Abstract
In dry areas, natural and artificial ponds experience frequent water level fluctuation, affecting conditions for some aquatic and amphibiotic taxa. Water beetles, bugs, and dragonflies make up much of pond diversity, and are responsive to changes in environmental conditions. Using a drought-prone pondscape within the Greater Cape Floristic Region biodiversity hotspot, we determine (1) the relative extent to which species richness, abundance, and composition are affected by pond water level fluctuation, (2) the effects of environmental variables and vegetation characteristics relative to fluctuating water levels, and (3) make recommendations to improve pondscape conservation. We found that the degree of fluctuation had a significant effect on beetle species richness, but had no significant effect on the other focal taxa. Water temperature, pH, and conductivity, and vegetation cover and composition were drivers of aquatic insect species richness, abundances, and assemblage structures. Habitat heterogeneity supported rich aquatic insect assemblages. We recommend that a range of ponds with various degrees of water level fluctuation should be maintained, along with naturally diverse marginal vegetation. Such a dynamic pondscape can contribute greatly towards maintenance of local and regional aquatic insect diversity in drought-prone regions and should be considered as a main focus in conservation efforts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, anthropogenically-induced climate change increases the frequency and duration of hydrological droughts (Dai, 2013; Trenberth et al., 2014). Most hydrological droughts occur over large spatial scales and induce accelerated environmental variability (Mosley, 2015), placing substantial ecological pressure on small freshwater water bodies (Rosset et al., 2010) and their associated terrestrial and aquatic communities (Bond et al., 2008; Pallarés et al., 2016). In addition to less frequent water replenishment, high ambient temperature during drought leads to increased evaporation rates in lentic (still) water bodies, and water levels drop continually as droughts progress (Furey et al., 2006). This leads to productive littoral zones becoming exposed (Bond et al., 2008) and some aquatic and amphibiotic species becoming increasingly threatened through the elimination of important habitat characteristics, including vegetation cover and water quality components.
‘Ponds’ are defined as small, natural or artificial lentic water bodies of 1 m2 to 5 ha in size, retaining water for > 4 months of the year (Biggs et al., 2017). Ponds support high diversity of aquatic species (Jeffries, 2005; Oertli et al., 2005; Céréghino et al., 2008; Hill et al., 2017a), are often rich in rare species (Davies et al., 2008), and are highly valued for their important role as structurally complex freshwater habitats (Biggs et al., 2017). Artificial ponds are integral features across agricultural landscapes (Raebel et al., 2012a), and in dry areas, many are used for irrigation outside the wet season (Apinda-Legnouo et al., 2014). These artificial habitats can function as local stepping stone habitats within highly fragmented agricultural landscapes (Simaika et al., 2016). Artificial ponds provide supplementary habitats for range-restricted species (Apinda-Legnouo et al., 2014) and increase local abundance and the area of occupancy for a range of aquatic taxa (Samways, 1989; Deacon et al., 2018). Species occupancy is further improved when ponds occur in networks across a landscape, collectively making up a pondscape (Hill et al., 2018).
Aquatic insects are important components of pond diversity and are highly responsive to changes in environmental conditions. Aquatic beetles (Coleoptera) and bugs (Hemiptera) are variably sensitive to water quality and can be used to indicate change in freshwater conditions (Winter et al., 2002). They fulfill many ecological roles as functional guilds and have several unique adaptations to a wide range of ecological conditions (Dickens & Graham, 2002). In turn, dragonfly (Odonata) adults, as well as their larvae, are excellent ecological indicators of water quality and habitat value, are dependent on freshwater and terrestrial habitats for life cycle completion, and are sensitive to changing environments related to water permanency and structural change (Clark & Samways, 1996; Grant & Samways, 2007; Simaika & Samways, 2011).
Multivariate and dynamic aquatic environments greatly determine insect population persistence (Gunderson et al., 2016), and several past investigations have indicated that lentic insects respond significantly to various habitat descriptors over short time scales. There is growing evidence that vegetation cover and composition, and chemical characteristics related to salinity, pH, oxygen content, and water temperature, are interactive and important determinants of insect occupancy, thus determining rich aquatic insect assemblages (Arribas et al., 2012; Apinda-Legnouo et al., 2014; Pallarés et al., 2017; Briggs et al., 2019). Yet, little attention has been given to the effect that seasonal water level fluctuation in artificial ponds has on aquatic insect assemblages, especially in drought-prone regions.
The effects of seasonal drought, and the associated fluctuation in water level, on aquatic insects and pondscape resilience is the main focus here. Our overall aim was to investigate the response of dragonfly larvae and adults, aquatic beetles, and aquatic bugs to dynamic pond conditions across an artificial pondscape. Our key objectives are to (1) determine whether species richness, abundance, and composition of beetles, bugs, and dragonflies are significantly affected by the degree of water level fluctuation, (2) determine the effects of environmental variables and vegetation composition on beetle, bug, and dragonfly species richness, abundance, and composition, and (3) make recommendations so as to promote effective conservation of dynamic pondscapes. We hypothesize that water level fluctuation has an impoverishing effect on insect species richness, composition, and abundance, and that the focal taxa respond equally to changing environmental conditions. We also expect that water chemistry components (i.e., temperature, dissolved oxygen, pH, and conductivity), as well as submerged and marginal vegetation characteristics, play a key interactive role in driving aquatic insect species richness, abundance, and composition in a drought-prone area.
