were analysed, in order to evaluate the genetic variability within the Pelasgus genus and check for undetected or cryptic genetic lineages that need further taxonomic investigation, as well as to enhance knowledge on the Pelasgus species’ current geographic distribution. The results of the study are expected to play a pivotal role in future monitoring programmes of Pelasgus species or populations that require focused conservation interventions.
Materials and methods
Sampling
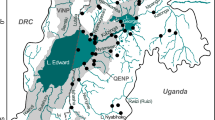
The fish samples were taken from various research surveys which had been conducted during a 10-year time span (2005–2014) and covered the entire known range of the Pelasgus genus in Greece. The samples were taken mostly from 2012 to 2014, within the framework of the National Monitoring Project for the assessment of the ecological quality of rivers in Greece, in order to fulfil the requirements of the Water Framework Directive 2000/60/EC. Additional samples were taken sporadically between 2005 and 2011, from ad hoc ichthyological investigations. All fish were captured using an electrofishing device, anesthetized with quinaldine or clove oil and then preserved in ethanol (98%). In total, 193 Pelasgus specimens were collected from 42 sampling sites (Fig. 1 and Supplementary Material Appendix 1, Table S1). Since morphological identification was not possible directly in the field, samples were originally assigned to species based on the Pelasgus distribution previously known according to Economou et al. (2007) and Barbieri et al. (2015) (Fig. 1). No post-fieldwork morphological identification was conducted, mainly due to the lack of an adequate number of adult individuals of both sexes.
Provenance of collected samples, museum samples (red numbers) and published sequences of Pelasgus species used in this study. Colour-coded polygons indicate the former known distribution of the Pelasgus species in the Balkan Peninsula (see inset). For detailed information see Supplementary Material Appendix 1, Table S1
DNA processing of newly collected specimens
Total genomic DNA was extracted from pelvic or pectoral fin clips, using a standard salt extraction protocol (Miller et al., 1988) with some modifications (available upon request). The DNA barcoding analysis was performed with mitochondrial cytochrome c oxidase subunit 1 (COI) gene, which is considered to be a standard marker for species-level identification in vertebrates. DNA amplification was achieved with Polymerase Chain Reaction (PCR) using primers VF2-t1 (5′-TGTAAAACGACGGCCAGTCAACCAACCACAAAGACATTGGCAC-3′) and FR1d-t1 (5′-CAGGAAACAGCTATGACACCTCAGGGTGTCCGAARAAYCARAA-3′), a pair of M13-tailed primers that Ivanova et al. (2007) have included in universal primer cocktails for fish barcoding. The PCR reaction mix (12.5 μl) contained 6.25 μl of Qiagen® Multiplex PCR Plus mix (1 ×), 1 μl of each primer (0.8 μM), 1 μl of Q-Solution (0.4 ×), RNase-free pure water and 20–50 ng of template DNA. The cycling conditions consisted of an initial step of 15 min at 95 °C, followed by 10 cycles of 35 s at 94 °C, 1.5 min touch-down from 52 °C to 49 °C, and 1.5 min at 72 °C, followed by 25 cycles of 35 s at 94 °C, 1.5 min at 55 °C, and 1.5 min at 72 °C and a final extension step at 72 °C for 10 min. After purification with ethanol/sodium acetate precipitation, PCR products were sequenced in both directions using primers M13F (5′-TGTAAAACGACGGCCAGT-3′) and M13R (5′-CAGGAAACAGCTATGAC-3′) (Messing, 1983). Sequencing reactions were carried out using the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Inc.) and products were run on an ABI 3730 capillary sequencer (Applied Biosystems) following the manufacturer’s instructions. Individual sequences were aligned with MEGA 6.06 (Tamura et al., 2013) and were re-examined manually by visual inspection of raw fluorogram data.
