Abstract
Pyropia is a genus of algae used in sushi. Nitrate (NO3−) is essential for its growth. Excess NO3− is flushed into seawater, as a result of anthropogenic activities, increasing the potential for higher than normal concentrations which could be harmful to both algae and humans. This study aims to evaluate the effect of NO3− on the morphology, ultrastructure, spore viability, autofluorescence of chloroplasts, and formation of cell wall, during germination of the red seaweed Pyropia acanthophora var. brasiliensis. To accomplish this, carpospores were cultivated at 24°C, 40 μmol photons m−2 s−1 with photoperiod of 12 h and exposed to 0, 25, 50, and 100 mM of NO3− for 2 days. Samples were cultured for another 5 days to measure the length of the germ tube. Concentrations of NO3− did not significantly influence the development, morphology, viability, or ultrastructure of the spores in the first 2 days. The absence of this nutrient did delay the ultrastructural development of the carpospore, decreasing autofluorescence of the chloroplast and delaying the formation of the cell wall and pyrenoid. Over the seven-day experimental period, higher concentrations of NO3− increased the size of the germ tube, indicating that this extra concentration boosted the development of P. acanthophora.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impact of human activity on the environment has been increasing over time. Release of aquatic pollutants has had the most striking effects because these pollutants destroy the quality of seawater. This, in turn, leads to an increase in the amount of water-soluble nitrate (NO3−). NO3− is an ion that occurs naturally in the environment as a nutrient from aerobic decomposition (Vesilind & Morgan, 2013), but its origin may be related to anthropogenic activity, such as the application of organic and inorganic fertilizers and the use of in situ sanitation systems (Baird & Cann, 2011). Beaches in Florianópolis have been suffering from the dumping of untreated sewage, and the number of affected beaches increases each year (Gauchazh, 2019). NO3− concentrations in seawater are not cited by inspection agencies, but the dumping of untreated sewage from thousands of homes is a major concern for biodiversity.
Concentrations of NO3− can produce mycosporine amino acids (MAAs) (Zheng & Gao, 2009; Barufi et al., 2011) and photosynthetic pigments (Barufi et al., 2011), as well as increase the growth rate (Jeanfils et al., 1993; Li et al., 2008) and viability (Bao Do & Cormier, 1991) of algae and terrestrial plants. In addition, favoring the production of these molecules by the presence of nitrogen (N) may mitigate the effects of some stressors, such as ultraviolet radiation (Barufi et al., 2011). Algae can take up NO3− so that even in the absence of free NO3−, algae can pick it up through enzyme nitrate reductase for immediate or reserve use. If the NO3− stock is full, the enzyme stops picking up this nutrient from the external environment, avoiding the expenditure of cellular energy (Martins, 2007). High concentrations of this nutrient may cause saturation in the production of photoprotection molecules. Accordingly, algae can also limit the entry of NO3− in the cell interior, thereby preventing deleterious effects (Barufi et al., 2011). On the other hand, high concentrations of NO3− are very harmful to humans. More specifically, the high intake of this molecule can cause methemoglobinemia, especially in children, blocking the oxygen transport capacity of hemoglobin, in addition to favoring the formation of cancer in the digestive tract, respiratory and cardiac problems, and poor infant development (Townsend et al., 2003; Camargo & Alonso, 2006). Therefore, the absorption of this nutrient by algae can minimize human problems generated by high concentrations of NO3−.
Pyropia, also known as Nori, includes algae used in the production of sushi, a food consumed worldwide (Zhang et al., 2003; Zhou et al., 2012). In addition, it is considered the most domesticated genus in the world (Schweikert et al., 2011). Algae of this genus have a biphasic life cycle, formed by alternating generations between macroscopic gametophytes (n), used in sushi production, and microscopic sporophytes (2n) (Blouin et al., 2011). Each generation releases a different spore type such that gametophytes release carpospores which will form phase 2n, whereas sporophytes will release conchospores which will form phase n (Blouin et al., 2011). Then the formation of carpospores will give rise to the conchocelis phase, which is vital to the continuation of the life cycle. Depending on the conditions, usually stress, the two phases can also release monospores, i.e., spores that regenerate their own phase (Blouin et al., 2011).