Sites and methods
Study sites
The sampled area was in the Greater Cape Floristic Region (GCFR), South Africa, an area supporting high local endemism and macroinvertebrate diversity (Dallas & Day, 2007) owing to a wide range of environmental conditions and available microhabitats (Darwall et al., 2009). The GCFR is a winter rainfall region and is dry in summer (Manning & Goldblatt, 2012). This Mediterranean-type ecosystem sees greatly fluctuating water levels of freshwater bodies throughout the year, with lowest levels in late summer (January–February).
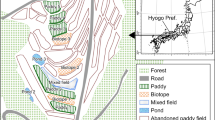
A total of eighteen ponds within the same geographic area (i.e., with the same potential pool of species to ensure no recruitment limitation (McCauley, 2006; Oertli et al., 2008; Raebel, 2012b)) and so constituting a pondscape (Boothby, 1997) were selected in Somerset West, Western Cape, South Africa (Fig. 1): ten ponds on the Erinvale Golf Estate (34.07369° S; 18.88788° E), four ponds on the Lourensford Wine Estate (34.07191° S; 18.88862° E), and four ponds on the Vergelegen Wine Estate (34.07639° S; 18.88989° E) (Fig. 1). Ponds were selected to represent a range of artificial pond types, as well as a gradient of fluctuation from early (high water levels) to late (lowest water levels) summer. The included pond types were permanent irrigation reservoirs and plastic-lined ornamental ponds with no additional substrate added, all of which have dramatically fluctuating water levels, yet did not dry up completely.
Data collection
Data were collected during two sampling seasons: high water level early summer (November–December 2017) and low water level late summer (January–February 2018). Dragonfly and damselfly larvae (Odonata; hereafter collectively referred to as ‘dragonflies’), water beetles (Coleoptera), and water bugs (Hemiptera) were sampled using an aquatic sweep net (300 mm × 300 mm; 1000-micron mesh size) for 2 min, within ten quadrats of 4 m × 1 m selected in the water along the edge of each pond. A minimum distance of 5 m between each quadrat and 2 m away from the water’s edge was maintained throughout sampling. Each netted sample was transferred to a sorting tray, and all focal individuals were hand-picked and placed in 70% ethanol for transport to the laboratory. Morpho-species were counted and identified using field identification guides and museum collections. All individuals are housed in the Stellenbosch University entomology museum. Visual surveys, using close-focus binoculars, were conducted to record adult dragonfly species richness and abundance on sunny, windless days between 08h00 and 16h00 for 45 min/pond. To confirm identification of cryptic species, individuals were caught with an aerial insect net and identified using Samways & Simaika (2016). One individual of each species was retained in the reference collection.
Environmental variables
Ten point-measurements of water parameters were measured for each pond (one point-measurement for each sampling quadrat), using a hand-held multi-parameter water quality meter. These variables included water temperature (°C), pH, conductivity (µS; as a proxy for salinity), and dissolved oxygen (mg/L), as well as sampling depth (m). Landscape environmental variables, including elevation (m a.s.l.) and pond size (m2), were also recorded. Marginal (dominant aquatic species, dominant terrestrial species, % grasses cover, % herbaceous cover, % reeds cover, % total vegetation cover, and vegetation height) and aquatic (% emergent vegetation and % submerged vegetation) vegetation structure and composition were estimated within each sampling quadrat. Degree of water level fluctuation was determined by measuring the exposed edge width in relation to high-water marks (m) during both the early and the late summer season for each pond. The percentage change was then calculated by subtracting the minimum edge width (lowest level of water fluctuation) from the maximum edge width (highest level of water fluctuation), which was then divided by the maximum edge width and multiplied by 100.
Statistical analyses
Species richness and abundance for each insect group (beetles, bugs, dragonfly adults, and larvae) were non-normally distributed (Shapiro-Wilk test), overdispersed (Pearson’s test), and spatially independent (Mantel test). We used generalized linear models (GLMs) with quasi-Poisson distributions to test the effects of environmental variables on species richness and abundance for each insect group. We performed model selection to determine which environmental variables contributed the most to variation in beetle species richness, beetle abundance, bug species richness, bug abundance, adult dragonfly species richness, adult dragonfly abundance, larval dragonfly species richness, and larval dragonfly abundance (response variables), respectively, using the dredge function in the MuMIn package for R (Barton, 2018). Wherever environmental variables were strongly correlated with one another, one was excluded from the final model set. The final model sets were built with eleven environmental variables in total: water temperature, pH, conductivity, sampling depth, pond size, % emergent vegetation, % submerged vegetation, % grasses cover, % herbaceous cover, % reeds cover, and % change in water level. The best model for each response variable was considered as the model with the lowest quasi-likelihood second-order Akaike’s information criterion value (QAICc) and delta quasi-likelihood second-order Akaike’s information criterion value (ΔQAICc).
