Abstract
Variations in morphological characteristics are frequently important determinants of the physiology, ecology and inter- and intra-specific interactions of organisms. Various causative drivers have been investigated for this morphological variation, such as sexual selection, variations in the available resources to exploit, habitat characteristics, and other environmental factors. However, it is not clear whether human disturbance has any selective role in creating morphological variations other than simply reducing the body size of individuals. We used a bioindicator, the Atlantic ghost crab Ocypode quadrata, to measure how human disturbance influences morphological claw variation. We examined the claw height and the volume of major and minor claws of crabs at 12 South Carolina sandy beaches with various degrees of human disturbance. Crabs from disturbed sites had smaller and shallower claws compared to crabs from pristine sites, and these differences varied with sex and with body size. Additionally, major claw volume and claw height also varied with crab body size, gender, and across beaches with different amounts of human disturbance. Other claw characteristics showed no difference between sexes with the degrees of human disturbance, or with variation in body size. Our results suggest that the magnitude of the human disturbance experienced by populations may have a selective role on the morphology of individual organisms that can differ between males and females. These morphological changes likely have ecological and physiological consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological variation plays a key role in the ecology, survival and physiology of organisms. Numerous studies show that morphological differences lead to differential exploitation of resources (Yamada & Boulding, 1998; Buck et al. 2003), potentially contributing to the physiological condition of organisms. Additionally, morphological differences can determine social structures and influence the outcome of intra- and inter-specific interactions (e.g., agonistic behaviors, predator–prey interactions) (Sneddon et al., 2000; Hernáez & João, 2018; Cannizzo et al., 2019; Rico-Guevara & Hurme, 2019).
Sexual dimorphism is a widespread phenomenon in nature (reviewed in Shine, 1989; Andersson, 1994; Rico-Guevara & Hurme, 2019), and various hypotheses have been proposed to explain mechanisms behind sexual dimorphism. An early and ongoing explanation is sexual selection (Shine, 1989; Claverie & Smith, 2010; Cothran & Jeyasingh, 2010; Rico-Guevara & Hurme, 2019). Sexual selection can cause sexual dimorphism if one sex, for example, preferentially selects larger mates to optimize reproductive success (Shine, 1989; Andersson, 1994; Maklakov et al., 2004). Additionally, sexual selection may lead to morphological divergence due to competition between members of the same sex for access to mates (Serrano-Meneses et al., 2007; Neff & Svensson, 2013; Clutton-Brock, 2017). Sexual dimorphism may also arise from resource availability if genders differ in their ability to exploit resources (Shine, 1989; Cothran & Jeyasingh, 2010; Nolen et al., 2017). Finally, habitat characteristics may have different selective impacts on each gender (Shine, 1989; Giri & Loy, 2008; Cannizzo et al., 2019).
Environmental factors may also lead to morphological variation across sites irrespective of gender. For example, crabs living in Argentinian lakes have a shorter but wider rostrum compared to conspecifics living in rivers (Giri & Loy, 2008). Also, mangrove tree crabs living in salt marshes have larger claws compared to conspecifics living in dock and mangrove habitats (Cannizzo et al., 2019). Given the occurrence of morphological variation across habitat types, we may also expect morphological variation across habitats of the same type that differ in quality. Such differences in habitat quality often result from human impacts on natural systems.
Claw morphology commonly varies in crustaceans. Claw size is an important factor that influences intra- and inter-specific interactions such as antagonistic and predator–prey interactions (Sneddon et al., 2000; Rico-Guevara & Hurme, 2019) and reproductive success (Shine, 1989; Andersson, 1994; Maklakov et al., 2004). However, claw growth requires a substantial energy investment (Berke & Woodin, 2008), and claw size is therefore influenced by many factors, including habitat type (Giri & Loy, 2008; Cannizzo et al., 2019) and diet quality (Smith & Palmer, 1994). Given the energy requirement, tradeoffs between claw growth and other body functions and behaviors such as general body growth, reproduction, decoration and predator avoidance are common in crustaceans (Berke & Woodin, 2008; Hultgren & Stachowicz, 2008; Hultgren & Stachowicz, 2011; Anderson et al., 2013).
Coastal regions experience strong human influences because of their desirable ecosystem services (Davenport & Davenport, 2006; Halpern et al., 2008; Barbier et al., 2011), and these influences are likely to become stronger in the future as the size of human coastal populations continue to increase (Vitousek et al., 1997; Davenport & Davenport, 2006; Halpern et al., 2008). Species living in coastal regions already show signs of stress (Vitousek et al., 1997; Halpern et al., 2008; Defeo et al., 2009), with populations responding to human influence by reducing individual organismal sizes and population densities (Neves & Bemvenuti, 2006; Hobbs et al., 2008; Cardoso et al., 2016; Schlacher et al., 2016; Gül & Griffen, 2018a, b; Suciu et al., 2018).