Pyropia acanthophora var. brasiliensis E.C. Oliveira & Coll is a red seaweed consumed around the world, thus having considerable commercial interest. In Brazil, the genus Pyropia has been reported since the nineteenth century (Milsten et al., 2015). There are three species of seaweed produced for human consumption: the Japonese kelp (Saccharina japonica (Areschoug) C.E.Lane, C.Mayes, Druehl & G.W.Saunders), wakame (Undaria pinnatifida (Harvey) Suringar), and Nori (Pyropia spp.), and of these only the Pyropia is present in Brazil (Simioni et al., 2019). No record of cultivating this seaweed is evident, but with its expansive Brazil coastline and the ease of adaptation to the environment that this algae has, it is easy to grow. However, even with its economic appeal, little information is currently available about the development of its spores or how NO3− can influence this decisive moment for propagation and production of the species. Therefore, we herein report the initial development of P. acanthophora carpospores with the aim of determining if the abiotic factor NO3− can cause changes in morphology, spore viability, and chloroplast autofluorescence.
Materials and methods
Collection site
Sambaqui Beach is north of Florianópolis, Santa Catarina, Brazil, at coordinates 27° 29′ S and 48° 32′ W. The beach comprises 14.17 hectares and a water volume of 425,100 m3, presenting an intermediate circulation, with predominant northerly winds, sand-muddy sediment, and a depth of 2.5 to 3.5 m (Nunes, 2010). With a mean of 33.8 ± 0.8 psu, salinity varies only slightly (Santos, 2009). The temperature of the seawater has a minimum average of 16.8 ± 0.8°C and maximum of 25.4 ± 1.1°C (Santos, 2009). On average, the amount of nitrate is 3.73 ± 0.01 mM (Pereira et al., 2019).
Collection and processing of biological material
The gametophytes of P. acanthophora var. brasiliensis were collected on the rocky shoreline of Sambaqui Beach, Florianópolis-SC, Brazil, from July to September 2017. Thalli were transported in plastic containers with seawater to the Laboratory of Plant Cell Biology (LABCEV), which is located in the Biological Sciences Center (CCB) of the Federal University of Santa Catarina (UFSC-Florianópolis). In the laboratory, thalli were washed with filtered seawater and screened for removal of the contaminants.
Soon after collection, fertile portions of P. acanthophora approximately 1.0 ± 0.05 cm were selected by light microscopy. These portions were placed in Petri dishes containing glass slides with filtered seawater droplets to which was added modified von Stosch medium 100% without NO3− (composed of MnCl2, EDTA, NaHPO4, FeSO4, biotin, cyanocobalamin, and thiamine) (Edwards, 1972) and left overnight in the dark at a temperature of 24 ± 1°C.
Conditions of cultivation
After the release, the obtained carpospores were cultivated at a temperature of 24 ± 1°C under photosynthetically active radiation (PAR) of 40 ± 10 μmol photons m−2 s−1 (fluorescent lamps, Philips C-5 Super 84 16 W/840,Brasil) (Light Radiometer Model PMA 2100, using a PMA 2132 sensor (Solar Light, USA)) and photoperiod of 12 h, starting at 8:00 h.
Experiments were performed with different concentrations of NO3−, totaling four experimental groups: 0 mM, 25 mM, 50 mM, and 100 mM of NO3− (concentrations based on prior algal research) (Do & Cormier, 1991; Jeanfils et al., 1993; Chen et al., 2011). Five replicates were made for each experimental group. The experimental period lasted 2 days. At the end of the experiment, the samples were processed for cytological analyses. All analyses were performed 24 and 48 h after release of carpospores. Data were presented as mean and standard deviation.
In addition, the spores of all treatments were cultivated for a further 5 days, totaling 7 days, to measure the length of the germ tube. This was done to allow us to confirm if NO3− had any long-term effects on the development of these carpospores.
Analysis of morphology and germination processes
The morphological characteristics of P. acanthophora carpospores were analyzed and photographed with an Olympus BX 41 epifluorescent microscope equipped with a QCapture Pro 5.1 image capture system. The analysis of spores was performed by evaluating the following characteristics: viable, germ-forming, and non-viable. The morphological characteristics were analyzed in five replicates. The counts were presented as a percentage and calculated by the ratio between the number of carpospores of each class and the total number of carpospores for each treatment.
Light microscopy (LM)
Samples were fixed in 3% glutaraldehyde solution, 0.1 M sodium cacodylate buffer, pH 7.2, and 2% sucrose. Prefixation was performed overnight at 4°C and followed by washing in 0.1 M sodium cacodylate buffer, pH 7.2 (Schmidt et al., 2009). Five replicates were made for each experimental group, and two of them were examined. Similarities based on the comparison among the replicates suggested that the analyses were reliable. The images were acquired with the OPTHD 3.7 Software capture system (USA), using an epifluorescence microscope (Leica DM 500, Switzerland).