Generalized linear models and generalized additive models (GAM) were constructed with the variables identified by model selection to test the fixed effects of important environmental variables on beetle species richness, beetle abundance, bug species richness, bug abundance, adult dragonfly species richness, adult dragonfly abundance, larval dragonfly species richness, and larval dragonfly abundance, respectively (eight models in total). All GLMs and GAMs were fitted by a Laplace approximation and quasi-Poisson distribution and were constructed in R (R Core Team, 2013) using the lme4 and mgcv packages (Bates & Sarkar, 2007; Wood, 2011).
Finally, to test for the effects of environmental variables on variation in species composition, GLMs with negative binomial distributions and multivariate extensions were constructed in R using the mvabund package (Wang et al., 2014). The manyglm function was used to fit separate GLMs for beetles, bugs, adult dragonflies, and larval dragonflies, including the most important environmental variables identified by interactive forward selection (i.e., each environmental variable was individually added to the analyses to determine their importance in each model). For each investigated group, analyses were permutated 999 times to allow for direct comparison of important variables. The datasets generated during the workflow and analyzed during the workflow are available in the Figshare repository (https://doi.org/10.6084/m9.figshare.8074832.v1).
Results
Collective species richness and abundance
A total of 11,715 focal aquatic insect individuals were collected, comprising fourteen dragonfly species (591 adult individuals, 1270 larval individuals), nine beetle morpho-species (1061 individuals), and seventeen bug morpho-species (8793 individuals). The full species list is given in Online Resource 1.
Model selection for species richness, abundance, and composition
Model selection identified six important environmental variables for aquatic insect species richness (% emergent vegetation, % submerged vegetation, % grasses cover, % reeds cover, % herbaceous cover, and conductivity) (Table 1). The remaining five variables (water temperature, pH, depth, pond size, and % change in water level) were not important in determining aquatic insect species richness. Nine important environmental variables were selected for aquatic insect abundance. These were % emergent vegetation, % grasses cover, % reeds cover, water temperature, conductivity, pH, depth, pond size and % change in water level (Table 1). Of the eleven investigated environmental variables, six were selected as important in determining species composition of the focal taxa. These were % emergent vegetation, % submerged vegetation, % grasses cover, water temperature, conductivity, and pH.
Effect of environmental variables on species richness, abundance, and composition
For beetles, both species richness and abundance were positively correlated with % emergent vegetation cover (Table 2; Online Resource 2). A decrease in % submerged vegetation cover and an increase in % grasses cover resulted in an increase in the beetle species richness. A decrease in % grasses cover and % change in water level saw an increase in beetle abundance. Beetle abundance also decreased with increasing sampling depth and was highest at about 15 cm. A further increase in water temperature resulted in an increase in beetle abundance, but was highest around 26°C. An increase in bug species richness was associated with a decrease in % herbaceous cover (Table 2; Online Resource 2). Bug abundance increased with an increase in pH and conductivity. Bug abundance also decreased with increasing % grasses cover and was lowest between 20 and 40% grasses cover.
Adult dragonfly species richness increased with an increase in conductivity, % submerged vegetation cover and % emergent vegetation cover, and was highest at 20% emergent vegetation cover (Table 2; Online Resource 2). None of the measured environmental variables had a significant effect on adult dragonfly abundance. Larval dragonfly species richness showed a marginal increase with increasing % reeds cover, yet larval dragonfly abundance decreased with an increase in % reeds cover and water temperature, and increased with an increase in conductivity (Table 2; Online Resource 2).
A significant shift in beetle species composition was associated with a change in % grasses cover and bug species composition shifted significantly with changes in conductivity levels (Table 3). None of the environmental variables investigated here had an influence in adult dragonfly species composition, yet larval dragonfly composition shifted significantly with changes in pH and conductivity levels.
Discussion
Aquatic habitat heterogeneity within ponds, defined by complex vegetation structure, multiple combinations of environmental variables, and variation in physical characteristics, is essential for maintaining diverse aquatic insect assemblages (Pan et al., 2015; Biggs et al., 2017; Hill et al., 2017b). Here, we found that fluctuating water levels had little effect on aquatic insect species richness, abundance, and composition and was only significant in the case of aquatic beetle abundance. Other environmental variables were also significant and are important to consider for understanding local processes and associated changes in insect species richness, abundance, and composition. Although there were some similarities between the taxa investigated here, there were also several key differences between insect responses to environmental variability, suggesting that different freshwater taxa are variably sensitive to the environmental and physical characteristics which collectively make up pond heterogeneity.
Effect of fluctuating water levels
The most surprising result was how remarkably tolerant the focal taxa were to various pond types and their fluctuating water levels, and mostly occupied fluctuating ponds in high abundance. Several aquatic insect groups colonize fluctuating ponds which have low predator pressure and are occupied by many prey species (e.g., larval Diptera). Their tolerance is presumably driven by a long history of adaptation to naturally extreme seasonal weather conditions and fluctuating water levels (Bird et al., 2019). There can be high functional redundancy among aquatic macroinvertebrates of drought-prone aquatic habitats making the assemblage overall resistant to severe drying events (Boersma et al., 2014), sometimes despite differences in environmental conditions among ponds during times of drought (Olds et al., 2011).
