Abstract
Didymosphenia geminata is a bloom-forming diatom that has invaded numerous temperate rivers globally. Proliferations of D. geminata can result in negative effects on invaded communities. Ecological theory suggests impacts may vary associated with trait variation in both invaded communities and the invader. Trait commonalities related to organism size are rarely considered, yet are expected to influence the outcomes of ecological (niche and neutral) processes and invader effects. We hypothesised that D. geminata would impact diversity and community composition, with effects varying between size classes, influenced by niche and spatial gradients. To examine this hypothesis, we surveyed 55 rivers along a gradient of D. geminata biomass in the South Island, New Zealand, collecting data on algal and invertebrate communities, 33 spatial predictors, and 111 physical and chemical predictors. Didymosphenia geminata biomass was associated with increased species richness in both algal and invertebrate assemblages, but blooms reduced beta-diversity resulting in more homogenous communities. Both niche and neutral processes influenced community assembly and invader effects, which varied between algae and invertebrates. However, D. geminata appeared to have a dominant influence on both communities, irrespective of organism size. These findings reinforce the substantial negative effect invasive species such as D. geminata can cause in invaded ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are a threat to biodiversity globally (Vitousek et al., 1997). Invasions can alter ecosystem structure, processes and functioning (Chapin et al., 2000). These changes often result in significant negative effects, but establishing causal mechanisms within ecosystems is difficult given the complexity of interactions between communities, invaders and invaded habitats (Didham et al., 2007; Vellend, 2010). Disentangling invasion impacts by accounting for co-occurring ecological processes known to influence community organisation may increase our ability to predict invasion effects where few general rules exist (Parker et al., 1999; Didham et al., 2007; Vellend, 2010). Here, we investigate the influence of an invasive, bloom-forming diatom Didymosphenia geminata (Lyngbye) M. Schmidt. (Bacillariophyceae) on the structure of benthic algae and macroinvertebrate communities in rivers by accounting for underlying environmental and spatial gradients.
Didymosphenia geminata is a stalk-forming benthic diatom which has attracted attention within its native northern hemisphere range due to the increased severity and frequency of nuisance proliferations (Bothwell et al., 2014). Within some temperate countries such as New Zealand and Chile, it is considered an aggressive invasive species (Kilroy & Unwin, 2011; Reid & Torres, 2014; Bray et al., 2016). The proliferations of D. geminata differ from other algae because they result from the production of mucopolysaccharide basal stalk material, rather than cells (Bothwell et al., 2014; Bray et al., 2017b). Stalk production is stimulated by phosphorus limitation (i.e. ~ 2 µg l−1 dissolved reactive phosphorus, DRP; Bothwell et al., 2014; Bray et al., 2017a). Blooms occur in hydrologically stable oligotrophic rivers, with occurrences common in the outflows of lakes and dams (Bray et al., 2016). Didymosphenia geminata proliferations increase algal biomass, altering periphyton appearance and assemblage composition (Kilroy et al., 2006). Invertebrate assemblages are also affected as D. geminata biomass increases, with increased taxa richness and variability, but often with greatly increased densities (Kilroy et al., 2009). Taxa that have been observed to increase in abundance with D. geminata include chironomid midges, micro-crustaceans, oligochaetes and nematode worms (Larned et al., 2007; Kilroy et al., 2009; Jellyman & Harding, 2016). Negative effects on ichthyofauna are less established, but include changes in condition and declines in abundance of certain taxa (Shearer et al., 2007; Bonnett et al., 2008; Jellyman & Harding, 2016). Impacts detected within and between trophic levels suggest changes to food-web structure and functioning (Rost et al., 2008). These changes appear to be driven by habitat alteration, which further influences behavioural and trophic interactions (Taylor, 2012).