Cytochemistry
The material was submitted to the following cytochemical tests:
Toluidine blue (TB-O)
This dye was used to identify acidic polysaccharides through metachromatic reaction. The slides containing the carpospores were treated with aqueous solution of 0.5% TB-O, acidified with 1 N HCl to pH 3.0 for 5 s, washed in distilled water, and air-dried (Mccully & Ragsdale, 1970; Gordon-Mills & McCandless, 1975).
Periodic acid–schiff (PAS)
This dye was used to identify the presence of neutral polysaccharides. Slides containing the carpospores were oxidized with 1% aqueous periodic acid solution for 3 min, washed with distilled water, and stained with Schiff’s reagent for 3 min. They were then washed with distilled water and air-dried (Gahan, 1984).
Transmission electron microscopy (TEM)
Samples exposed to nitrate concentrations for 48 h were prefixed in 2.5% glutaraldehyde solution, 0.1 M sodium cacodylate buffer, pH 7.2, and 0.2 M sucrose. Prefixation was performed overnight at 4°C and followed by three washes in 0.1 M sodium cacodylate buffer, pH 7.2. Afterwards, specimens were postfixed in 1% osmium tetroxide (OsO4) in 0.1 M cacodylate buffer, pH 7.2 (Pueschel, 1979). Subsequently, the material was washed three times in 0.1 M cacodylate buffer, pH 7.2, and then dehydrated in acetone. After dehydration, the material was infiltrated into LR White resin (Sigma). The material was polymerized in horizontal molds in an oven at 55°C for 24 h. Ultrafine sections were made with a diamond razor, using a PowerTome XL ultramicrotome and contrasted with uranyl acetate and lead citrate, according to the procedures of Reynolds (1963). The samples were observed and photographed in a Jeol (JEM) 1011 transmission electron microscope at the Central Laboratory of Electronic Microscopy (LCME) of UFSC-Florianópolis. Five replicates were performed for each experimental group, and two replicates were examined. Similarities based on the comparison between the replicates suggested that the analyses were reliable.
Confocal laser scanning microscopy (CLSM)
The samples were fixed in 3.7% paraformaldehyde in phosphate-buffered saline (PBS). Blade material and coverslip were observed in a confocal microscope (Leica TCS SP-5; Wetzlar, Germany) at the Central Electron Microscopy Laboratory (LCME) of UFSC. Chloroplast autofluorescence was excited at the wavelength of 488 nm and emitted from 510 to 750 nm (red) (Zitta et al., 2013). To analyze the presence of cell wall, the samples were incubated with 10 mg ml−1 of Calcofluor White M2R (18909 Calcofluor White Stain - Sigma) for 15 min (Kim & Fritz, 1993; Bouzon et al., 2006). Cell wall fluorescence was observed by excitation at the wavelength of 405 nm and emission from 410 to 490 nm (cyan). The LAS-AF Lite (Leica) program was used for final image processing. Five replicates were made for each experimental group, and two of them were examined. Similarities based on comparison among the replicates suggested that the analyses were reliable.
Germ tube measurement after 7 days of development
After 7 days of development, the length (mm) of the germ tube of the carpospores (n = 50 for each treatment) treated with 0, 25, 50, and 100 mM of NO3− was measured by the ImageJ 1.51 K program in order to verify if NO3− had any effects on the development. Data were presented as mean and standard deviation.
Statistical analysis
The data of germ tube measurement passed the Shapiro–Wilk normality test and have heteroscedasticity (Bartlett test). Data were then submitted to Analysis of Unifactorial Variance (ANOVA), the independent factor being NO3− concentration (P ≤ 0.05). In order to evaluate what levels actually differ from what other levels and in which direction, out of all the possible combinations of all available levels was performed the post hoc test (Tukey). On the other hand, the data of development of carpospores (%) were not within the normal curve and have homoscedasticity (Bartlett test). Therefore, data were then submitted to Kruskal–Wallis test (P ≤ 0.05), with the independent factor being NO3− concentration. All analyses were performed with Statistica v.10.0.
Results
Analysis of morphology and germination processes
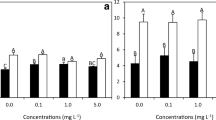
In the first 24 h of development, the carpospores were distributed into three groups: viable, germ-forming, and non-viable. In all treatments, spores around 10 μm in diameter were visualized as having a brownish stain, corresponding to the chloroplast (Fig. 1a–d). Cells elongated to form a protuberance at one of the poles to form a germ tube (Fig. 1a–d, arrows). All treatments presented viable and germ-like spores without (P = 0.46) showing any statistical difference among them (Fig. 2a). With the exception of [25], all treatments presented non-viable spores, but in treatments that presented non-viable spores, no statistical difference was noted (P = 0.22) (Fig. 2a).