The Atlantic ghost crab Ocypode quadrata (Fabricius, 1787) is a semi-terrestrial crab that lives on sandy shores (Milne & Milne, 1941). It is widely used as a bioindicator of human impacts because of its strong responses to different types of impacts caused by human (Wolcott & Wolcott, 1984; Neves & Bemvenuti, 2006; Gül & Griffen, 2018a, b; Suciu et al., 2018) and natural disturbances (Hobbs et al,. 2008; Gül & Griffen, 2019a). As with other species, ghost crabs also display smaller individual size and lower population abundance on disturbed shores (Schlacher et al., 2016; Gül & Griffen, 2018a, 2018b). In addition, ghost crabs also alter their burrowing behavior and associated energetic demands under human influence (Gül & Griffen, 2019b).
Various mechanisms have been proposed to explain smaller individual sizes of ghost crabs under human disturbance. For instance, vehicles on the beach lead to direct mortality by crushing (Wolcott & Wolcott, 1984; Schlacher et al., 2007), and larger crabs may experience higher mortality because they emerge from their burrows more often than smaller crabs (Wolcott & Wolcott, 1984). In addition to mortality caused by vehicles, direct handling by beachgoers at disturbed sites may influence body sizes since larger crabs are captured more frequently (Gül & Griffen, 2018b). Moreover, reduced food availability and/or food quality likely contribute to lower growth rates in crabs at disturbed sites (Stelling-Wood et al., 2016; Kiskaddon et al., 2019). Variations in energetic balance due to changes in behaviors and daily activities in ghost crabs between sites that differ in the strength of human disturbance may further contribute to differences in body size of crabs (Gül & Griffen, 2019b). Thus, there are several mechanisms that may all contribute to the observed decrease in body size of crabs at disturbed sites. Yet the impacts of human disturbance on size-independent morphological features have received less attention and are limited to some bird and lizard species (Smith et al., 2008; Marnocha et al., 2010; Winchell et al., 2016; Putman et al., 2019).
The impacts of human disturbances on natural populations have been widely investigated by observing the population densities and individual sizes of bioindicator species (Carignan & Villard, 2002; Spellerberg, 2005; Cortes et al., 2013). However, it is not clear whether human disturbance plays a selective role in eliciting morphological differences other than the reductions in body size that have been reported. If human impacts can be shown to correlate with other forms of morphological change, this may further assist in forecasting the future conditions of disturbed populations as human use of coastal areas continues to rise. Therefore, we used the Atlantic ghost crab as a model organism to understand whether human disturbance can be correlated with variation in claw morphology across sites with different amounts of human disturbance. Changes in burrowing behaviors (i.e. burrow fidelity and longevity) in ghost crabs leads to increased energetic demand at sites with higher human disturbance levels (Gül & Griffen, 2019b). At the same time, energy storage and reproductive potential of ghost crabs both decline (Gül & Griffen in review) as a direct result of reduced abundance of common ghost crab prey items at disturbed sites (González et al., 2014; Cardoso et al., 2016; Frota et al., 2019; Laitano et al., 2019; Gül & Griffen in review). This energy imbalance may lead to growth-reproduction tradeoffs (Stearns, 1992). Additionally, male Ocypodidae species tend to have larger claws compared to female conspecifics to increase the chance of being selected for reproduction (McLain & Pratt, 2007; Reaney, 2009) and to increase success in agonistic encounters against other males. Females, on the other hand, grow smaller claws compared to their male conspecifics (Hartnoll, 1974), potentially reflecting a tradeoff to store more energy for egg production instead of investing in claw growth. We therefore predicted that female ghost crabs would have smaller claw sizes at sites with high human disturbance compared to conspecifics from pristine and moderately impacted sites due to this potential tradeoff between the allocation of limited energy to reproduction vs. growth. We further predicted that males would have smaller claw sizes at sites with human disturbance, but that these changes would be less dramatic compared to females due to a faster claw growth in males of this species compared to females (Haley 1969).
Materials and methods
Study sites
We examined variation in the size of major and minor claws of crabs on twelve sandy beaches with different degrees of human disturbances on the South Carolina coast (Fig. 1, Table 1). Although the coast of South Carolina possesses ocean-exposed sandy shores, the wave energy affecting the beaches is relatively low because the continental shelf is relatively broad (Kana, 1988). Low wave energy makes the South Carolinian beaches preferable destinations for vacations, and while beach activity may be beneficial for the local economy, it alters local faunal populations and alters the geo-morphological features of heavily used sites (Gül and Griffen, 2018a).