DNA processing of museum specimens
Museum material used in this study is depicted in Supplementary Material Appendix 1, Table S2. Besides 18 samples of P. epiroticus [(Steindachner, 1895) syntypes collected at Ioannina Lake (Lake Pamvotis) Greece, in 1892], DNA from 15 additional museum samples was extracted from all 5 branchial arches from the right side of the specimens. The protocol followed all the necessary requirements for working with the museum DNA, including a DNA clean room, and sterilised and UV-irradiated utensils. The tissue was first air-dried to remove residual ethanol and DNA was extracted with QIAamp® DNA Mini and Blood Mini Kit (Qiagen) following the manufacturer’s protocol. All extractions included extraction controls, to ensure there was no contamination of the buffers. The barcoding region of cytochrome oxidase I (COI) was amplified with primers FishF1 (5′-TCAACCAACCACAAAGACATTGGCAC-3′) and FishR1 (5′-TAGACTTCTGGGTGGCCAAAGAATCA-3′) (Ward et al., 2005), but since museum DNA is typically degraded (Wandeler et al., 2007), complementary/new primers were designed amplifying up to 320 bp long fragments (Supplementary Material Appendix 1, Table S3). The adjacent fragments overlapped for at least 30 bp—an additional contamination control. In addition, all PCRs included negative and positive controls. PCR volume was 25 μl, with 2.5 μl of buffer, 2 μl MgCl2 (2.0 mM), 1 μl of Enhancer, 500 μM dNTPs, 0.25 μl of each primer (25 pmol/μl) and 0.2 μl of AmpliTaq Gold® 360 DNA Polymerase (1 unit). The volume of the DNA varied according to the measured concentrations, the aim being 10–20 ng/μl of final DNA concentration in 25 μl reaction. Touch-down PCR protocol was used for all fragments together with an increased number of cycles (45). The detailed PCR protocol for all pairs of primers is presented in Table S4. Purification of PCR products was performed with the Qiagen PCR purification kit, and purified PCR products were sequenced (in both directions) by LGC Genomics (Berlin, Germany) with the above-mentioned PCR primers. After amplification, the fragments were aligned with MEGA 6.06 (Tamura et al., 2013) and composed into a single sequence. Composed sequences were added to the final dataset.
Barcoding and phylogenetic relations
COI sequences of fresh and museum samples were aligned with COI sequences of Pelasgus species retrieved from GenBank (Supplementary Material Appendix 1, Table S1) using ClustalW as implemented in MEGA 7 (Kumar et al., 2016). Amino acid translations were examined to ensure the absence of stop codons in the alignment. The final multiple alignment was then processed with DAMBE5 (Xia, 2013), to define unique haplotype sequences. As networks are very helpful for inferring haplotype clustering, the haplotypes were used for the construction of a haplotype network with the median-joining network method (Bandelt et al., 1999) and default settings as implemented in the programme Network 5.0.0.0 (Fluxus Technology Ltd., http://www.fluxus-engineering.com). Maximum Likelihood Phylogeny was investigated with the online programme PHYML 3 (Guindon et al., 2010) as implemented in the platform ATGC (http://www.atgc-montpellier.fr/). Model selection was made automatically by the programme PHYML 3 with the Smart Model Selection application (Lefort et al., 2017), while branching support was surveyed with aBayes method (Anisimova et al., 2011) and bootstrapping (1,000 replicates).
An arbitrary distance-based approach was used at first, in order to check for species delimitation based on sequence divergence values within and among putative species. According to this approach a species can be correctly identified when the mean distance to the most closely related species (nearest neighbour) is higher than the maximum intraspecific distance (April et al., 2011). Divergence over sequence pairs were estimated with MEGA7 (Kimura, 1980; Kumar et al., 2016), after performing Model Selection with the same software (Nei & Kumar, 2000; Kumar et al., 2016).
A computationally efficient distance-based method was also employed, in order to delimit the species based on pairwise sequence distances between all Pelasgus individuals within the dataset. The Automatic Barcode Gap Discovery (ABGD) analysis (Puillandre et al., 2012a) checks the frequency distribution of pairwise distances between all sequences and locates the so called “barcode gap” that represents the limit under which distances are statistically more likely to be intraspecific rather than interspecific. ABGD then provides a set of a priori threshold intraspecific values for partitioning the sequences into groups, each representing a hypothetical species (the method is described in detail by Puillandre et al., 2012a). The ABGD analysis was performed with a web interface tool (http://wwwabi.snv.jussieu.fr/public/abgd/), using the default value for the relative gap width (X = 1.5) and K2P as distance metric (the best substitution model according to Model Selection analysis performed in MEGA). The number of steps was set to 20 and the number of bins to 50. Pmin and Pmax were set to 0.01 and 0.03, respectively.
Groups indicated by ABGD analysis were further evaluated with Species Delimitation Plugin (SPD) in Geneious Prime® v11.0.4+ 11 (Biomatters Ltd., https://www.geneious.com/). SPD provides several metrics to support the exploration of putative species boundaries in a gene tree (Masters et al., 2011). In this tree-based approach the user can assign clades to putative species and the plugin computes statistics relating to the probabilities of observed monophyly or exclusivity having occurred by chance in a coalescent process (Masters et al., 2011). The Maximum Likelihood phylogenetic tree estimated by PHYML was imported in Geneious and SPD was utilized to generate PID (Strict and Liberal) statistics that provide probabilities of making a correct identification of a hypothetical sample under strict or relaxed cladistic criteria (Masters et al., 2011) and Rosenberg’s PAB statistic that infers the probability of reciprocal monophyly under the null model of random coalescence (Rosenberg, 2007). Rodrigo’s (RD) statistic (Rodrigo et al., 2008) was excluded since this calculation is not relevant when the underlying tree has not been estimated under a strict molecular clock (Masters et al., 2010; Malaquias et al., 2017).