Dragonflies and bugs in drought-prone areas are known for their rapid development, a physiological adaptation enabling these insect taxa to outlive seasonal aquatic habitats and remain at natal ponds as the subsequent winter rains are inevitable (Suhling et al., 2015; Bird et al., 2019). Flying over the sun-scorched rocky terrain to seek new ponds would appear to be a high-risk strategy, yet some dry climate species are opportunistic colonizers and have good flight ability. This enables dispersing individuals to reach favorable habitats when conditions in their natal habitats deteriorate (Suhling et al., 2005; Lytle, 2015). Only beetle abundance was significantly lower in ponds with high degrees of water level fluctuation, indicating that beetles are more sensitive to water level fluctuation compared to the other focal taxa investigated here. The least fluctuating ponds likely offer more constant conditions for aquatic beetles through the continual presence of water and breeding microhabitats, both of which are important features for maintaining a diverse insect assemblage (e.g., Bilton et al., 2008).
Effects of other environmental variables
The focal taxa studied here were variably responsive to water temperature gradients. Higher water temperature (and the associated drop in dissolved oxygen content with increasing temperature) had no limiting effect on water beetles, as they are atmospheric breathers, and are morphologically equipped to occupy ponds characterized by high water temperature and low dissolved oxygen content. This is consistent with previous findings for beetles in the same region (Deacon et al., 2019), another region of South Africa (Deacon et al., 2018), and in Europe (Arribas et al., 2012). These findings are further supported by beetle species richness being highest along the shallow and productive edges of ponds, where there was mostly abundant marginal vegetation cover and higher water temperature (Samways et al., 1996). This tolerance to elevated water temperature may also be an adaptation for surviving fluctuating water levels, as water temperature increases as water levels drop. Though the effects of water temperature on aquatic bugs and adult dragonflies were unclear, dragonfly larvae showed a strong preference for cool water. Dragonfly larvae are gill breathers, and cooler water associated with relatively high dissolved oxygen content provides them with optimal ecological conditions to ensure survival.
We found that bugs and dragonfly larvae were most abundant in ponds characterized by high conductivity. Yet, generalist bug genera, e.g., Anisops, Enithares, and Sigara, and some generalist dragonfly genera, e.g., Crocothemis and Ischnura, were associated with high conductivity, while most others occupied ponds with relatively low conductivity. Generalist species may be better adapted to saline conditions and dominate ponds with higher salinity (Hart et al., 1991; James et al., 2003; Polhemus, 2008), since increasing salinity places sensitive species at risk of desiccation as drought conditions progress (Friday, 1987; Pallarés et al., 2016). Our results are consistent with previous findings for aquatic insects occupying natural and artificial ponds in the GCFR (Apinda-Legnouo et al., 2014; Deacon et al., 2019), suggesting that aquatic insect assemblages, at least in the case of bugs and dragonflies, differ significantly among ponds characterized by varying conductivity levels. However, the aquatic insects investigated here are highly mobile as adults, and may move between ponds with varying conductivity levels to feed and breed (Hart et al., 1991), leading to high species richness occasionally associated with ponds characterized by high conductivity.
We found that bugs were most abundant in ponds with alkaline pH conditions, likely also resulting from generalist species having a competitive advantage over specialist species in ponds which deviate from neutral pH conditions. Larval dragonfly species composition shifted along the pH gradient investigated here, and Crocothemis species were mostly found in alkaline pH conditions, while other genera, e.g., Ceratogomphus and Trithemis, mostly occupied ponds with close to neutral pH conditions. Yet other genera, e.g., Africallagma, Ischnura and Anax, occurred across the whole pH gradient investigated here. This suggests that pH gradients drive differences between aquatic insect assemblages (da Rocha et al., 2016), and larvae from different dragonfly genera are variably sensitive to pH gradients. Some have a range of physiological adaptations allowing them to tolerate various ecological conditions until they are ready to emerge as adults, at least in this drought-prone area. Differences in pH levels among ponds had no strong effects on beetles, indicating that they are well adapted to the range of pH conditions they may encounter in various ponds (Griffiths et al., 2015).
We found no significant effects of pond size on any of the investigated taxa. This indicates that ponds of variable sizes contribute equally to regional diversity, and that ponds are important for maintaining rich biodiversity at the landscape scale, as has been found elsewhere (Mosscrop et al., 2015; Biggs et al., 2017). This is related to ponds providing a wide range of near-natural habitat conditions, so promoting occupancy by widespread and locally endemic species (Williams et al., 2010). The ponds investigated here are also interspersed and connected at a local landscape scale and likely serve as stepping stone habitats for highly mobile and opportunistic insect taxa (Davy-Bowker, 2002; Bird et al., 2019).