Organisational and biodiversity patterns are determined by both species traits and ecological filters (sensu Hutchinson 1957), but also by speciation, dispersal and stochastic demographic processes which are encompassed by neutral theory (Hubbell, 2001). Aspects of both niche and neutral processes determine patterns of community composition and diversity (α as richness and β as turnover; Anderson et al., 2011; Rosindell et al., 2012) in stream algae (Vanormelingen et al., 2008), and macroinvertebrates (Thompson & Townsend, 2006). The strength of these processes is expected to differ between algae and invertebrates, related to differences in trait composition (Allen et al., 2006; Farjalla et al., 2012). Given fundamental differences related to trait commonalities between organism size classes, predictions may be made about how size mediates the effects of invaders. Organism size is an ecologically integrative trait, influencing both patterns of assembly in communities (Allen et al., 2006), invasion success and, potentially, invader effects. Several predictions regarding the effects of D. geminata, and patterns of community assembly otherwise may be made. The ‘everything (small) is everywhere but the environment selects’ (EiE) hypothesis (Baas Becking 1934 in Fontaneto, 2011; Farjalla et al., 2012) posits that where microorganisms have high dispersal, patterns of assembly are primarily determined by niche filtering, with little spatial structuring. In contrast, the ‘size-plasticity’ hypothesis identifies larger organisms (e.g. macroinvertebrates) should exhibit greater niche structuring, given greater developmental constraints, greater complexity, decreasing trait plasticity and increasing specialisation (Farjalla et al., 2012). Larger organisms may also have comparatively lower dispersal abilities therefore should exhibit increasing spatial structuring. Both hypotheses argue for increasing niche determinism associated with either decreasing (EiE) or increasing organism size (‘size-plasticity’).
In the following study, we examined the influence of D. geminata on algal and invertebrate communities comparing homogenisation, α (richness), and β (measured as community turnover) diversity (Anderson et al., 2006, 2011), while accounting for co-occurring spatial and physical gradients (sensu Vincent et al., 2006; Farjalla et al., 2012). The present study accompanies Bray et al. (2016) which examined D. geminata habitat associations using the same survey data, but the present study examines the following new questions:
- 1.
Does D. geminata, accounting for other gradients, have an observed influence on diversity (α and β), community composition and homogenisation?
- 2.
Do key ecological processes vary with organism size and therefore influence D. geminata impacts? This question may be separated into the following:
- (a)
Do smaller organisms exhibit greater ecological determinism (i.e. ‘EiE’), resulting in a greater observed influence of D. geminata?
- (b)
Are larger organisms structured to a greater extent by niche processes (i.e. ‘size-plasticity’), resulting in a greater observed influence of D. geminata?
- (c)
Alternatively, do differences in dispersal ability influence community structure?
- (a)
Methods
Study sites
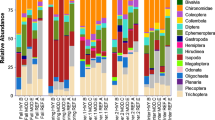
A survey was conducted at 55 sites in lotic waterways within the South Island, New Zealand during the summer of 2009–2010 (see Bray, 2014; Bray et al., 2016 for further detail). Site selection was stratified by D. geminata biomass and geographic region. Sites were divided into the following biomass categories; ‘High-biomass’, ‘Low-biomass’, ‘Positive’, and ‘Reference’ sites (Fig. 1). High-biomass sites were based on an a priori biomass proliferation limit of > 50 ash-free dry mass (AFDM) g m−2 with D. geminata dominating the periphyton (Biggs & Price, 1987). Low-biomass sites included those that had D. geminata present within the periphyton, but did not reach the proliferation limit. Positive sites had live suspended D. geminata cells in the water column, but no detected benthic cells. Reference sites had no living D. geminata cells within the periphyton or the water column (see Bray et al. (2016) for a site list).
Study sites within the South Island of New Zealand. D. geminata has not been detected in the North Island, so it was excluded from the survey. High-biomass sites were dominated by D. geminata and exceeded > 50 AFDM g m−2. Low-biomass sites had D. geminata within the periphyton but did not meet this proliferation limit. Positive sites had suspended D. geminata cells but no benthic cells detected. Reference sites had no D. geminata cells in the periphyton or within the water column
Data collection
At each study location, a 50-m reach was selected that included at least one riffle-run-pool complex. Physical measurements were made at each site, including an assessment of channel stability (stability index; Pfankuch, 1975). Depth and water velocity (Marsh McBirney Flowmate Model, 2000) were measured across a transect perpendicular to flow in the wetted channel. Lower bank width was also measured (or estimated where it could not be physically measured). Upper bank or bankfull width was estimated using ARC-GIS. Shear stress at the substrate was calculated using Froude’s number; Fr = V/(D g)0.5, where V = mean channel water velocity (m s−1); D = mean depth (m); g = acceleration due to gravity (9.81 m s−2). Riparian shading was calculated where possible from the centre of each reach using a spherical mirrored crown Densitometer (Harding et al., 2009). The relative area occupied by substrate (Wentworth particle size) was categorised using random transects, with substrate (SI; Jowett & Richardson, 1990) and embeddedness indices calculated (Harding et al., 2009).