Results
DNA processing of fresh and museum specimens
All 193 fresh samples were successfully amplified and sequenced for the partial COI gene (Supplementary Material Appendix 1, Table S1). From the available museum specimens (Supplementary Material Appendix 1, Table S2), one non-type from Louros River drainage and six syntype specimens from Lake Pamvotis were successfully amplified for the complete COI region (Supplementary Material Appendix 1, Table S1). For the remaining museum specimens, only a part of the COI gene was successfully amplified in 10 of them (Supplementary Material Appendix 1, Table S2), and thus these were not included in further analyses.
Barcoding and phylogenetic relations
In total, 240 sequences (200 from fresh and museum samples and 40 from the GenBank) were aligned, resulting in 51 unique haplotypes (Supplementary Material Appendix 1, Table S1). The length of the aligned COI dataset was 636 bp, with 136 variable and 118 parsimony-informative sites. No insertions, deletions or stop codons after translation were detected in the sequences, which supports the high quality of the sequencing and the absence of any nuclear-mitochondrial pseudogenes (numts).
The median-joining haplotype network constructed with all available sequences (Fig. 2) indicates the existence of seven main haplotype groups (I–VII). Colours in the figure are assigned to Pelasgus species’ geographic ranges and thus each haplotype is depicted with a colour according to the location(s) from which it was sampled. The existence of more than one colour in some groups indicates the presence of the putative species in locations outside their already known geographic range. Except for Groups I (P. minutus) and II (P. prespensis) where no discrepancy was observed, in all the other groups there was a lack of agreement between former known distribution and genetic assignment to a greater or lesser extent (for details see Supplementary Material Appendix 1, Table S1).
Median-joining network of 51 COI haplotypes including those identified in the current study along with those retrieved from GenBank (see Supplementary Material Appendix 1, Table S1 for accession numbers and coding). Each circle represents a haplotype and its size is proportional to haplotype frequency. Small red nodes represent possible median vectors while numbers indicate the number of nucleotide differences between haplotypes (minor differences of 1 or 2 nucleotides are not given)
The same pattern was also corroborated by the phylogenetic tree (Fig. 3); haplogroups I–VI assigned to P. minutus, P. prespensis, P. thesproticus, P. marathonicus, P. stymphalicus and P. laconicus, respectively, are monophyletic and correspond to well-resolved clades (branch support values > 0.96 aBayes/ > 75% bootstraps). The haplogroup/clade VII is also monophyletic and strongly supported (branch support value 0.99/91%). This clade is further divided into two subclades: the first one (VIIa) includes four haplotypes (H37, H40, H41, H42) that were retrieved from the museum syntypes of P. epiroticus from Lake Pamvotis. The second subclade (VIIb) includes mainly haplotypes retrieved from samples collected in the Louros and Arachthos Rivers drainages, which were previously considered to belong to P. thesproticus species range.
Maximum Likelihood phylogenetic tree. Pelasgus prespensis and P. minutus were used as outgroups for rooting the tree. Support values are provided as numbers (aBayes support) and coloured dots (% bootstraps). ABGD delimitation is also shown (group numbering same with network). Haplotypes shown in red were sampled in the type locality of each putative species
Divergence over haplotype pairs was estimated with Kimura 2 parameter model (with Gamma distribution G = 0.22) which is considered to be the best (according to Model Selection performed) to describe the substitution pattern (Supplementary Material Appendix 1, Table S5). Mean intra- and inter-specific divergence values are presented in Table 1. The mean genetic divergence within groups ranged from 0.16% in P. prespensis to 1.39% in P. epiroticus. The lowest interspecific distance was between P. marathonicus and P. stymphalicus (3.04%) while the highest distance was between P. minutus and P. epiroticus (18.29%). The highest pairwise genetic distance between samples assigned to P. epiroticus was 2.7% (between H39–H47, H39–H50, H45–H47, H45–H50 in Supplementary Material Appendix 1, Table S5), which is lower than the mean distance of P. epiroticus to the most closely-related species P. thesproticus (4.17%).
As well as this descriptive approach, the results of the species delimitation analysis are schematically presented in Fig. 3. ABGD analysis (Supplementary Material Appendix 2, Fig. S1) proposed the partition of specimens into groups (candidate species) based on various a priori thresholds of intraspecific divergence and the distribution of pairwise genetic distances of all sequences. Initial partition for thresholds ranging from 0.010 to 0.0141 defined seven groups corresponding to the seven already recognized Pelasgus species. For thresholds 0.0141–0.0283, the number of delimited groups falls to four. In this case, only P. prespensis, P. minutus and P. laconicus are well defined, while all the other specimens seem to belong to a single group/species. For thresholds higher than 0.03, all the specimens belong to just one species. Recursive partition for 0.015 threshold also provided the seven groups that correspond to the known Pelasgus species.