Light microscopy of carpospores of the red seaweed P. acanthophora var. brasiliensis exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 24 and 48 h. a–d. Carpospores after 24 h of development exhibiting brownish staining, ~ 10 μm in diameter, and the presence of a protuberance in one of the poles of the cell for formation of germ tube (arrows). e–h. Carpospores after 48 h of development exhibiting the presence of germ tube longer than 20 μm
Development of carpospores (%) of the red macroalga P. acanthophora var. brasiliensis exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 24 and 48 h. Values presented with mean (n = 5, mean ± standard deviation). Same letters indicate no significant differences according to ANOVA and Tukey’s test (P ≤ 0.05)
After 48 h of development, the carpospores were distributed in the same 3 groups: viable, germ-forming, and non-viable. Spores from all treatments were well developed, forming a germ tube larger than 20 μm in length (Fig. 1e–h). For the first 48 h of development, the carpospores were classified in the same three groups mentioned above. All treatments presented viable spores without germ tube (P = 0.08) and without statistical differences among them (Fig. 2b), as well as viable spores with the presence of the germ tube (P = 0.19) and non-viable spores (P = 0.43), again without statistical differences among groups (Fig. 2b).
Light microscopy (LM)
After 24 h, samples of all treatments stained with AT-O (Fig. 3a–d) showed a positive metachromatic reaction in the central region with a higher amount of acidic polysaccharides and a weaker reaction in the periphery of the cytoplasm. After 48 h, the samples (Fig. 3e–h), which presented spores with germ tubes, showed a metachromatic reaction on the cell wall, which could be visualized both in the carpospore cells and in the formed germ tube.
Light microscopy of the carpospores of the red seaweed P. acanthophora var. brasiliensis stained with TB-O and exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 24 and 48 h. a–d Carpospores after 24 h of development showing a positive metachromatic reaction in the central region and a weaker reaction in the periphery of the cytoplasm. e–h. Carpospores after 48 h of development showing metachromatic reaction in the cell wall, as observed in both carpospore cells and the formed germ tube. Fig. 4 Light microscopy of carpospores of the red seaweed P. acanthophora var. brasiliensis stained with PAS and exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 24 and 48 h. a, b. Carpospores after 24 h of development and treated with nitrate concentrations of [0] and [25] exhibiting a positive reaction in the central region, a weaker reaction along the periphery of the cytoplasm, and polarization for germ tube formation (arrow). c, d. Carpospores with the same development time and treated with nitrate concentrations of [50] and [100] presented this staining pattern for samples a and b, but with a higher intensity. e–h Carpospores after 48 h of development with positive reaction throughout the spore, especially in the thick cell wall (arrows)
After 24 h, samples of treatments [0] and [25] stained with PAS (Fig. 4a, b) presented a positive reaction in the central region and a weaker reaction in the periphery of the cytoplasm, indicating the presence of neutral polysaccharides. In this same period, the samples exposed to [50] and [100] (Fig. 4c, d) also presented this staining pattern, but with a much stronger intensity when compared to the other two samples. In particular, sample [0] presented polarization for germ tube formation (Fig. 4a, arrow).
Light microscopy of carpospores of the red seaweed P. acanthophora var. brasiliensis stained with PAS and exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 24 and 48 h. a, b Carpospores after 24 h of development and treated with nitrate concentrations of [0] and [25] exhibiting a positive reaction in the central region, a weaker reaction along the periphery of the cytoplasm, and polarization for germ tube formation (arrow). c, d Carpospores with the same development time and treated with nitrate concentrations of [50] and [100] presented this staining pattern for samples a and b, but with a higher intensity. e–h Carpospores after 48 h of development with positive reaction throughout the spore, especially in the thick cell wall (arrows)
Transmission electron microscopy of carpospores of the red seaweed P. acanthophora var. brasiliensis exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 48 h. a–c Carpospores treated with concentration of [0] exhibited a spherical shape with chloroplasts (C) and mitochondria (*) and a slightly developed cell wall (CW). d–f Carpospores treated with nitrate concentration of [25] exhibiting cell division (arrow), pyrenoid formation (Py), organized thylakoids (arrows), and the presence of a cell wall (CW). g–i Carpospores treated with nitrate concentration of [50] exhibiting polarization (arrow), plastoglobuli (P), and cell wall (CW). j–l Carpospores treated with nitrate concentration of [100] with polarization (arrow), pyrenoid formation (Py), and vesicles (V) with cell wall-forming material, as well as a developed cell wall (CW)
After 48 h, the samples (Fig. 4e–h) with germ tube formation also showed a positive reaction throughout the spore, especially in the thick cell wall (Fig. 4e–h, arrows). Samples of treatments [50] and [100] showed a more intense staining, similar to that observed after 24 h, but the cell wall did not appear very thick when compared to treatments at [0] and [25] (Fig. 4e, f).