The New Zealand freshwater geodatabases River Environments Classification (REC, Snelder & Biggs, 2002) and the Freshwater Ecosystems of New Zealand (FENZ, Leathwick et al., 2010) were used to gather GIS modelled variables. Variables were gathered based on reach identifiers (NZReach scores derived from the REC, Snelder & Biggs, 2002). Reach identifiers were determined from coordinates obtained at the site using a handheld GPS (Garmin 60CSX). A categorical estimate of the percentage of flow affected by upstream lentic waterbodies (lakes, dams and wetlands) was determined using ARC-GIS 10.2. The estimated percentage contribution was ranked between 1 and 5, as follows: (1) On-channel still waterbodies influencing < 5% of flow at the site; (2) On-channel still waterbodies contributing 5%–< 20% of flow. (3) On-channel still waterbodies contributing 20%– < 60% of flow. (4) On-channel lentic waterbodies contributing 60–100% of flow. Reach and catchment geologies were extracted from the Quarter Million Mapping Program (QMAPS, GNS; Graham, 2009), and the River Environment Classification (REC, Snelder & Biggs, 2002). ARC-GIS 10.2. was used for mapping and obtaining geodatabase data (ESRI, 2012).
A total of 10 water chemistry variables (i.e. total alkalinity, dissolved calcium, dissolved magnesium, total nitrogen (TN), nitrate–nitrite, total Kjeldahl nitrogen (TKN), dissolved reactive phosphorus (DRP), total dissolved phosphorus (TDP), total phosphorus (TP), organic phosphorus and reactive silica (SiO2)) were analysed using standards following APHA methods (R. J. Hill Laboratories Ltd, Hamilton, New Zealand; see Bray et al., 2016 for water chemistry methods).
Ten quantitative benthic periphyton samples were collected at equidistant points from a transect across the wetted channel at each site. Samples were pooled and the composite sample was homogenised for 30 s using a hand blender. Aliquots were taken for analysis of taxonomic composition, relative abundance and biovolume preserved with Lugol’s iodine, and the remainder frozen for biomass analysis. Ash-free dry mass was calculated following methods in Biggs and Kilroy (2000). Chlorophyll a extraction occurred on filtered subsamples immersed in 90% ethanol in a 78°C water bath for 5 min, followed by 12 h of refrigeration to ensure complete extraction (Biggs & Kilroy, 2000). Chlorophyll a was then assessed using a Trilogy Fluorometer, with adjustments for turbidity (Turner Designs, California, USA). Correction for phaeopigments occurred by reanalysis after addition of 0.1 ml of 0.3 M hydrochloric acid. Relative abundance counts of taxonomic samples were conducted using an Olympus BX50 microscope, with a minimum of 300 algal cells identified. Algal identifications were carried out using a variety of texts (Krammer & Lange-Bertalot, 1988, 1991a, b, 1995; Biggs & Kilroy, 2000; John et al., 2002) and biovolumes were calculated using Hillebrand et al. (1999) or obtained from online USGS datasets (http://diatom.ansp.org/nawqa/biovol2001.aspx).
Invertebrate collections involved three replicate Surber samples (0.06 m2; 250 µm mesh) taken from riffle habitats. These were preserved in ethanol (70%) and processed in the laboratory. Taxa were identified and enumerated to the lowest possible taxonomic resolution using Winterbourn et al. (2000). Where sub-sampling was required for large samples, scans for rare taxa were also conducted and included in density data.
Data analysis
A total of 144 physical and spatial variables were analysed against biological data. 18 physical variables were derived from measurements collected at each site, 14 comprised water chemistry variables, 13 were GIS calculated, 54 were obtained from GIS geodatabases and 33 spatial variables were generated using through Principal Coordinates of Neighbour Matrices analysis (PCNM; Borcard & Legendre, 2002).