SDP results for the seven main clades of Fig. 3 are presented in Table 2. The more relaxed cladistic probabilities (P ID Liberal) were above 0.96 for all species. In addition values of P ID Strict, i.e. the mean probability for the prediction, of making a correct identification of an unknown specimen of the focal species using placement on a tree and the criterion that it must fall within, though not sister to, the species clade, were high for most species (> 0.85), indicating that the genetic distinctiveness of the clades is valid. Low values of P ID Strict in P. prespensis (0.58) and P. minutus (0.81) could be justified by the small number of reference taxa (haplotypes) in these two species. The Rosenberg’s PAB values were significant for all putative species (P < 0.05) and highly significant for P. marathonicus, P. stymphalicus, P. epiroticus, P. thesproticus and P. laconicus (P < 0.001) confirming the delimitation of seven species defined by ABGD analysis.
Distribution of haplotypes/species
Spatial occurrence of COI haplotypes (P. prespensis and P. minutus excluded) is depicted in Figs. S2, S3 and S4 (Supplementary Material Appendix 2). Each disc summarizes the number and relative abundance of haplotypes. This presentation gives a detailed picture of the geographic range of putative species and is also indicative of their phylogeographic structure and genetic diversity.
Pelasgus epiroticus, excluding the population of the Pamvotis Lake (type locality) where the species is nowadays considered to be extinct, seems to be still present in the neighbouring Zaravina Lake in Epirus (Supplementary Material Appendix 2, Fig. S2), as well as in the Stymphalia Lake and Kandyla Springs in the Peloponnese (Supplementary Material Appendix 2, Fig. S3). A subclade of P. epiroticus is, according to our analysis, distributed in the drainages of rivers that flow into the Amvrakikos Gulf (Supplementary Material Appendix 2, Fig. S2). In total, 13 haplotypes of this species were discovered from the analysis of 51 fresh samples from these sites. Two of these (H37 and H40) were also present in the museum samples of Pamvotis Lake. Haplotypes H41 and H42 derived from museum samples have not been discovered in any of the fresh samples.
Pelasgus thesproticus distribution (Supplementary Material Appendix 2, Fig. S2) includes Corfu Island, the Acheron River drainage, the Paramithia Lakes’ drainage (type locality of the species) and Kalamas Estuary (a total of six sampling sites, Supplementary Material Appendix 1, Table S1). In total, 7 haplotypes were retrieved from 30 samples. H04 is the most common and abundant haplotype that was found at all the mainland sampling locations. The populations of Corfu Island, on the contrary, exhibit private haplotypes (H01, H02, H03).
The range of P. stymphalicus expands northward to Trichonis Lake and the Astakos Stream in Aitoloakarnania and southward to Pamissos River in the Peloponnese (Fig. S3). This species is located in more than 15 sites (the type locality, Stymphalia Lake, included), according to 13 haplotypes that were retrieved from 71 samples (for details see also Supplementary Material Appendix 1, Table S1). The most abundant haplotypes (e.g. H20, H21, H22, H23, H26) are confined to just one or two drainages of adjacent regions. For example, haplotypes that were discovered in the Peloponnese are absent in the Aitoloakarnania and vice versa. Only one haplotype assigned to P. stymphalicus (H16) is located far from the known species’ range, in Sperchios River (see Supplementary Material Appendix 2, Fig. S4).
Pelasgus laconicus is distributed in the central and southern Peloponnese (Fig. S3). Four haplotypes have been assigned to 22 samples from 6 different sites. Besides the Evrotas River, the type locality of the species, P. laconicus is located in the Sminos and Tripotamos Rivers and the Manari Springs of Alphios River Basin. There is also a sporadic presence in the lower Alphios River, along with P. stymphalicus.
Pelasgus marathonicus is widespread in eastern Greece from the Pinios River (Thessaly) drainage in the north to the Erasino Stream (Attica) in the south, including the drainages of Sperchios, Viotikos Kifissos and Assopos Rivers (Supplementary Material Appendix 2, Fig. S4). Despite the large geographic range of this species, only 6 haplotypes were recognized in 39 samples. Haplotype H13 was prevalent in all of the 10 sites that were included in the analysis.