Transmission electron microscopy (TEM)
When analyzed in transmission electron microscopy, treatment [0] samples after 48 h presented a spherical carpospore without polarization for germ tube formation, but with the presence of many chloroplast arms (C), cell wall (CW) (Fig. 5a–c), and some mitochondria (*) (Fig. 5b, c). For treatment [25] samples, TEM revealed cell divisions (Fig. 5d, arrow), chloroplast with a well-developed pyrenoid (Py), well-organized thylakoids (Fig. 5e, f, arrow), and cell wall (CW) formation (Fig. 5f). Treatment [50] samples presented a polarized carpospore in advance of forming the germ tube, but with a disorganized cytoplasm (Fig. 5g, arrow). Plastoglobuli (P) (Fig. 4h), organized thylakoids, and cell wall development (PC) were present (Fig. 5i). For treatment [100] samples, TEM showed polarization of the carpospore (Fig. 5j, arrow), presence of pyrenoid (Py), vesicles (V) with material to form a cell wall, as well as actual cell wall (CW) and organized thylakoids (Fig. 5j–l), but with a disorganized cytoplasm.
Confocal microscopy (CM)
When analyzed under confocal microscopy, samples developed after 24 h presented a single, starred central chloroplast with autofluorescence (Fig. 6a–d). Chloroplast fluorescence intensity was the same for all four nitrate concentrations, but the size of chloroplast arms was smaller in treatment [0]. A positive cell wall reaction was evident throughout the spore and exhibited the same intensity for all treatments (Fig. 6a–d).
Confocal microscopy of the red seaweed P. acanthophora var. brasiliensis carpospores exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 24 and 48 h. a–d Carpospores after 24 h of development with a central chloroplast, single and starred, with autofluorescence (red). e–h Carpospores after 48 h of development with autofluorescence of the chloroplast in both the spore cell and germ tube. a–h A positive reaction of the cell wall (blue) was also evident throughout the cell and in the germ tube of the carpospores
After 48 h, all samples (Fig. 6e–h) with germ tube already formed exhibited autofluorescence of the chloroplast in both spore cell and germ tube (Fig. 6e–h). A positive reaction of the cell wall was also evident throughout the cell and in the germ tube of the carpospore (Fig. 6e–h).
Germ tube measurement after 7 days of development
After 7 days of development, the carpospores exposed to [50] and [100] presented the longest germ tube with a mean of 65.88 ± 17.55 and 64.85 ± 15.65 µm, respectively. The carpospores treated with [0] presented the shortest germ tube with a mean of 36.15 μm (Fig. 7a–e).
Light microscopy of carpospores of the red seaweed P. acanthophora var. brasiliensis exposed to different concentrations of nitrate (0, 25, 50, and 100 mM) in the laboratory for 7 days (a–d) and the average germ tube length of the same samples (e) (n = 50, mean ± standard deviation). Different letters indicate significant differences according to ANOVA and Tukey’s test (P ≤ 0.05)
Discussion
At only 48 h of development, young P. acanthophora carpospores did not show any significant sensitivity after exposure to the NO3− concentrations used in this study, showing the same developmental rate, overall morphology, cell organization, and chloroplast autofluorescence. However, when exposure was extended to 7 days, the higher concentrations of NO3− resulted in correspondingly longer germ tube formation, evidencing that such nitrate concentrations assisted in the development of carpospores.
On the other hand, the absence of NO3− caused a retardation in carpospore development, delaying the polarization of the cell to germ tube formation and, consequently, cell wall formation. However, the carpospores with higher concentrations of NO3− showed cellular polarization, well-developed cell wall, and the presence of pyrenoids, a region where RuBisCo enzyme accumulation occurs, evidencing a good and rapid development of the carpospores. The presence of empty vesicles was also observed, evidencing the production and use of material for cell wall construction, as previously observed by Bouzon et al. (2005). However, with this fast development, a disorganized cytoplasm was observed. In the case of carpospores that had an excellent and rapid development in high nutrient concentrations, great failures were not observed, only a cellular disorganization.
NO3− did affect chloroplast formation and autofluorescence such that the absence of this nutrient resulted in the failure of chloroplast arms to form and the autofluorescence of this organelle to decrease. NO3− is a source of nitrogen for the formation of phycobiliproteins (Chaloub et al., 2015), the molecular basis of photosynthetic pigments (Glazer, 1994). Thus, we suggest that the presence of NO3− favors the production of photosynthetic pigments, as previously observed by Barufi et al., (2011), Miranda et al., (2014), and Ribeiro et al., (2017).