Effects of vegetation cover
The focal taxa showed significant responses to vegetation characteristics, as previously shown for the region (Apinda-Legnouo et al., 2014; Deacon et al., 2019). Beetles, adult dragonflies, and larval dragonflies showed increasing species richness with increasing emergent marginal vegetation cover. This is not surprising as most aquatic beetles only periodically dive into the water column, and require marginal vegetation as substrate to rest on and to crawl out of the water (Verberk et al., 2001). Similar to beetles, dragonfly larvae require marginal substrate to hunt, seek refuge from predators, and crawl out of the water to emerge as adults. Most adult dragonflies hold their territories close to the water and require marginal vegetation as perching substrate (Samways & Simaika, 2016). Bugs were the only taxon here to show lower species richness with increasing herbaceous marginal cover, as most species sampled here were not reliant on marginal vegetation, and dwell on or just below the water surface to feed on smaller insects close to the surface in open water (Fairchild et al., 2003; Griffiths et al., 2015).
Bug and larval dragonfly abundances were also negatively correlated with increasing marginal vegetation cover, suggesting that vegetated margins reduce the availability of suitable microhabitats where bugs locate prey, since most hunt in open water (Fairchild et al., 2003; Griffiths et al., 2015). Although dragonfly larvae prefer vegetated margins of ponds, inter- and intraspecific competition for finding prey and/or suitable perching substrates along the vegetated margins of ponds is likely high, leading to the lower abundance we observed here. In the case of beetles, most species and individuals occurred along pond margins where vegetation penetrates the water surface, while lower beetle abundance was associated with increasing below-surface vegetation. However, most of the beetle species sampled here were diving beetles (Dytiscidae), which rarely make use of the entirely submerged vegetation. Instead, they mostly seek refuge among emergent vegetation along the shallow margins of ponds (Verberk et al., 2001; Stals, 2003), enabling them to exit the water when required (Yee & Kehl, 2015). Beetles were the only taxon to show a shift in species composition according to changes in % grasses cover where there was abundant grasses along the margins. The most common beetles were from the genera Cybister and Berosus, while other genera, e.g., Gyrinus and Rhantus, were associated with open water. This indicates that beetles have a strong preference for specific locations in ponds and that they are well adapted to the microhabitats they occupy.
Implications for conservation
The findings presented here contribute to our increasing knowledge of the drivers of biodiversity at the landscape scale. The focal aquatic insects were variably sensitive to environmental conditions, indicating strong adaptation to highly dynamic systems. Ponds are significant components of freshwater ecosystems and have high conservation value in general (Chester & Robson, 2013), as they make a substantial contribution to the local and regional diversity (Osborn & Samways, 1996; Biggs et al., 2017) and increase ecological resilience by providing a wide range of suitable habitats. In general, the insects investigated here appear to be well adapted to water level fluctuation in the drought-prone GCFR, yet responses to water level fluctuation, chemical properties, and vegetation characteristics are variable among insect taxa. It is therefore essential that ponds characterized by high habitat heterogeneity are maintained (Harabiš & Dolný, 2012) to account for the regional species pool. Effective management of ponds, in terms of their physical and chemical properties, should form part of guidelines to maximize conservation of aquatic insects and higher taxa in local aquatic ecosystems, which supports findings from the various climates of Austria (Schindler et al., 2003), Italy (Della Bella et al., 2005), Poland (Biggs et al., 2004), the United Kingdom (Bilton et al., 2008), the Cerrado region of Brazil (De Marco et al., 2014) as well as the east coast of the USA (Fairchild et al., 2003). Therefore, conserving a range of ponds, including ponds with constant water levels and fluctuating ponds, ponds with diverse aquatic and marginal vegetation, and those with a range of environmental gradients, is the optimal goal. This conservation strategy should be included in conservation efforts in South Africa, as has been done under the Habitats Directive in the European Union (Céréghino et al., 2008).
References
Apinda-Legnouo, E. A., M. J. Samways & J. P. Simaika, 2014. Value of artificial ponds for aquatic beetle and bug conservation in the Cape Floristic Region biodiversity hotspot. Aquatic Conservation: Marine and Freshwater Ecosystems 24: 522–535.
Arribas, P., J. Velasco, P. Abellán, D. Sánchez-Fernández, C. Andújar, P. Calosi, A. Millán, I. Ribera & D. T. Bilton, 2012. Dispersal ability rather than ecological tolerance drives differences in range size between lentic and lotic water beetles (Coleoptera: Hydrophilidae). Journal of Biogeography 39: 984–994.
Bates, D. M. & D. Sarkar, 2007. lme4: Linear mixed-effects models using S4 classes. R package version 1.1-12.
Barton, K., 2019. MuMIn: Multi-model inference. R package version 1.42.1. Retrieved from https://CRAN.R-project.org/package=MuMIn.
Biggs, J., D. T. Bilton, P. Williams, P. Nicolet, L. Briggs, B. Eeles & M. Whitfield, 2004. Temporary ponds of eastern Poland: an initial assessment of their importance for nature conservation. Archive of Science 57: 73–83.
Biggs, J., S. von Fumetti & M. Kelly-Quinn, 2017. The importance of small waterbodies for biodiversity and ecosystem services: implications for policy makers. Hydrobiologia 793: 3–39.