Discussion
This study aimed to expand our knowledge on the current geographic distribution and genetic diversity of the Pelasgus species in Greece, by analysing COI sequences from 28 drainages and 47 locations. Our data revealed seven groups, corresponding to the seven previously known species; however, in some of them (P. epiroticus, P. thesproticus, P. stymphalicus) unexpected distribution ranges were detected. The study included type specimens of P. epiroticus in a molecular survey for the first time. Most importantly, this study identifies some “hotspots” (species, genetic lineages or geographic areas), for which priorities for conservation and further in-depth studies are urgently required.
The first identified hotspot is the discovery of an extant population of P. epiroticus, which was found by using the barcodes of its type specimens (museum samples). The species, originally found in Lake Pamvotis, nowadays appears to be extinct from its type locality. However, the present study has revealed the species’ presence in Zaravina Lake, confirming a report from the 1990s (referred to as Paraphoxinus epiroticus in Zalidis & Mantzavelas, 1996). The most abundant haplotype retrieved from samples in Zaravina L. (H37) is one of the four haplotypes retrieved from museum syntypes from Pamvotis Lake. The discovery of P. epiroticus in Zaravina Lake indicates that the conservation of this population should be of high priority, while its apparent extinction from the Pamvotis Lake confirms its critically endangered status.
Haplotypes assigned to P. epiroticus (subclade b) were found in populations of Arachthos and Louros, as well as in some small freshwater systems associated with the Amvrakikos Gulf (Krikellos, Vutumias, Vlychos—Sites 11, 12 and 13 in Fig. 1), previously believed to host P. thesproticus or P. stymphalicus. However, this finding should be treated with caution and populations should be the target of separate conservation actions with no translocation between the subclades. A prerequisite for further conservation management would also be a morphological study of specimens belonging in the two subclades to confirm genetic results.
Haplotypes clustering to P. epiroticus were also detected in Stymphalia Lake and the neighbouring Kandyla Springs in the Peloponnese. In the case of the Kandyla Springs, this can be attributed to unintentional translocation, deriving from stockings of grass and silver carps from Lake Pamvotis (Kottelat & Barbieri, 2004) carried out in the 1980s. Two haplotypes (H37 and H40) occur in common between Kandyla samples and museum syntypes from Pamvotis Lake. Unintentional stockings are also probably the reason for the presence of the species in Stymphalia Lake (Economou et al., 1999), although the only haplotype assigned to P. epiroticus in this lake (H29) has not been detected elsewhere. The co-existence of haplotypes clustered to P. epiroticus and P. stymphalicus in Stymphalia Lake (the type locality of P. stymphalicus) creates suspicions that hybridization between these two species could have happened after the accidental translocation. Although no reference for known hybridization events in Pelasgus species still exists, this possibility cannot be excluded. After all, hybridization is not exceptional in cyprinids and has been reported for another species in the Peloponnese (Perea et al., 2016).
Targeted ichthyologic surveys for specimen collections from the wider areas of both Zaravina and Pamvotis Lakes, as well as detailed morphological, ecological and genetic studies of the two populations from Kandyla Springs and Stymphalia Lake are urgently required to confirm genetic results by morphometry and to delineate more finely the current geographic range of the species.
According to our analysis, P. thesproticus is represented by only one clade, distributed in Corfu, the Acheron drainage, the Paramithia Lakes’ drainage and Kalamas River. Thus, the species range of P. thesproticus is much smaller than formerly expected while the range of P. epiroticus is rather larger (see above). In Geiger et al. (2014), two clades were detected in this geographic area. The first clade was formed from the populations of the Acheron and Kalamas Rivers, which they assigned to Pelasgus sp. and the second from the Arachthos population that they designated as P. thesproticus. In the present study, specimens from the Paramithia Lakes (site Choskova), the type locality of P. thesproticus, were included for the first time, as well as previously mentioned type specimens of P. epiroticus. This allowed us to correctly assign the Acheron population to the same clade with populations of P. thesproticus from Corfu, the Kalamas River and the Paramithia Lakes, while the Arachthos population is tentatively assigned to P. epiroticus. A biogeographic pattern similar to the one mentioned above, was also revealed in a genetic study on the critically endangered Corfu killifish Valencia letourneuxi (Vogiatzi et al., 2014), where two groupings were identified in Northwestern Greece. The first included populations inhabiting rivers flowing into the Amvrakikos Gulf (where P. epiroticus subclade b was identified) and the second, more northerly group, included the populations from the Kalamas and Acheron Rivers, as well as Corfu Island (where P. thesproticus was identified). The genetic divergence of the populations of P. thesproticus in Corfu Island from the mainland populations, probably due to their long geographic isolation, indicates that the Corfu populations should be treated as distinct conservation units, with future translocation and restocking actions to take place only within the island.