We conducted our field observations in July 2018. We chose study sites using the urbanization index, modified from González et al. (2014), which was created based on observations and the quantitative evaluations of the variables shown in Table 1 during the summer of 2016 and 2017. The index was calculated using the formula X’ = (X − Xmin)/(Xmax − Xmin), where X represents the value for each of the variables in Table 1 and Xmin and Xmax represent the minimum and the maximum values for the variables. The urbanization index score for each beach was obtained by averaging the individual X’ values for the different metrics that comprise the score. The index ranges from 0 (low human impact) to 1 (extreme human impact). During the summer of 2016 and 2017, we observed the beach cleaning technique, buildings, and other amenities next to each beach. We also counted the numbers of vehicles and beach visitors over approximately 1 km of beach as a proxy for the intensity of the visitors and intensity for the mechanical influence if there was any. We counted the number of people on each site over a 2 h period during the weekdays from 0800 to 1000. We observed vehicles during the nights and counted the vehicles on the beach. Because the use of off-road vehicles on the South Carolina sandy beaches are not allowed, we observed the beach cleaning vehicles, vehicles that are used by the municipality agents and life guards.
Claw morphology
To determine differences in claw size between male and female ghost crabs under various intensities of human disturbance, we collected opportunistically-encountered adult crabs of a wide range of sizes around the strandline over approximately 1-km of beach during the night at each site using a dip net. We sampled the crabs during night times at approximately similar hours, because Atlantic ghost crabs are nocturnal organisms and they spend the day in their burrows (Lucrezi and Schlacher, 2014). We sampled crabs around the strandline because they forage around the strandline on carrion, wrack material, coquina clam, mole crabs etc. (Wolcott, 1978). We measured the carapace width as well as the length, height, and width of both the major (crusher) and minor (cutter) claws using a Vernier caliper (± 0.1 mm) as shown in Fig. 2. We excluded individuals with injuries such as missing limbs or injuries on the carapace. Various claw dimensions such as claw length, claw depth or claw height can be an indicator for the claw size; however, these metrics may change independently across habitats (Edgell & Rochette, 2007). Therefore, we used volumes of both claws (i.e., product of the claw dimensions, length × height × width) as morphological claw characteristics of the crabs. We also used the variation in major and minor claw height as a proxy for the claw closing force (Lee, 1993; Yamada & Boulding, 1998; Sneddon et al., 2000). We first log-transformed the data for the volume and the height of the claws and for carapace width to linearize these allometric relationships as suggested by Tukey (1977). While problems exist when using logarithmic transformations of allometric data, these problems are largely associated with using the resulting linear equations to extrapolate and make predictions, or when predicted values from least squares regression are detransformed back to arithmetic units (Smith, 1993). We did not do either of these here, and we therefore avoid biases that can occur with transformed allometric data. Following transformation, we verified that all relationships between the carapace width and claw volume or height for both claws and for each sex were linear. We then used linear mixed effects models (lmerTest, package in R, Kuznetsova et al., 2018) to examine whether claw volumes and claw height (i.e., a proxy for claw closing force) varied between sexes, with crab size (i.e., carapace width) and with the extent of human disturbances, treated as a continuous variable using the urbanization index as described above. We also included two-way and three-way interaction terms in our models. To control for spatial (latitudinal) differences associated with individual sites, we included the study site nested in latitude as a random factor in all mixed models. Mixed models were followed by a Tukey’s HSD test for multiple comparisons between sexes (“lsmeans” package in R, Lenth & Hervé, 2018). Further, because of the difficulty of interpreting main effects in the presence of significant three-way interactions, we also conducted linear regressions for males and females separately, and with only the urbanization index or the carapace width used as a predictor variable. W do not present the results of these individual repressions here, but have used them to interpret main effects in the analyses that included sex, urbanization index, and carapace width together.
Results
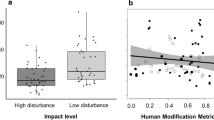
We examined a total of 331 adult ghost crabs (153 males and 178 females). We found that claw morphology of ghost crabs varied with the intensity of human disturbance, with crab body size, and with sex. Specifically, we found that the major (i.e., crusher) claw volume of O. quadrata was larger in males than in females (Tukey HSD, p < 0.05), but increased in both sexes as body size increased (Fig. 3a, Table 2a). Further, major claw volume decreased as human influence on a beach increased, but this relationship was stronger for male than for female crabs (significant interaction term between urbanization index and sex, Table 2a). Further, the three-way interaction between sex, crab size, and urbanization index was significant (Table 2a). Minor claw volume also increased with crab carapace width, but was not different between the sexes (Tukey HSD, p > 0.05). In addition, minor claw volume decreased as human disturbance become stronger, but the extent of this decrease was greater for male than for females (Fig. 3b, significant interaction between urbanization index and sex, Table 2b). The three-way interaction between human impacts, crab body size, and sex was significant for this analysis as well. No major influence of the random factors was detected on the major or minor claw volumes (Table 2a, b).