Bilton, D. T., L. C. McAbendroth, P. Nicolet, A. Bedford, S. D. Rundle, A. Foggo & P. M. Ramsay, 2008. Ecology and conservation status of temporary and fluctuating ponds in two areas of southern England. Aquatic Conservation: Marine and Freshwater Ecosystems 19: 134–146.
Bird, M. S., M. C. Mlambo, R. J. Wasserman, T. Dalu, A. J. Holland, J. A. Day, M. H. Villet, D. T. Bilton, H. M. Barber-James & L. Brendonck, 2019. Deeper knowledge of shallow waters: reviewing the invertebrate fauna of southern African temporary wetlands. Hydrobiologia 827: 89–121.
Boersma, K. S., M. T. Bogan, B. A. Henrichs & D. A. Lytle, 2014. Invertebrate assemblages of pools in arid-land stream have high functional redundancy and are resistant to severe drying. Freshwater Biology 59: 491–501.
Bond, N. R., P. S. Lake & A. H. Arthington, 2008. The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600: 3–16.
Boothby, J., 1997. Pond conservation: towards a delineation of pondscape. Aquatic Conservation: Marine and Freshwater Ecosystems 7: 127–132.
Briggs, A., J. S. Pryke, M. J. Samways & D. Conlong, 2019. Macrophytes promote aquatic insect conservation in artificial ponds. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1190–1201.
Céréghino, R., A. Ruggiero, P. Marty & S. Angélibert, 2008. Biodiversity and distribution patterns of freshwater invertebrates in farm ponds of a south-western French agricultural landscape. Hydrobiologia 597: 43–51.
Chester, E. T. & B. J. Robson, 2013. Anthropogenic refuges for freshwater biodiversity: their ecological characteristics and management. Biological Conservation 168: 64–75.
Clark, T. E. & M. J. Samways, 1996. Dragonflies (Odonata) as indicators of biotope quality in the Kruger National Park, South Africa. Journal of Applied Ecology 33: 1001–1012.
da Rocha, F. C., E. M. de Andrade, F. B. Lopes, F. J. de Paula Filho, J. H. Costa Filho & M. D. da Silva, 2016. Physical-chemical determinant properties of biological communities in continental semi-arid waters. Environmental Monitoring and Assessment 188: 1–15.
Dai, A., 2013. Increasing drought under global warming in observations and models. Nature Climate Change 3: 52.
Dallas, H. F. & J. A. Day, 2007. Natural variation in macroinvertebrate assemblages and the development of a biological banding system for interpreting bioassessment data - a preliminary evaluation using data from upland sites in the south-western Cape, South Africa. Hydrobiologia 575: 231–244.
Darwall, W., K. Smith, D. Allen, M. Seddon, G. M. Reid, V. Clausnitzer & V. J. Kalkman, 2009. Freshwater biodiversity: a hidden resource under threat. Wildlife in a changing world - An Analysis of the 2008 IUCN Red List of Threatened Species, 43.
Davies, B., J. Biggs, P. Williams, M. Whitfield, P. Nicolet, D. Sear, S. Bray & S. Maund, 2008. Comparative diversity of aquatic habitats in the European agricultural landscape. Agriculture, Ecosystems and Environment 125: 1–8.
Davy-Bowker, J., 2002. A mark and recapture study of water beetles (Coleoptera: Dytiscidae) in a group of semi-permanent and temporary ponds. Aquatic Ecology 36: 435–446.
De Marco, P., D. S. Nogueira, C. C. Correa, T. B. Vieira, K. D. Silva, N. S. Pinto, D. Bichsel, A. S. V. Hirota, R. R. S. Vieira, F. M. Carneiro, A. A. Bispo de Oliveira, P. Carvalho, R. P. Bastos, C. Ilg & B. Oertli, 2014. Patterns in the organization of Cerrado pond diversity in Brazilian pasture landscapes. Hydrobiologia 723: 87–101.
Deacon, C., M. J. Samways & J. S. Pryke, 2018. Artificial reservoirs complement natural ponds to improve pondscape resilience in conservation corridors in a biodiversity hotspot. PloS One 13: e0204148.
Deacon, C., M. J. Samways & J. S. Pryke, 2019. Aquatic insects decline in abundance and occupy low-quality habitats to survive hydrological droughts. Freshwater Biology 64: 1643–1654.
Della Bella, V., M. Bazzanti & F. Chiarotti, 2005. Macroinvertebrate diversity and conservation status of Mediterranean ponds in Italy: water permanence and mesohabitat influence. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 583–600.
Dickens, C. W. & P. M. Graham, 2002. The South African Scoring System (SASS) version 5 rapid bioassessment method for rivers. African Journal of Aquatic Science 27: 1–10.
Fairchild, G. W., J. Cruz, A. M. Faulds, A. E. Z. Short & J. F. Matta, 2003. Microhabitat and landscape influences on aquatic beetle assemblages in a cluster of temporary and permanent ponds. Journal of North American Benthological Society 22: 224–240.
Friday, L. E., 1987. The diversity of macro invertebrate and macrophyte communities in ponds. Freshwater Biology 18: 87–104.