Concerning the rest of the Pelasgus species that are well resolved, the occurrence of P. laconicus in both the Evrotas River and the upper Alfios Basin was confirmed (Barbieri et al., 2015). This is attributed to the fact that in past geological times the upper sections of these two rivers were connected (Skoulikidis et al., 2009). Moreover, the detection of both P. laconicus and P. stympalicus in the lower Alfios confirms the previous studies that reported the co-existence of these two species in the lower part of the drainage (Economou et al., 2007; Barbieri et al., 2015). The data on the distribution of P. marathonicus supports previous ichthyologic surveys that reported the presence of the species in Attika and Beotia (Viotikos Kiffisos, Asopos, Erasinos, Marathon Lake), as well as in the Sperchios and Pinios (Thessaly) Basins. The presence of one haplotype assigned to P. stymphalicus in the Sperchios Basin is possibly a result of accidental translocation, as the species is used as live bait in the nearby lake systems of Attiko-Beotia and in the Peloponnese that, due to their proximity to Athens, attract many anglers. Finally, the distribution of P. stymphalicus falls within the range reported in previous studies (Economou et al. 2007; Kottelat & Freyhof, 2007a).
DNA barcoding is an important tool for the detection of cryptic diversity, since it can assign atypical specimens to genetic lineages that may correspond to new species. However, such single-gene approaches are considered only indicative for taxonomic revisions and cannot replace comprehensive taxonomic analysis (Hajibabaei et al., 2007). Incautious utilization of barcoding for species delimitation has been criticized due to certain pitfalls of this method associated with introgressive hybridization and/or incomplete lineage sorting (Funk & Omland, 2003; Chase et al., 2005). Prior to formal taxonomic changes, results from the phylogeny of the barcoding gene should be tested with additional types of data and respective analyses, such as morphological and/or ecological data and multi-locus coalescent-based methods (Puillandre et al., 2012b; Satler et al., 2013). However, in this study the barcoding approach has proved very useful for clarifying the distribution and haplotype diversity of Pelasgus species, with private haplotypes present even in neighbouring drainages or adjacent regions, indicating significant population structuring and isolation. While further analyses (e.g., genetic analysis of several nuclear loci) are required to identify evolutionary significant units and to formulate proper conservation actions, the present study indicates some of the Pelasgus populations that should be prioritized for conservation management. Finally, we have also demonstrated how barcoding of type material can reveal crucial information for the protection of endangered species.
Conclusion
The current study has provided an insight into the distribution of the Pelasgus species in Greece and has also identified certain hotspots that require further taxonomic investigations. More specifically: (a) the use of P. epiroticus museum samples from its type locality, from which the species is apparently now extinct, was extremely valuable since it revealed for the first time the presence of an extant population in Zaravina Lake; (b) it revealed haplotypes of P. epiroticus in systems well outside its native range due to translocations; (c) it revealed a subclade of P. epiroticus (Amvrakikos Gulf systems) with important conservation implications; (d) it resolved the distribution of P. thesproticus, and finally (e) it confirmed the previously reported distribution of P. stymphalicus, P. laconicus and P. marathonicus.
References
Anisimova, M., M. Gil, J. F. Dufayard, C. Dessimoz & O. Gascuel, 2011. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biology 60: 685–699.
Apostolidis, A. P., M. T. Stoumboudi, E. Kalogianni, G. Cote & L. Bernatchez, 2011. Genetic divergence among native trout Salmo trutta populations from southern Balkans based on mitochondrial DNA and microsatellite variation. Journal of Fish Biology 79: 1950–1960.
April, J., R. Mayden, R. Hanner & L. Bernatchez, 2011. Genetic calibration of species diversity among North America’s freshwater fishes. Proceedings of the National Academy of Sciences of USA 108: 10602–10607.
Bandelt, H. J., P. Forster, & A. Röhl, 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16: 37–48.
Barbieri, R., S. Zogaris, E. Kalogianni, M. T. Stoumboudi, Y. Chatzinikolaou, S. Giakoumi, Y. Kapakos, D. Kommatas, N. Koutsikos, V. Tachos, L. Vardakas & A. Economou, 2015. Freshwater Fishes and Lampreys of Greece. Monographs on Marine Sciences, Vol 8 Hellenic Centre for Marine Research, Athens.
Buj, I., R. Šanda, S. Zogaris, J. Freyhof, M. F. Geiger & J. Vukić, 2019. Cryptic diversity in Telestes pleurobipunctatus (Actinopterygii; Leuciscidae) as a consequence of historical biogeography in the Ionian Freshwater Ecoregion (Greece, Albania). Hydrobiologia 835: 147–163.
Chase, M. W., N. Salamin, M. Wilkinson, J. M. Dunwell, R. P. Kesanakurthi, N. Haidar & V. Savolainen, 2005. Land plants and DNA barcodes: short-term and long-term goals. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 1889–1895.