To examine patterns of β-diversity, non-metric multidimensional scaling (NMDS) and ordinations were conducted on algal and invertebrate relative abundance data. “adonis” and “betadisper” functions in the R package “vegan” were then employed to test for community shifts between biomass categories and to test for community homogenisation, respectively (Oksanen et al. 2008). Post hoc Tukey’s tests were used to differentiate betadisper categories. Rare taxa (< 3 presences) were removed, as their removal slightly increased explained variation in community analyses. No data transformation was conducted on relative abundance data. Bray–Curtis was used as a measure of dissimilarity (Anderson et al., 2011).
NMDS and distanced-based redundancy analysis (dbRDA) were used to explore relationships between D. geminata, other biotic, physical and spatial variables. Biotic, physical, chemical and spatial drivers of the algal community were assessed on relative algal biovolumes (NMDS and dbRDA). Backwards stepwise selection for dbRDA, and permutation tests for NMDS (Vegan:envfit) variable selection (α < 0.05) were used in model reduction procedures. Significance testing was based on 999 Monte Carlo permutation tests for these analyses. Prior to analysis, collinearity was also assessed among variables, with exclusion of the weaker variable based on Pearson’s coefficient values > 0.6. All environmental and biotic explanatory data were centred and standardised before analysis.
Partial least squares path modelling (PLS-PM) was used to investigate direct and indirect effects between physical and chemical variables, D. geminata biomass, % Ephemeroptera, Plecoptera and Trichoptera (EPT) and algal AFDM. PLS-PM models underwent model reduction where indices with factor loadings below ~ 0.5, and paths with significance P > 0.1 were excluded. Significance was tested using 100 bootstraps. PLS-PM was employed using PLS-Graph (PLSG Version 3, California, US). PLS-PM was preferentially employed over covariance-based approaches, as the method allows rapid model reduction and selection (Chin, 2001; see Supplementary Material S3 for further details).
Variation partitioning (vegan:varpart) allowed further assessment of likely biotic, environmental and spatial associations with community composition. These analyses were conducted on relative abundance data, using Bray–Curtis as the measure of dissimilarity. Spatial and environmental variables were reduced using backwards and forwards stepwise selection based on redundancy analysis (RDA) and Akaike information criterion (AIC) values to exclude non-significant variables. Fractions were tested for significance using 200 permutations, partialling competing matrices in a stepwise fashion (Borcard & Legendre, 1994; Peres-Neto et al., 2006).
Correlation between spatial, physical and ecological communities was explored through PCNM vectors (Principal Coordinates of Neighbour Matrices). PCNM detects and quantifies fine- and broad-scale spatial gradients from longitude and latitude (Borcard & Legendre, 2002). Kruskal–Wallis ANOVA was also used to examine patterns between geographic regions. R Studio (0.97.318; RStudio Team, 2015) using R (2.15.2; R Development Core Team, 2010) was used for all community analyses.
Results
Diversity, homogenisation and community changes
Algal richness was positively correlated with D. geminata biomass (Pearson’s r = 0.35, P < 0.01). Relative abundance counts of quantitative periphyton samples identified 193 taxa (Supplementary Material S1). Algal beta-diversity declined as D. geminata biomass increased, with an apparent community shift (“adonis”, P < 0.001), and increasing community homogenisation (“betadisper”, P < 0.0001; Fig. 2a). Homogeneity of group dispersions identified that High-biomass D. geminata sites had more homogeneous communities. High-biomass sites were also dominated by D. geminata biomass, where stalk biovolumes accounted for 96% of mean algal biomass (range 84.5–99.9%). Didymosphenia geminata cells only accounted for 9% (range 0.5–66%) of relative community cell counts, when stalk fragments were excluded within High-biomass sites. High-biomass sites were diverse with 116 algal taxa across the 14 sites. Within these sites, small diatoms were numerous and diverse, accounting for much of the remaining cell biovolume and the majority of cell counts. Common taxa included Achnanthidium minutissimum Kützing and Encyonema minutum (Hilse ex Rabenh.) Mann in Round, Epithemia sorex Kützing, Synedra ulna var. biceps (Kützing) Schönfeldt, Fragilaria capucina Desmazières, Rhopalodia novae-zelandiae Hustedt, Rossithidium linearis (W.Smith) Round & Bukhtiyarova, Cocconeis placentula Ehrenberg, Diatoma tenuis C.Agardh were common. Other non-bacillariophytes such as Spirogyra sp., Tolypothrix tenuis Kützing, Tolypothrix distorta Kützing, Oedogonium sp. and Mougeotia cf. depressa (Hassal) Whittrock also commonly occurred. Many Low-biomass sites were again characterised by D. geminata dominance however were below the > 50 g m−2 AFDM limit set, while in others D. geminata comprised a minor component of taxa counts and biovolume. Positive and Reference sites had diverse assemblages, typically dominated by taxa of Bacillariophyta, with Chlorophyta, and Cyanophyta also common.