Macroalgae are very efficient organisms in the rapid absorption of nutrients because they have mechanisms for the storage of these ions, facilitating the use of these organisms in the recovery of contaminated or nutrient-rich areas, and acting as true biological filters (Carneiro, 2007). It is well known that algae can perform phycoremediation of NO3−. Some of these representative algae are Chlorella vulgaris Beijerinck and Scenedesmus quadricauda (Turpin) Breb (Kshirsagar, 2013), Chondrus crispus Stackh. and Palmaria palmata (L.) Kuntze (Corey et al., 2013), Hypnea cervicornis J. Agardh (Ribeiro et al., 2013), Hypnea aspera Kützing (Ribeiro et al., 2017) and even algae of the same genus as that studied herein, such as Pyropia yezoensis (Ueda) M.S. Hwang & H.G. Choi (Wu et al., 2017). Phycoremediation of NO3− typically refers to the cleaning of sewage and fertilizers present in the sea, but it can also be directly associated with the mutualism established between algae and seafood. Mutualism occurs when animals release their waste, mainly nitrate, nitrite, and ammonia, which, in turn, are used as nutrients by algae (Abe et al., 2002). The algae then release oxygen for the respiration and development of animals. For example, the red macroalga Kappaphycus alvarezii (Doty) Doty ex P.C.Silva in P.C.Silva, Basson & Moe develops very well on the effluents present in the cultivation of shrimp Litopenaeus vannamei Boone (Pires, 2014). Pires, (2014) also analyzed the quality of the shrimp effluent and found the presence of 1.91 mg L−1 of NO3− (approximately 31 mM NO3−). When using this effluent in the culture of K. alvarezii, the alga developed very well without the need for Von Stosch enriched seawater medium. As we have seen in our previous work, P. acanthophora var. brasiliensis grew well in high concentrations of NO3−, indicating that it could be cultivated in environments with high levels of this nutrient and, possibly, perform phycoremediation in places with intense discharge of this molecule, or in large seafood farms. Phycoremediation, also known as green treatment, is the process whereby algae are used to remove excess nutrients from the water, preventing large-scale pollution from occurring (Sharma & Khan, 2013).
Although no statistical difference was observed at concentrations of 0 and 100 mM of NO3−, we detected a tendency toward a greater amount of non-viable spores, suggesting that these are limiting concentrations of this nutrient for the development of spores of the species. According to Barufi et al. (2011), NO3− concentrations favor the increase of MAAs, increasing antioxidant activity, thereby eliminating ROS-induced photoinhibition caused by stressors, but high concentrations of NO3− lead to saturation of the production of photoprotective molecules, corroborating the observed saturation in our study.
Conclusion
The present study demonstrated that different concentrations of NO3− do not affect the development, morphology, viability, or ultrastructure of the carpospores, nor did they interfere with the morphology and autofluorescence of the chloroplast in the first 2 days of development. On the other hand, the absence of this nutrient does delay the ultrastructural development of the carpospore, decreases the autofluorescence of the chloroplast, and delays formation of the cell wall and pyrenoid. However, after 7 days, higher nitrate concentration resulted in a longer germ tube, indicating that this nutrient in higher concentrations boosts the development of P. acanthophora var. brasiliensis, in turn suggesting the viability of this species as a tool for phycoremediation in contaminated places or places where mutualism is established between algae and seafood.
References
Abe, K., A. Imamaki & M. Hirano, 2002. Removal of nitrate, nitrite, ammonium and phosphate ions from water by the aerial microalga Trentepohlia aurea. Journal of Applied Phycology 1: 129–134.
Baird, C. & M. Cann, 2011. Química Ambiental, 4a ed. Bookman, Porto Alegre.
Bao Do, C. & F. Cormier, 1991. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Reports 9: 500–504.
Barufi, J. B., N. Korbee, M. C. Oliveira & F. L. Figueroa, 2011. Effects of N supply on the accumulation of photosynthetic pigments and photoprotectors in Gracilaria tenuistipitata (Rhodophyta) cultured under UV radiation. Journal of Applied Phycology 23: 457–466.
Blouin, N. A., J. A. Brodie, A. C. Grossman, P. Xu & S. H. Brawley, 2011. Porphyra: A marine crop shaped by stress. Trends in Plant Science 16(1): 29–37.
Bouzon, Z. L., L. C. Ouriques & E. C. De Oliveira, 2005. Ultrastructure of tetraspore germination in the agar-producing seaweed Gelidium floridanum (Gelidiales, Rhodophyta). Phycologia 44: 409–415.