Crivelli, A.J., 2006. Pelasgus epiroticus. The IUCN Red List of Threatened Species 2006: e.T61256A12454831.
Economou, A. N., R. Barbieri, C. Daoulas, T. Psarras, M. T. Stoumboudi, I. Bertahas, S. Giakoumi & A. Patsias, 1999. Endangered Freshwater Fish of Western Greece and Peloponnese: Distribution, Abundance, Threats and Recommended Conservation Measures. PENED, Technical Report. Anavyssos Attiki: Hellenic Centre for Marine Research, Institute of Inland Waters; 1999 (in Greek).
Economou, A. N., S. Giakoumi, L. Vardakas, R. Barbieri, M. Stoumboudi & S. Zogaris, 2007. The freshwater ichthyofauna of Greece – an update based on a hydrographic basin survey. Mediterranean Marine Science 8: 91–166.
Funk, D. J., & K. E. Omland, 2003. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology, Evolution, and Systematics 34: 397–423.
Geiger, M. F., F. Herder, M. T. Monaghan, V. Almada, R. Barbieri, M. Bariche, P. Berrebi, J. Bohlen, M. Casal-Lopez, G.B. Delmastro, G.P.J. Denys, A. Dettai, I. Doadrio, E. Kalogianni, H. Kärst, M. Kottelat, M. Kovačić, M. Laporte, M. Lorenzoni, Z. Marčič, M. Özuluğ, A. Perdices, S. Perea, H. Persat, S. Porcelotti, C. Puzzi, J. Robalo, R. Šanda, M. Schneider, V. Šlechtová, M. Stoumboudi, S. Walter & J. Freyhof, 2014. Spatial heterogeneity in the Mediterranean biodiversity hotspot affects barcoding accuracy of its freshwater fishes. Molecular Ecology Resources 14: 1210–1221.
Guindon, S., J.F. Dufayard, V. Lefort, M. Anisimova, W. Hordijk & O. Gascuel, 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321.
Hajibabaei, M., G. A. C. Singer, P. D. N. Hebert & D. A. Hickey, 2007. DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends in Genetics 23: 167–172.
IUCN, 2019. The IUCN Red List of Threatened Species. [available on internet at http://www.iucnredlist.org]. Accessed 21 March 2019.
Ivanova, N. V., T. S. Zemlak, R. H. Hanner & P. D. N. Hebert, 2007. Universal primer cocktails for fish DNA barcoding. Molecular Ecology Notes 7: 544–548.
Kimura M., 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16:111-120.
Kottelat, M. & R. Barbieri, 2004. Pseudophoxinus laconicus, a new species of minnow from Peloponnese, Greece, with comments on the West Balkan Pseudophoxinus species (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 15: 147–160.
Kottelat, M. & J. Freyhof, 2007a. Handbook of European Freshwater Fishes. Berlin: Kottelat, Cornol and Freyhof.
Kottelat, M. & J. Freyhof, 2007b. Pelasgus, a new genus name for the Balkan species of Pseudophoxinus (Teleostei: Cyprinidae). Ichthyological Exploration of Freshwaters 18: 103–108.
Kumar S., G. Stecher & K. Tamura, 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33:1870-1874.
Lefort, V., J.E. Longueville & O. Gascuel, 2017. SMS: Smart Model Selection in PhyML. Molecular Biology and Evolution 34: 2422–2424.
Leonardos, I., I. Paschos & M. Prassa, 2005. Threatened fishes of the world: Phoxinellus epiroticus (Steindachner 1895) (Cyprinidae). Environmental Biology of Fishes 72: 250.
Leonardos, I. D., I. Kagalou, M. Tsoumani & P. S. Economidis, 2008. Fish fauna in a Protected Greek lake: biodiversity, introduced fish species over a 80-year period and their impacts on the ecosystem. Ecology of Freshwater Fish 17: 165–173.
Malaquias, M. A. E., L. T. Ohnheiser, T. R. Oskars, & E. Willassen, 2017. Diversity and systematics of philinid snails (Gastropoda: Cephalaspidea) in West Africa with remarks on the biogeography of the region. Zoological Journal of the Linnean Society 180: 1–35.
Masters, B. C., V. Fan & H. A. Ross, 2010. Species delimitation plugin manual. https://assets.geneious.com/plugins/3rdparty/Species_Delimitation_Plugin_Manual.pdf
Masters, B. C., V. Fan & H.A. Ross, 2011. Species delimitation – a Geneious Plugin for the exploration of species boundaries. Molecular Ecology Resources 11: 154–157.
Messing, J., 1983. New M13 vectors for cloning. Methods in Enzymology 101: 20–78.
Miller, S. A., D. D. Dykes & H. F. Polesky, 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research 16: 1215.