Furey, P. C., R. N. Nordin & A. Mazumder, 2006. Littoral benthic macroinvertebrates under contrasting drawdown in a reservoir and a natural lake. Journal of North American Benthological Society 25: 19–31.
Grant, P. B. C. & M. J. Samways, 2007. Montane refugia for endemic and Red Listed dragonflies in the Cape Floristic Region biodiversity hotspot. Biodiversity and Conservation 16: 787–806.
Griffiths, C., J. Day & M. Picker, 2015. Freshwater Life: A field guide to the plants and animals of southern Africa. Penguin Random House South Africa.
Gunderson, A. R., E. J. Armstrong & J. H. Stillman, 2016. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annual Review of Marine Science 8: 357–378.
Harabiš, F. & A. Dolný, 2012. Human altered ecosystems: suitable habitats as well as ecological traps for dragonflies (Odonata): the matter of scale. Journal of Insect Conservation 16: 121–130.
Hart, B. T., P. Bailey, R. Edwards, K. Hortle, K. James, A. McMahon, C. Meredith & K. Swadling, 1991. A review of the salt sensitivity of Australian freshwater biota. Hydrobiologia 210: 105–144.
Hill, M. J., J. Biggs, I. Thornhill, B. A. Briers, D. G. Gledhill, J. C. White, P. J. Wood & C. Hassall, 2017a. Urban ponds as an aquatic biodiversity resource in modified landscapes. Global Change Biology 23: 986–999.
Hill, M. J., R. G. Death, K. L. Mathers, D. B. Ryves, J. C. White & P. J. Wood, 2017b. Macroinvertebrate community composition and diversity in ephemeral and perennial ponds on unregulated floodplain meadows in the UK. Hydrobiologia 793: 95–108.
Hill, M. J., C. Hassall, B. Oertli, L. Fahrig, B. J. Robson, J. Biggs, M. J. Samways, N. Usio, N. Takamura, J. Krishnaswamy & P. J. Wood, 2018. New policy directions for global pond conservation. Conservation Letters 11: e12447.
James, K. R., B. Cant & T. Ryan, 2003. Responses of freshwater biota to rising salinity levels and implications for saline water management: a review. Australian Journal of Botany 51: 703–713.
Jeffries, M., 2005. Small ponds and big landscapes: the challenge of invertebrate spatial and temporal dynamics for European pond conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 541–547.
Lytle, D. A., 2015. Chapter 37: Order Hemiptera. In: Thorp, J. H & D. C. Rogers, editors. Thorp and Covich’s freshwater invertebrates: Ecology and general biology. Amsterdam: Elsevier, pp. 1953-1979.
Manning, J. & P. Goldblatt, 2012. Plants of the Greater Cape Floristic Region 1: the Core. Cape flora, Strelitzia 29. South African National Biodiversity Institute, Pretoria South Africa.
McCauley, S. J., 2006. The effects of dispersal and recruitment limitation on community structure of odonates in artificial ponds. Ecography 29: 585–595.
Mosley, L. M., 2015. Drought impacts on the water quality of freshwater systems: review and integration. Earth-Science Reviews 140: 203–214.
Mosscrop, L. E., A. M. Paterson, A. M. DeSellas, J. Kurek, R. Weeber & J. P. Smol, 2015. Long-term stability of cladoceran assemblages in small, shallow, Canadian Shield lakes experiencing marked calcium declines. Aquatic Sciences 77: 547–561.
Oertli, B., J. Biggs, R. Céréghino, P. Grillas, P. Joly & J. B. Lachavanne, 2005. Conservation and monitoring of pond biodiversity: introduction. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 535–540.
Oertli, B., N. Indermuehle, S. Angélibert, H. Hinden & A. Stoll, 2008. Macroinvertebrate assemblages in 25 high alpine ponds of the Swiss National Park (Cirque of Macun) and relation to environmental variables. Hydrobiologia 597: 29–41.
Olds, B. P., B. C. Peterson, K. D. Koupal, K. M. Farnsworth-Hoback, C. W. Schoenebeck & W. W. Hoback, 2011. Water quality parameters of a Nebraska reservoir differ between drought and normal conditions. Lake and Reservoir Management 27: 229–234.
Osborn, R. & M. J. Samways, 1996. Determinants of adult dragonfly assemblage patterns at new ponds in South Africa. Odonatologica 25: 49–58.
Pallarés, S., J. Velasco, A. Millán, D. T. Bilton & P. Arribas, 2016. Aquatic insects dealing with dehydration: do desiccation resistance traits differ in species with contrasting habitat preferences? PeerJ 4: e2382.
Pallarés, S., M. Botella-Cruz, P. Arribas, A. Millán & J. Velasco, 2017. Aquatic insects in a multistress environment: cross-tolerance to salinity and desiccation. Journal of Experimental Biology 220: 1277–1286.
Pan, B.-Z., H.-Z. Wang, M. T. Pusch & H.-J. Wang, 2015. Macroinvertebrate responses to regime shifts caused by eutrophication in subtropical shallow lakes. Freshwater Science 34: 942–952.