Using claw height as a proxy, we found that the closing strength of the major claw reflected the patterns described above for the major claw volume. Specifically, the height of the major claw was greater in males than in females (Tukey HSD, p < 0.05) and increased with crab carapace width (Fig. 4a, Table 2c). Major claw height also decreased as human impacts become stronger, but this decrease was greater for males than for females (significant interaction between the urbanization index and sex, Table 2c). The three-way interaction was again significant. Finally, we found that the height of the minor claw increased with body size and decreased with human impacts, but neither of these relationships differed between sexes (Fig. 4b, Table 2d). Random factors showed no major effects on the major and minor claw closing forces (Table 2c, d).
In addition, we analyzed these same results by comparing series of nested models using log likelihood. Result of this analysis agreed qualitatively with the results presented here, with the exception that the log likelihood approach found that the best model to examine minor claw height included the three-way interaction between sex, carapace width, and degree of human impact.
Discussion
We have shown that the volume and the height of the crusher and cutter claws of O. quadrata are positively related to crab body size and are negatively related to the degree of human impact on a beach. Further, we have shown that changes in claw morphology with crab body size and with human impacts across beaches are generally more extreme for male than for female crabs. Since claw size and claw closing strength can directly influence sexual selection (Shine, 1989; Claverie & Smith, 2010; Cothran & Jeyasingh, 2010), foraging efficiency (Yamada & Boulding, 1998; Edgell & Rochette, 2009; Jaroensutasinee & Jaroensutasinee, 2015), success in agonistic behaviors (Sneddon et al., 2000; Cannizzo et al., 2019, reviewed in Rico-Guevara & Hurme, 2019), and mating success (Serrano-Meneses et al., 2007), changes in the claw morphology have the potential to influence the ecology and the physiology of O. quadrata. The results of this research therefore have important implications for ghost crab populations on sandy shores and for our understanding of the broad potential influences of human disturbances on the ecological and physiological changes of organisms.
The changes in the claw sizes as a response to the levels of the human disturbance for males and females reported here may possibly reflect plastic variation in the strength of the agonistic behaviors under human influence. Agonistic behaviors are widespread in crustaceans (Dalosto et al., 2013; Ayres-Peres et al., 2015), which commonly use claws as an important weapon for winning these encounters (Andersson, 1994; Rico-Guevara & Hurme, 2019), and ghost crabs are no exception (Lucrezi & Schlacher, 2014). The strength of agonistic behaviors is directly related to density in crustaceans, and individuals living at higher population densities often become better competitors, likely due to selection for stronger claws used in agonistic encounters (Calsbeek, 2009). Claw volumes of both male and female crabs declined proportionately with increasing strength of human disturbance. As a bioindicator of human disturbance on the sandy shores, population abundance and the individual sizes of Atlantic ghost crabs decline dramatically under the influence of human disturbance (Wolcott & Wolcott, 1984; Neves & Bemvenuti, 2006; Hobbs et al., 2008; Gül & Griffen, 2018a, b; Suciu et al., 2018). Thus, there are potentially weaker agonistic behaviors at sites under strong human influences. Moreover, lower claw closing strength (i.e., claw height), after accounting for the crab body size, was found in crabs living at the sites in which the human disturbance is strongest (e.g., ui > 0.9). Previous work has shown that the ghost crab density was lowest in those sites (Gül & Griffen, 2018a, b).
Differences in the size of both claws here may also result from variation in the available energy for crabs living in sites with different levels of human disturbance. Specifically, both male and female crabs had smaller crusher and cutter claws after accounting for the body size at beaches under human influence compared to their conspecifics at pristine sites. Besides reducing ghost crab populations, human disturbance dramatically influences the main prey items of ghost crabs such as bean clams, mole crabs and sandy beach coleoptera (González et al., 2014; Cardoso et al., 2016; Frota et al., 2019; Laitano et al., 2019; Gül & Griffen in review). Diet type and overall resource availability can influence the claw morphology and body size in other species (Shine, 1989; Smith & Palmer, 1994; Cothran & Jeyasingh, 2010). If this is true for ghost crab populations, it is logical to conclude that reductions in preferred food lead to smaller claw sizes in ghost crabs at disturbed sites. However, this explanation raises the question of why limited food would not influence the major claw volume of male crabs in the same way that it influences females (i.e., why do the patterns differ for the two genders)? Hartnoll (1974) reported that male crabs generally grow their claws faster compared to the females after reaching maturity. Similar results have been reported for ghost crabs. Haley (1969) showed that claws of male ghost crabs grow faster compared to those of females at all size classes. This differential growth may result from sexual selection (Schenk & Wainwright, 2001). Therefore, it can be hypothesized that male ghost crabs grow their major claws faster even under human disturbance to attract females, as fiddler crabs do (i.e. fiddler crabs and ghost crabs belong to the same phylogenetic family, Ocypodidae) (McLain & Pratt, 2007; Reaney, 2009), or for use in mate guarding. Overall, the results here show that human disturbance influences both major and minor claw sizes, but that impacts appear to be larger on major claws, and different patterns between male and female crabs are consistent with increased focus on claw growth in males for reproductive purposes.