Nei, M. & S. Kumar, 2000. Molecular Evolution and Phylogenetics. Oxford University Press, New York.
Perdikaris, C., C. Nathanailides, E. Gouva, C. Karipoglou & I. Paschos, 2005. Collapse of Epirus minnow, Phoxinellus epiroticus (Steindachner, 1895) fisheries in Lake Pamvotis, Greece. Ichthyological Exploration of Freshwaters 16: 171–174.
Perea, S., J. Vukić, R. Šanda & I. Doadrio, 2016. Ancient mitochondrial capture as factor promoting mitonuclear discordance in freshwater fishes: a case study in the genus Squalius (Actinopterygii, Cyprinidae) in Greece. PLoS ONE 11: e0166292.
Puillandre, N., A. Lambert, S. Brouillet & G. Achaz, 2012a. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21: 1864–1877.
Puillandre, N., M. V. Modica, Y. Zhang, L. Sirovich, M. C. Boisselier, C. Cruaud, M. Holford & S. Samadi, 2012b. Large-scale species delimitation method for hyperdiverse groups. Molecular Ecology 21: 2671–2691.
Rodrigo A. G., F. Bertels, J. Heled, R. Noder, H. Shearman & P. Tsai, 2008. The perils of plenty: what are we going to do with all these genes? Philosophical Transactions of the Royal Society London. Series B, Biological Sciences 363: 3893–3902.
Rosenberg N. A., 2007. Statistical tests for taxonomic distinctiveness from observations of monophyly. Evolution 61: 317–323.
Satler, J. D., B. C. Carstens & M. Hedin, 2013. Multilocus species delimitation in a complex of morphologically conserved trapdoor spiders (Mygalomorphae, Antrodiaetidae, Aliatypus). Systematic Biology 62: 805–823.
Skoulikidis, N. T., A. N. Economou, K. C. Gritzalis & S. Zogaris, 2009. Rivers of the Balkans. In Tockner, K, C. T. Robinson & U. Uehlinger (eds), Rivers of Europe. Academic/Elsevier, San Diego.https://doi.org/10.1016/B978-0-12-369449-2.00011-4
Tamura, K., G. Stecher, D. Peterson, A. Filipski & S. Kumar, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729.
Vardakas, L., E. Kalogianni, C. Papadaki, T. Vavalidis, A. Mentzafou, D. Koutsoubas & T. N. Skoulikidis, 2017. Defining critical habitat conditions for the conservation of three endemic and endangered cyprinids in a Mediterranean intermittent river before the onset of drought. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 1270–1280.
Vogiatzi, E., E. Kalogianni, B. Zimmerman, S. Giakoumi, R. Barbieri, P. Paschou, A. Magoulas, D. Tsaparis, N. Poulakakis & C. S. Tsigenopoulos, 2014. Reduced genetic variation and strong genetic population structure in the freshwater killifish Valencia letourneuxi (Valenciidae) based on nuclear and mitochondrial markers. Biological Journal of the Linnean Society 111: 334–349.
Wandeler, P., P.E.A. Hoeck & L.F. Keller, 2007. Back to the future: museum specimens in population genetics. Trends in Ecology and Evolution 22: 634–642.
Ward, R. D., T. S. Zemlak, B. H. Innes, P. R. Last & P. D. N. Hebert, 2005. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 1847–1857.
Xia, X., 2013. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Molecular Biology and Evolution 30: 1720–1728.
Zalidis, G. & A. Mantzavelas, 1996. Inventory of Greek wetlands as natural resources. Wetlands 16: 548–556.
Zardoya, R., P. S. Economidis & I. Doadrio, 1999. Phylogenetic relationships of Greek Cyprinidae: molecular evidence for at least two origins of the Greek cyprinid fauna. Molecular Phylogenetics and Evolution 13: 122–131.
Acknowledgements
Field work to collect genetic material was conducted mainly within the framework of the Programme “Monitoring of ecological water quality of rivers, coastal and transitional waters of Greece” (Article 8 Directive 2000/60/ΕU), (2012–2015), funded by the European Regional Development Fund (ESPA). For field work and collection of specimens, a permit was secured by HCMR from the Ministry of Environment and Energy of Greece. We are grateful to Mrs. Margaret Eleftheriou for comments and linguistic editing of the manuscript. We also thank the two anonymous reviewers who improved the final version of this manuscript with their constructive criticism.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Christian Sturmbauer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Tsaparis, D., Konstantinidis, I., Palandacic, A. et al. DNA barcoding provides new insights on the distribution, systematics and conservation of the freshwater genus Pelasgus (Cypriniformes: Cyprinidae) in Greece. Hydrobiologia 848, 1163–1176 (2021). https://doi.org/10.1007/s10750-021-04526-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04526-9