A total of 153 invertebrate taxa were identified (Supplementary Material S2) and invertebrate richness increased with increasing D. geminata biomass (Pearson’s r = 0.33, t53 = 2.6, P < 0.05), with greater richness in both Low- and High-biomass sites than other sites. However, Simpsons diversity (ANOVA, F3,50 = 1.09, P = 0.36) and the Berger–Parker index of dominance identified no change between categories (ANOVA, F3,50 = 1.19, P = 0.32). Invertebrate densities showed no trend based on D. geminata biomass categories (ANOVA, F3,50 = 2.16, P = 0.11) or D. geminata biomass (Pearson’s r = 0.15, t53 = 1.10, P = 0.28), however, did increase with total algal AFDM (Pearson’s r = 0.31, t53 = 2.36, P < 0.05). Invertebrate β-diversity (compositional turnover) based on relative abundance data (Fig. 3b) showed weakened but significant compositional turnover between categories (“adonis” P < 0.005), with increased homogeneity (“betadisper”, P < 0.05). High-biomass sites were dominated by non-biting midges (Diptera: Chironomidae) comprising up to 58% (mean 40%) with high but variable densities of Orthocladiinae (with a maximum of ~ 25,100 individuals per m2), but also the cladoceran Chydoridae (with a maximum of ~ 40,000 individuals per m2). Ephemeroptera (primarily the leptophlebiid Deleatidium spp.) comprised 13% of counts within High-biomass sites, while Trichoptera (primarily the hydroptilid Oxyethira albiceps) accounted for 12% and Crustacea (comprised primarily of cladocerans and ostracods) approximately 11%. Mollusca dominated by the hydrobiid Potamopyrgus antipodarum and Oligochaetes were also common. Low-biomass, Positive sites and Reference sites were more variable, including taxa from a broader range of families.
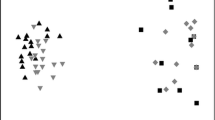
Constrained ordinations of community data performed with distance-based redundancy analysis (dbRDA). a Algal dbRDA (relative abundances by biovolume, with D. geminata relative abundance counts removed and biomass added as a predictor); and b benthic invertebrate dbRDA using relative abundances of individuals. The Bray–Curtis dissimilarity index was used for both ordinations with significant variables (P < 0.05) presented (see also Supplementary Materials 2 and 3 for community taxa lists)
Physical, chemical and spatial gradients related to community change
Changes in algal community composition were strongly associated with D. geminata biomass (Table 1a). Other parameters including the stability index (Pfankuch), bankfull width, temperature, PCNM7 and lentic influence, also correlated with unconstrained community change (P < 0.05; Fig. 3a; Table 1a). Similarly, dbRDA identified D. geminata biomass (P < 0.01), PCNM17 (P < 0.01), temperature (P < 0.05), total nitrogen (TN; P < 0.05), reach stability (Pfankuch; P < 0.05) as significant drivers of algal composition (see Supplementary Material S1 for full algal dbRDA results; Fig. 3a).
D. geminata biomass was strongly associated with invertebrate community change, but other spatial, physical and chemical variables were also associated with these changes (Fig. 2b; Table 1b). dbRDA identified the Pfankuch stability index (P < 0.05) as the strongest driver of invertebrate composition, followed by D. geminata (P < 0.05), lentic influence (P < 0.05), PCNM8 (P < 0.01), temperature (P < 0.01), silica (P < 0.05) and total phosphorus (P < 0.05; see Supplementary Material S2 for full invertebrate dbRDA results; Fig. 3b).