We only examined variation in claw size and did not directly measure claw closing strength. However, previous studies have shown that there is a close relationship between claw height and the closing strength across species (Lee, 1993; Yamada & Boulding, 1998; Sneddon et al., 2000), as well as between claw size and resource availability and foraging efficiency (Smith & Palmer, 1994; Yamada & Boulding, 1998; Buck et al., 2003; Edgell & Rochette, 2009; Cothran & Jeyasingh, 2010). Ghost crabs mostly feed on beach clams and mole crabs on the Southern coast (Wolcott, 1978), and these organisms can be opened and/or crushed even by crabs with weak claws due to their soft bodies and shells, suggesting that the dietary implications of reduced claw size in this system may be moderate. Further research is required to understand whether the lower claw height influences the diet of ghost crabs.
The correlation between the intensity of human disturbance and claw size of ghost crabs suggests yet another mechanism by which human disturbances may lead to reduced body size and density of this bioindicator species (Wolcott & Wolcott, 1984; Neves & Bemvenuti, 2006; Hobbs et al., 2008; Schlacher et al., 2016; Gül & Griffen, 2018a, b; Suicu et al., 2018). If the available energy sources are limited under human influence, as hypothesized above, then species may not obtain enough energy at the most disturbed sites. In that case, both males and females may exhibit a tradeoff between their growth and their reproductive activity. Females may spend more of the limited energy that they gain on egg production instead of claw growth, while males may spend more energy for increasing their chance of gaining access to a female by growing their major claws instead of their body. Further studies are required to test this hypothesis, such as by investigating the differences in daily activities (e.g., burrow size and behavior) between males and females, as well as differences in the energy requirement for reproduction and for claw growth.
Additionally, habitat characteristics and environmental factors can influence the morphological variation in organisms (Giri & Loy, 2008; Cannizzo et al., 2019). Ghost crabs are widely used as a bioindicator of human disturbance due to their lower population abundance and smaller individual sizes in disturbed habitats (Schlacher et al., 2016). Environmental and geo-morphological factors such as sand grain size, sand compaction, and beach slope and width may contribute to changes in prey availability (Tewfik et al., 2016) and subsequently to these changes in ghost crab demographics at disturbed sites (Turra et al., 2005; Gül and Griffen, 2018b). Similarly, these physical beach characteristics may be a direct contributing factor to variation in ghost crab claw volumes and closing strengths across sites. Beach width, slope, and grain size vary across South Carolina beaches with different levels of human disturbance (Gül and Griffen, 2018a, b). However, during the research reported here we did not collect data on beach characteristics that would be needed to test the hypothesis of a direct link between beach characteristics and claw morphology. Therefore, it is not possible to determine whether the changes in claw morphology documented here are the direct result of human impacts, or whether human impacts have an indirect effect on claw morphology via changes in habitat characteristics. This question remains for future research.
In conclusion, human disturbance appears to play a key role in determining the size and closing strength of the major and minor claws of O. quadrata, a bioindicator species, and this role is sex-specific. One plausible reason for this is a shortage in food (i.e., energy limitation) at disturbed sites. This mechanism would also explain the reported decrease in overall body size on beaches influenced by human disturbance. However, the potential influences of claw size on foraging efficiency and success in mating and in agonistic encounters remains to be tested. Understanding the possible influences of such morphological variation on the ecology and physiology of individuals will further our understanding of population level responses of this bioindicator species to various levels of human disturbance.
References
Andersson, M., 1994. Sexual Selection. Princeton University Press, Princeton, New Jersey.
Anderson, J. R., A. J. Spadaro, J. A. Baeza & D. C. Behringer, 2013. Ontogenetic shifts in resource allocation: colour change and allometric growth of defensive and reproductive structures in the Caribbean spiny lobster Panulirus argus. Biological Journal of the Linnean Society 108: 87–98.
Ayres-Peres, L., P. B. Araujo, C. G. Jara, A. V. Palaoro & S. Santos, 2015. How variable is agonistic behavior among crab species? A case study on freshwater anomurans (Crustacea: Decapoda: Aeglidae). Journal of Zoology 297(2): 115–122.
Barbier, E. B., S. D. Hacker, C. Kennedy, E. W. Koch, E. C. Siter & B. R. Silliman, 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81(2): 169–193.
Berke, S. K. & S. A. Woodin, 2008. Energetic costs, ontogenetic shifts and sexual dimorphism in spider crab decoration. Functional Ecology 22: 1125–1133.
Buck, T. L., G. A. Breed, S. C. Pennings, M. E. Chase, M. Zimmer & T. H. Carefoot, 2003. Diet choice in an omnivorous salt-marsh crab: different food types, body size, and habitat complexity. Journal of Experimental Marine Biology and Ecology 292: 103–116.