Bouzon, Z. L., L. C. Ouriques & E. C. Oliveira, 2006. Spore adhesion and cell wall formation in Gelidium floridanum (Rhodophyta, Gelidiales). Journal of Applied Phycology 18: 287–294.
Camargo, J. A. & Á. Alonso, 2006. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environment International 32: 831–849.
Chaloub, R. M., N. M. S. Motta, S. P. de Araujo, P. F. de Aguiar & A. F. da Silva, 2015. Combined effects of irradiance, temperature and nitrate concentration on phycoerythrin content in the microalga Rhodomonas sp. (Cryptophyceae). Algal Research Elsevier B.V. 8: 89–94.
Chen, J., F. Wu, W. Wang, C. Zheng, G. Lin, X. Dong, J. He, Z. Pei & H. Zheng, 2011. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. Journal of Experimental Botany 62: 4481–4493.
Corey, P., J. K. Kim, J. Duston, D. J. Garbary & B. Prithiviraj, 2013. Bioremediation potential of Palmaria palmata and Chondrus crispus (Basin Head): effect of nitrate and ammonium ratio as nitrogen source on nutrient removal. Journal of Applied Phycology 25: 1349–1358.
Do, C. B. & F. Cormier, 1991. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Reproduction 9: 500–504.
Edwards, P., 1972. Cultured red alga to measure pollution. Marine Pollution Bulletin 3: 184–188.
Gahan, P. B., 1984. Plant histochemistry and cytochemistry. Academic Press, Cambridge.
Gauchazh, 2019. Florianópolis tem 20 pontos impróprios para banho; veja a lista. https://gauchazh.clicrbs.com.br/ambiente/noticia/2019/01/florianopolis-tem-20-pontos-improprios-para-banho-veja-a-lista-cjqjfocil0pkb01rxmzl3vjoq.html.
Glazer, A. N., 1994. Phycobiliproteins – a family of valuable, widely used fluorophores. Journal of Applied Phycology 6: 105–112.
Gordon-Mills, E. M. & E. L. McCandless, 1975. Carrageenans in the cell walls of Chondrus crispus Stack. (Rhodophyceae, Gigartinales) I. Localization with fluorescent antibody. Phycologia 14: 275–281.
Jeanfils, J., M. F. Canisius & N. Burlion, 1993. Effect of high nitrate concentrations on growth and nitrate uptake by free-living and immobilized Chlorella vulgaris cells. Journal of Applied Phycology 5: 369–374.
Kim, G. H. & L. Fritz, 1993. Gamete recognition during fertilization in a red alga, Antithamnion nipponicum. Protoplasma 174: 69–73.
Kshirsagar, A. D., 2013. Bioremediation of wastewater by using microalgae: An experimental study. International Journal of Life Sciences Biotechnology and Pharma Research 2: 339–346.
Li, Y., M. Horsman, B. Wang, N. Wu & C. Q. Lan, 2008. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Applied Microbiology and Biotechnology 81: 629–636.
Martins, A. P., 2007. Efeitos da disponibilidade do nitrato no metabolismo do nitrogênio em variantes pigmentares de Hypnea musciformis (Wulfen in Jacqu.) J.V. Lamour. (Gigartinales, Rhodophyta). Dissertation of Botany Institute.
McCully, K. S. & B. D. Ragsdale, 1970. Production of Arteriosclerosis by Homocysteinemia. The American Journal of Pathology 61: 1–7.
Milstein, D., A. S. Medeiros, E. C. Oliveira & M. C. Oliveira, 2015. Native or introduced? A re-evaluation of Pyropia species (Bangiales, Rhodophyta) from Brazil based on molecular analyses. European Journal of Phycology 50(1): 37–45.
Miranda, R. D. S., R. O. Mesquita, N. S. Freitas, J. T. Prisco & E. Gomes-Filho, 2014. Nitrate: Ammonium nutrition alleviates detrimental effects of salinity by enhancing photosystem II efficiency in sorghum plants. Revista Brasileira de Engenharia Agrícola e Ambiental 18: 8–12.
Nunes, B. G., 2010. Monitoramento do ambiente do cultivo experimental da alga Kappaphycus alvarezii na Praia de Sambaqui, Florianópolis/SC.
Pereira, D. T., B. Pereira, A. Fonseca, F. Ramlov, M. Maraschin, F. Álvarez-Gómez, F. L. Figueroa, É. C. Schmidt, Z. L. Bouzon & C. Simioni, 2019. Effects of ultraviolet radiation (UV ‐A + UV ‐B) on the antioxidant metabolism of the red macroalga species Acanthophora spicifera (Rhodophyta, Ceramiales) from different salinity and nutrient conditions. Photochemistry and Photobiology XX: 1–11.