Partial least squares path analysis examined direct and indirect associations between physical and chemical variables and the abundance of taxa known to be sensitive to a range of stressors (i.e. %EPT). Declining %EPT appeared most strongly influenced by a direct relationship with D. geminata biomass and total AFDM, rather than other physical and chemical variables. Total AFDM (R2= 0.40) explained more variance in %EPT than D. geminata biomass (R2 = 0.32), indicative of a general response to periphyton biomass rather than uniquely to D. geminata biomass. Increasing nutrient concentrations and disturbance mediated the effect of D. geminata biomass on %EPT, while water velocity had a direct positive effect on %EPT (see Supplementary Material S3 for PLS statistics and diagrams).
Variation partitioning of algal community relative biovolumes (D. geminata excluded) explained only 14% of community variation. 7.6% was explained by D. geminata biomass (P < 0.01), 8.2% by environmental variables (temperature, silica, Pfankuch, TN; P < 0.05) and 2.2% was purely spatial (PCNM 3 and 2; P < 0.05; Fig. 4a). Conversely, 32% of variation in the invertebrate community was explained, with 5.6% explained by D. geminata biomass alone (P < 0.05), 15.7% by environmental predictors (the lentic index, temperature, silica, TP and Pfankuch; P < 0.005), and 10.5% by spatial variables (PCNM variables 5, 2, 27, 4 and 8; P < 0.001; Fig. 4b; see also Supplementary Material S4 for full variance partitioning results).
a, b Results from variation partitioning (Vegan:varpart). Area-proportional Euler diagrams showing the variation explained by competing drivers within, a the algal community (relative abundance) and b the invertebrate community (relative abundance). Diagrams show the pure effects of (D) D. geminata, (E) abiotic environmental drivers, and (S) space while the intersections represent covariance among drivers (e.g. DES). Abiotic environmental and spatial drivers derived from Principal Coordinates of Neighbours Matrices analysis (PCNM) were selected using backwards stepwise selection procedures (P < 0.05). All pure fractions were significant at P < 0.05. Variation is based on adjusted R2 as an unbiased estimator (Peres-Neto et al., 2006; Supplementary Material S4)
Discussion
Invasions by non-native organisms threaten freshwater biodiversity, but the effects of invasive species can be difficult to predict. Invader effects may vary associated with a myriad of factors, including trait differences among invaded communities and the invader, niche requirements, trophic position, dispersal abilities, phenotypic plasticity and the effects of the invader on habitats (Parker et al., 1999). Accounting for these co-occurring ecological processes is necessary to better understand the effects of an invasion.
Didymosphenia geminata proliferations and community changes
Increasing D. geminata biomass was associated with increasing homogenisation and community changes in both algal and invertebrate assemblages (Fig. 2a, b). Similar to our results, both Sivarajah et al. (2015) and Gillis & Lavoie (2014) found that D. geminata proliferations altered benthic diatom community composition and biomass. Other studies have reported similar but inconsistent patterns for invertebrates, with increasing taxa dominance but variable changes to density (Kilroy et al., 2006, 2009; Larned et al., 2007). With increasing D. geminata biomass, we found increases in oligochaeta, chironomids, cladoceran and nematode taxa groups, with a concomitant decline in sensitive EPT fauna, confirming observations by Larned et al. (2007) and Kilroy et al. (2009). A variety of mechanisms may interact to cause D. geminata impacts on algal and invertebrate communities. These included trophic interactions (e.g. direct effects as food resources change) and habitat alteration (e.g. indirect effects mediated through competition and predation). Taylor (2012) identified habitat alteration was the primary mechanism causing changes to invertebrates. However, a range of complex direct and indirect interactions may also occur (Table 2).