Calsbeek, R., 2009. Experimental evidence that competition and habitat use shape the individual fitness surface. Journal of Evolutionary Biology 22: 97–108.
Cannizzo, Z. J., S. K. Nix, I. C. Whaling & B. D. Griffen, 2019. Individual morphology and habitat structure alter social interactions in a range-shifting species. Diversity 11: 6.
Cardoso, R. S., C. A. M. Barboza, V. B. Skinner & T. M. B. Cabrini, 2016. Crustaceans as ecological indicators of metropolitan sandy beach health. Ecological Indicators 62: 154–162.
Carignan, V. & M. A. Villard, 2002. Selecting indicator species to monitor ecological integrity: a review. Environmental Monitoring and Assessment 78(1): 45–61.
Claverie, T. & I. P. Smith, 2010. Allometry and sexual dimorphism in the chela shape in the squat lobster Munida rugosa. Aquatic Biology 8: 179–187.
Clutton-Brock, T., 2017. Reproductive competition and sexual selection. Philosophical Transactions of the Royal Society B 372: 20160310.
Cortes, R. M. V., S. J. Hughes, V. R. Pereira & S. D. P. Varandas, 2013. Tools for bioindicator assessment in rivers: the importance of spatial scale, land use patterns and biotic integration. Ecological Indicators 34: 460–477.
Cothran, R. D. & P. D. Jeyasingh, 2010. Condition dependence of a sexually selected trait in crustacean species complex: importance of the ecological context. Evolution 64(9): 2535–25346.
Dalosto, M. M., A. V. Palaoro, J. R. Costa & S. Santos, 2013. Aggressiveness and life underground: the case of burrowing crayfish. Behaviour 150: 3–22.
Davenport, J. & J. L. Davenport, 2006. The impact of tourism and personal leisure transport on coastal environments: a review. Estuarine, Coastal and Shelf Science 67(1–2): 280–292.
Defeo, O., A. McLachlan, D. S. Schoeman, T. A. Schlacher, J. Dugan, A. Jones, M. Lastra & F. Scapini, 2009. Threats to sandy beach ecosystems: a review. Estuarine, Coastal and Shelf Science 81(1): 1–12.
Edgell, T. C. & R. Rochette, 2007. Geographic correlation between reciprocally adaptive traits of an exotic decapod predator and native gastropod prey: evidence of an arms race? Evolutionary Ecology Research 9: 579–597.
Edgell, T. C. & R. Rochette, 2009. Prey-induced changes to a predator’s behaviour and morphology: implications for shell–claw covariance in the northwest Atlantic. Journal of Experimental Marine Biology and Ecology 382: 1–7.
Frota, G. P., T. M. B. Cabrini & R. S. Cardoso, 2019. Fluctuating asymmetry of two crustacean species on fourteen sandy beaches of Rio de Janeiro State. Estuarine, Coastal and Shelf Science 223: 138–146.
Giri, F. & A. Loy, 2008. Size and shape variation of two freshwater crabs in Argentinean Patagonia: the influence of sexual dimorphism, habitat, and species interactions. Journal of Crustacean Biology 28(1): 37–45.
González, S. A., K. Yáñez-Navea & M. Muñoz, 2014. Effect of coastal urbanization on sandy beach coleoptera Phaleria maculate (Kulzer, 1959) in northern Chile. Marine Pollution Bulletin 83(1): 265–274.
Gül, M. R. & B. D. Griffen, 2018a. Impacts of human disturbance on ghost crab burrow morphology and distribution on sandy shores. PLoS ONE 13(12): e0209977.
Gül, M. R. & B. D. Griffen, 2018b. A reliable bioindicator of anthropogenic impact on the coast of South Carolina. Southeastern Naturalist 17(2): 357–364.
Gül, M. R. & B. D. Griffen, 2019a. Combined impacts of natural and anthropogenic disturbances on the bioindicator Ocypode quadrata (Fabricius, 1787). Journal of Experimental Marine Biology and Ecology. https://doi.org/10.1016/j.jembe.2019.151185.
Gül, M. R. & B. D. Griffen, 2019b. Burrowing behavior and burrowing energetics of a bioindicator under human disturbance. Ecology and Evolution. https://doi.org/10.1002/ece3.5853.
Gül, M.R. & B.D. Griffen, in review. Diet, energy storage, and reproduction in a bioindicator species across beaches with different levels of human disturbance. Ecological Indicators.
Haley, S. R., 1969. Relative growth and sexual maturity of the Texas ghost crab, Ocypode quadrata (Fabr.). (Brachyura, Ocypodidae). Crustaceana 17: 285–297.