Polhemus, J. T., 2008. Aquatic and semiaquatic Hemiptera. In: Merrit, R.W., K.W. Cummins and M.B., editors. An introduction to the aquatic insects of North America. Dubuque: Kendall/Hunt Publishing Copp, pp. 385-423.
R Development Core Team., 2013. R Development Core Team. Austria, Vienna.
Raebel, E. M., T. Merckx, R. E. Feber, P. Riordan, D. W. Macdonald & D. J. Thompson, 2012a. Identifying high-quality pond habitats for Odonata in lowland England: implications for agri-environment schemes. Insect Conservation and Biodiversity 5: 422–432.
Raebel, E. M., T. Merckx, R. E. Feber, P. Riordan, D. W. Macdonald & D. J. Thompson, 2012b. Multi-scale effects of farmland management on dragonfly and damselfly assemblages of farmland ponds. Agriculture, Ecosystems and Environment 161: 80–87.
Rosset, V., A. Lehmann & B. Oertli, 2010. Warmer and richer? Predicting the impact of climate warming on species richness in small temperate water bodies. Global Change Biology 16: 2376–2387.
Samways, M. J., 1989. Farm dams as nature reserves for dragonflies (Odonata) at various altitudes in the Natal Drakensberg Mountains, South Africa. Biological Conservation 48: 181–187.
Samways M. J. & J. P. Simaika, 2016. Manual of freshwater assessment for South Africa: Dragonfly biotic index. Suricata 2. Pretoria: South African National Biodiversity Institute.
Samways, M. J., R. Osborn & I. Van Heerden, 1996. Distribution of benthic invertebrates at different depths in a shallow reservoir in the KwaZulu-Natal Midlands. Koedoe 39: 69–76.
Schindler, M., C. Fesl & A. Chovanec, 2003. Dragonfly associations (Insecta: Odonata) in relation to habitat variables: a multivariate approach. Hydrobiologia 497: 169–180.
Simaika, J. P. & M. J. Samways, 2011. Comparative assessment of indices of freshwater habitat conditions using different invertebrate taxon sets. Ecological Indicators 11: 370–378.
Simaika, J. P., M. J. Samways & P. P. Frenzel, 2016. Artificial ponds increase local dragonfly diversity in a global biodiversity hotspot. Biodiversity and Conservation 25: 1921–1935.
Stals, R. & I. J. de Moor, 2007. Coleoptera. Guides to the freshwater invertebrates of Southern Africa. Water Research Commission, Pretoria: 1–263.
Suhling, F., G. Sahlén, J. Kasperski & D. Gaedecke, 2005. Behavioral and life history traits in temporary and perennial waters: comparisons among three pairs of sibling dragonfly species. Oikos 108: 609–617.
Suhling, F., I. Suhling & O. Richter, 2015. Temperature response of growth of larval dragonflies – An overview. International Journal of Odonatology 18: 15–30.
Trenberth, K. E., A. Dai, G. Van Der Schrier, P. D. Jones, J. Barichivich, K. R. Briffa & J. Sheffield, 2014. Global warming and changes in drought. Nature Climate Change 4: 17.
Verberk, W. C. E. P., G.-J. A. van Duinen, T. M. J. Peeters & H. Esselink, 2001. Importance of variation in water-types for water beetle fauna (Coleoptera) in Korenburgerveen, a bog remnant in the Netherlands. Proceedings of the Section Experimental and Applied Entomology 12: 121–128.
Wang, Y. I., U. Naumann, S. T. Wright & D. I. Warton, 2012. mvabund – an R package for model-based analysis of multivariate abundance data. Methods in Ecology and Evolution 3: 471–474.
Williams, P., J. Biggs, A. Crowe, J. Murphy, P. Nicolet, A. Meatherby & M. Dunbar, 2010. Countryside survey report from 2007, Technical report No 7/07. Lancaster, UK, Pond Conservation and NERC/Centre for Ecology and Hydrology.
Winter, J. G., K. M. Somers, P. J. Dillon, C. Paterson & R. A. Reid, 2002. Impacts of golf courses on macroinvertebrate community structure in Precambrian Shield streams. Journal of Environmental Quality 31: 2015–2025.
Wood, S. N., 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society B 73: 3–36.
Yee, D. A., & S. Kehl, 2015. Chapter 39: Order Coleoptera. In: Thorp, J. H & D. C. Rogers, editors. Thorp and Covich’s freshwater invertebrates: Ecology and general biology. Amsterdam: Elsevier, pp. 2056–2154.
Acknowledgements
Special thanks to G. Leckie at Erinvale Golf Estate, S. Reece and J. West at Lourensford Wine Estate, and J. van Rensburg at Vergelegen Wine Estate for granting access to the study sites.
Funding
This study was funded by Mondi Group. The funder was not involved in the design of the study, data analysis, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jooste, M.L., Samways, M.J. & Deacon, C. Fluctuating pond water levels and aquatic insect persistence in a drought-prone Mediterranean-type climate. Hydrobiologia 847, 1315–1326 (2020). https://doi.org/10.1007/s10750-020-04186-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04186-1