Pires, C. M., 2014. Análise do potencial de fertilização da macroalga Kappaphycus alvarezii com efluentes oriundos da carcinicultura de Litopenaeus vannamei em sistema de bioflocos. Trabalho de Conclusão de Curso da Universidade Federal de Santa Catarina.
Pueschel, C. M., 1979. Ultrastructure of tetrasporogenesis in Palmaria Palmata (Rhodophyta). Journal of Phycology 15: 409–424.
Reynolds, E. S., 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of Cell Biology 17: 208–212.
Ribeiro, A. L. N., K. E. Tesima, J. M. Souza & N. S. Yokoya, 2013. Effects of nitrogen and phosphorus availabilities on growth, pigment, and protein contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta). J. Journal of Applied Phycology 25(4): 1151–1157.
Ribeiro, A. L. N., V. G. Chiozzini, E. S. Braga & N. S. Yokoya, 2017. Physiological responses and biofilter potential of Hypnea aspera (Rhodophyta, Gigartinales) cultivated in different availabilities of nitrate, ammonium, and phosphate. Journal of Applied Phycology 29(2): 683–694.
Santos, F. W. M., 2009. Período de permanência de cordas do mexilhão Perna perna (L., 1758) em cultivo. Trabalho de Conclusão de Curso da Universidade Federal de Santa Catarina.
Schmidt, É. C., L. A. Scariot, T. Rover & Z. L. Bouzon, 2009. Changes in ultrastructure and histochemistry of two red macroalgae strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales), as a consequence of ultraviolet B radiation exposure. Micron 40: 860–869.
Schweikert, K., J. E. S. Sutherland, C. L. Hurd & D. J. Burritt, 2011. UV-B radiation induces changes in polyamine metabolism in the red seaweed Porphyra cinnamomea. Plant Growth Regulation 65: 389–399.
Sharma, G. K. & S. A. Khan, 2013. Bioremediation of sewage wastewater using selective algae for manure production. International Journal of Environmental Engineering Management 4: 573–580.
Simioni, C., L. Hayashi & M. C. Oliveira, 2019. Seaweed resources of Brazil: what has changed in 20 years? Botanica Marina 62(5): 433–441.
Townsend, A. R., R. W. Howarth, F. A. Bazzaz, M. S. Booth, C. C. Cleveland, S. K. Collinge, A. P. Dobson, P. R. Epstein, E. A. Holland, D. R. Keeney, M. A. Mallin, C. A. Rogers, P. Wayne & A. H. Wolfe, 2003. Human health effects changing nigrogen cycle. Frontiers in Ecology and the Environment 1: 240–246.
Vesilind, P. A. & S. M. Morgan, 2013. Introdução à engenharia ambiental. Cengage Learning, São Paulo.
Wu, H., J. K. Kim, Y. Huo, J. Zhang & P. He, 2017. Nutrient removal ability of seaweeds on Pyropia yezoensis aquaculture rafts in China’s radial sandbanks. Aquatic Botany 137: 72–79.
Zhang, Q., N. Li, G. Zhou, X. Lu, Z. Xu & Z. Li, 2003. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodophyta) in aging mice. Pharmacological Research 48: 151–155.
Zheng, Y. & K. Gao, 2009. Impacts of solar uv radiation on the photosynthesis, growth, and uv-absorbing compounds in Gracilaria lemaneiformis (Rhodophyta) grown at different nitrate concentrations. Journal of Phycology 45: 314–323.
Zhou, C., X. Yu, Y. Zhang, R. He & H. Ma, 2012. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydrate Polymers Elsevier Ltd. 87: 2046–2051.
Zitta, C. S., T. Rover, L. Hayashi & Z. L. Bouzon, 2013. Callus ontogeny of the Kappaphycus alvarezii (Rhodophyta, Gigartinales) brown tetrasporophyte strain. Journal of Applied Phycology 25: 615–629.
Acknowledgements
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for the financial support to Zenilda Laurita Bouzon (process 449712/2014-1). This study is part of the Ph.D. thesis of the first author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Iacopo Bertocci
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, D.T., Ouriques, L.C., Bouzon, Z.L. et al. Effects of high nitrate concentrations on the germination of carpospores of the red seaweed Pyropia acanthophora var. brasiliensis (Rhodophyta, Bangiales). Hydrobiologia 847, 217–228 (2020). https://doi.org/10.1007/s10750-019-04083-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04083-2