Halpern, B. S., S. Walbridge, K. A. Selkoe, C. V. Kappel, F. Micheli, C. D’Agros, J. Bruno, K. S. Casey, C. Ebert, H. E. Fox, R. Fujita, D. Heinemann, L. S. Lenihan, E. M. P. Madin, M. T. Perry, E. R. Selig, M. Spalding, R. Steneck & R. Watson, 2008. A global map of human impact on marine ecosystems. Science 319: 948–952.
Hartnoll, R. G., 1974. Variation in growth pattern between some secondary sexual characters in crabs (Decapoda Brachyura). Crustaceana 27: 131–136.
Hernáez, P. & M. C. A. João, 2018. Social structure, sexual dimorphism and relative growth in the ghost shrimp Callichirus seilacheri (Bott, 1955) (Decapoda, Axiidea, Callianassidae) from the tropical eastern Pacific. Marine Biology Research 14(8): 856–867.
Hobbs, C. H., C. B. Landry & J. E. Perry, 2008. Assessing anthropogenic and natural impacts on ghost crabs (Ocypode quadrata) at Cape Hatteras National Seashore, North Carolina. Journal of Coastal Research 24(6): 1450–1458.
Hultgren, K. & J. J. Stachowicz, 2008. Alternative camouflage strategies mediate predation risk among closely related co-occurring kelp crabs. Oecologia 155: 519–528.
Hultgren, K. & J. J. Stachowicz, 2011. Camouflage in decorator crabs: integrating ecological, behavioural and evolutionary approaches. In Stevens, M. & S. Merlaita (eds.), Animal camouflage. Cambridge University Press, Cambridge: 214–229.
Jaroensutasinee, F. W. T. M. & K. Jaroensutasinee, 2015. Effects of sexual dimorphism and body size on feeding behaviour of the fiddler crab, Uca bengali Crane, 1975. Crustaceana 88(2): 231–242.
Kiskaddon, E., K. Chernicky & S. Bell, 2019. Resource use by and trophic variability of Armases cinereum (Crustacea, Brachyura) across human-impacted mangrove transition zones. PLoS ONE 14(2): e0212448.
Kuznetsova, A., P.B. Brockhoff, R.H.B. Christensen, 2018. Package “lmerTest” https://cran.r-project.org/web/packages/lmerTest/lmerTest.pdf
Laitano, M. V., N. M. Chiaradia & J. D. Nuñez, 2019. Clam population dynamics as an indicator of beach urbanization impacts. Ecological Indicators 101: 926–932.
Lee, S. Y., 1993. Chela height is an acceptable indicator of chela strength in Carcinus maenas (Linnaeus, 1758) (Decapoda, Brachyura). Crustaceana 65: 115–116.
Lenth, R.V. & M. Hervé, 2018. Package “lsmeans” https://cran.r-project.org/web/packages/lsmeans/lsmeans.pdf
Lucrezi, S. & T. A. Schlacher, 2014. The ecology o ghost crabs-a review. Oceanography and Marine Biology-Annual Review 52: 201–256.
Maklakov, A. A., T. Bilde & Y. Lubin, 2004. Sexual selection for increased male body size and protandry in a spider. Animal Behaviour 68(5): 1041–1048.
Marnocha, E., J. Pollinger & T. B. Smith, 2010. Human-induced morphological shifts in an island lizard. Evolutionary Applications 4(2): 388–396.
McLain, D. K. & A. E. Pratt, 2007. Approach of females to magnified reflections indicates that claw size of waving fiddler crabs correlates with signaling effectiveness. Journal of Experimental Marine Biology and Ecology 343(2): 227–238.
Milne, L. J. & M. J. Milne, 1941. Notes on the behavior of the ghost crab. American Naturalist 80: 362–380.
Neff, B. D. & E. I. Svensson, 2013. Polyandry and alternative mating tactics. Philosophical Transactions of the Royal Society B 368: 20120045.
Neves, F. M. & C. E. Bemvenuti, 2006. The ghost crab Ocypode quadrata (Fabricius, 1787) as a potential indicator of anthropogenic impact along the Rio Grande do Sul coast. Brazil. Ecological Indicators 133(4): 431–435.
Nolen, Z. J., P. E. Allen & C. W. Miller, 2017. Seasonal resource value and male size influence male aggressive interactions in the leaf footed cactus bug, Narnia femorata. Behavioural Processes 138: 1–6.
Packard, G. C. & T. J. Boardman, 1999. The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comparative Biochemistry and Physiology B 122: 37–44.
Putman, B. J., M. Gasca, D. T. Blumstein & G. B. Pauly, 2019. Downsizing for downtown: limb lengths, toe lengths, and scale counts decrease with urbanization in western fence lizards (Sceloporus occidentalis). Urban Ecosystems 22: 1071–1081.
Reaney, L. T., 2009. Female preference for male phenotypic traits in a fiddler crab: do females use absolute or comparative evaluation? Animal Behaviour 77: 139–143.
Rico-Guevara, A. & K. J. Hurme, 2019. Intrasexually selected weapons. Biological Reviews 94: 60–101.
Serrano-Meneses, M. A., A. Córdoba-Aguilar, V. Méndez, J. Layen & T. Székely, 2007. Sexual size dimorphism in the American rubyspot: male body size predicts male competition and mating success. Animal Behaviour 73: 987–997.
Schenk, S. C. & P. C. Wainwright, 2001. Dimorphism and the functional basis of claw strength in six brachyuran crabs. Journal of Zoology 255: 105–119.
Schlacher, T. A., S. Lucrezi, R. M. Connolly, C. H. Peterson, B. L. Gilby, B. Maslo, A. D. Olds, S. J. Walker, J. X. Leon, C. M. Huijbers, M. A. Weston, A. Turra, G. A. Hyndes, R. A. Holt & D. S. Schoeman, 2016. Human threats to sandy beaches: a meta-analysis of ghost crabs illustrates global anthropogenic impacts. Estuarine, Coastal and Shelf Science 169: 56–73.
Schlacher, T. A., L. M. C. Thompson & S. Price, 2007. Vehicles versus conservation of invertebrates on sandy beaches: quantifying direct mortalities inflicted by off-road vehicles (ORVs) on ghost crabs. Marine Ecology 28: 354–367.
Shine, R., 1989. Ecological causes for the evolution of sexual size dimorphism: a review of the evidence. The Quarterly Review of Biology 64: 419–461.
Smith, R. J., 1993. Logarithmic transformation bias in allometry. American Journal of Physical Anthropology 90: 215–228.
Smith, L. D. & A. R. Palmer, 1994. Effects of manipulated diet on size and performance of Brachyuran crab claws. Science 264: 710–712.
Smith, T. B., B. Milá, G. F. Grether, H. Slabbekoorn, I. Serpil, W. Buermann, S. Saatchi & J. P. Pollinger, 2008. Evolutionary consequences of human disturbance in a rainforest bird species from Central Africa. Molecular Ecology 17: 58–71.
Sneddon, L. U., F. A. Huntingford, A. C. Taylor & J. F. Orr, 2000. Weapon strength and competitive success in the fights of shore crabs (Carcinus maenas). Journal of Zoology 250: 397–403.
Spellerberg, I., 2005. Monitoring ecological change, 2nd ed. Cambridge University Press, Cambridge, UK.
Stearns, S. C., 1992. The evolution of life histories. UK, Oxford University Press, Oxford.
Stelling-Wood, T. P., G. F. Clark & A. G. B. Poore, 2016. Responses of ghost crabs to habitat modification of urban sandy beaches. Marine Environmental Research 116: 32–40.
Suciu, M. C., D. C. Tavares & I. R. Zalmon, 2018. Comparative evaluation of crustaceans as bioindicators of human impact on Brazilian sandy beaches. Journal of Crustacean Biology. https://doi.org/10.1093/jcbiol/ruy027.
Tewfik, A., S. S. Bell, K. S. McCann & K. Morrow, 2016. Predator diet and trophic position modified with altered habitat morphology. PLoS ONE 11(1): e0147759.
Tukey, J. W., 1977. Exploratory data analysis. Addison-Wesley, Reading, MA.
Turra, A., M. A. O. Gonçalves & M. R. Denadai, 2005. Spatial distribution of the ghost crab Ocypode quadrata in low-energy tide-dominated sandy beaches. Journal of Natural History 39(23): 2163–2177.
Vitousek, P. M., H. A. Mooney, L. Lubchenco & J. M. Melillo, 1997. Human domination of Earth’s ecosystems. Science 277: 494–499.
Winchell, K. M., R. G. Reynolds, S. R. Prado-Irwin, A. R. Puente-Rolón & L. J. Revell, 2016. Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70(5): 1009–1022.
Wolcott, T. G., 1978. Ecological role of ghost crabs, Ocypode quadrata (Fab.) on an ocean beach: scavengers or predators? Journal of Experimental Marine Biology and Ecology 31: 67–82.
Wolcott, T. G. & D. L. Wolcott, 1984. Impact of off-road vehicles on macroinvertebrates of a mid-Atlantic beach. Biological Conservation 29(3): 217–240.
Yamada, S. B. & E. G. Boulding, 1998. Claw morphology, prey size selection and foraging efficiency in generalist and specialist shell-breaking crabs. Journal of Experimental Marine Biology and Ecology 220: 191–211.
Acknowledgements
We thank the Baruch Institute, Coastal Carolina University, and DeBordieu Colony management for permission to access their facilities. This research was funded by the Ministry of National Education, Republic of Turkey (awarded to MRG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gül, M.R., Griffen, B.D. Changes in claw morphology of a bioindicator species across habitats that differ in human disturbance. Hydrobiologia 847, 3025–3037 (2020). https://doi.org/10.1007/s10750-020-04308-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04308-9