Within algal assemblages D. geminata invasion may be related to trophic interactions (direct effects) or habitat alteration (indirect effects), or a combination of effects (Taylor, 2012). For example, D. geminata may alter habitats, causing changes to grazer and predator abundances, further influencing algal structure and composition (Larned et al., 2007; Taylor, 2012). Invertebrate grazing may indirectly influence algal composition and structure though the removal of old cells, providing a stimulatory response (Power et al., 1985; Opsahl et al., 2003). Larned et al. (2007) observed Deleatidium spp., Pycnocentrodes spp., and Potamopyrgus spp. were observed to ingest D. geminata, while the dominant taxonomic group Chironomidae did not, with results indicating overall consumption likely had little effect on biomass, suggesting weak direct trophic connections. Processes such as habitat transformation or ecosystem engineering (Falk-Petersen et al., 2006) may be occurring with D. geminata invasions. This may explain its large relative direct effect compared to other niche filters (e.g. hydrologic stability with a strong indirect effect), and the greater relative effect on stream algae compared to stream invertebrates (Dudley et al., 1986; Jones et al., 1994; Farjalla et al., 2012; Taylor, 2012).
Similarly, a variety of potential mechanisms may drive D. geminata effects on invertebrates. These include (1) negative indirect effects where suitable substrate and habitats are smothered, likely to occur with Ephemeroptera that are unable to burrow; (2) positive indirect effects where habitats are created for invertebrate taxa (e.g. oligochaetes); (3) negative direct effects where invertebrate taxa are unable to consume, or gain sufficient nutrition to survive within D. geminata mats (Larned et al., 2007); (4) positive direct effects through consumption of algae facilitated by D. geminata blooms; (5) mediation of trophic interactions such as predation; or (6) some combination of effects (Larned et al., 2007; Taylor, 2012).
Contrasting strengths of spatial decay and niche determinism in stream communities
Central to the neutral theory of ecology is the assumption that there is ecological equivalence among species (Hubbel, 2001). This assumption has polarised ecologists, as it minimises the myriad of deterministic processes that influence communities (Alonso et al., 2006; Thompson & Townsend, 2006; Vellend, 2010; Vellend et al., 2014). However, this assumption is a deliberate simplification and has stimulated a wealth of research on the importance of these processes (Alonso et al., 2006; Vellend et al., 2014). An emerging consensus suggests dispersal, demographic stochasticity, speciation (encompassed by neutral theory) and deterministic (niche) processes collectively contribute to patterns of community assembly (Vellend, 2010; Vellend et al., 2014). Thereby shifting the focus towards the relative importance of these processes (Alonso et al., 2006; Thompson & Townsend, 2006; Vellend et al., 2014).
Invertebrate communities exhibited greater structuring associated with niche and spatial gradients with a smaller relative effect of D. geminata, compared to algal communities. In algal communities physical and chemical predictors explained less variation, and communities exhibited little turnover associated with spatial gradients, but D. geminata appeared to have a greater relative influence (Fig. 4). These differences in community responses might be expected given the varying niche preferences, abundances, growth rates, reproductive strategies, trophic positions and dispersal abilities of algal and invertebrate communities (Allen et al., 2006; Sweetman et al., 2010; Farjalla et al., 2012; Soininen, 2014). We evaluated two hypotheses related to algal and invertebrate communities; (a) the “everything is everywhere but the environment selects” hypothesis (Bass Becking in Fontaneto, 2011) predicts smaller sized organisms should be niche limited to a greater extent, contrasting with (b) the “size-plasticity” hypothesis, where larger organisms are predicted to exhibit less phenotypic plasticity, thereby strengthening environmental filtering (Farjalla et al., 2012). These two hypotheses predict different outcomes, and suggest niche determinism strengthens among either, (a) smaller-, or (b) larger-bodied organisms.
Reduced compositional turnover in algal communities across spatial gradients, evident here, has previously been observed for small-bodied organisms (Shurin et al., 2009; Farjalla et al., 2012). This may be predicted based on dispersal ability alone (Nekola & White, 1999). Common factors that increase colonisation success rates in smaller sized organisms (< 2 mm) include increased dispersal via passive transport; asexual reproduction which nullifies Allee effects; and dormant phases which facilitate dispersal (Sarnelle & Knapp, 2004). Microorganisms should also be less affected by barriers, and be more likely to disperse, which may further compensate for low persistence, as covered by source–sink dynamics, rescue effects, and mass-effect perspectives (Brown & Kodric-Brown, 1977; Leibold et al., 2004). Consequently, microorganisms generally exhibit low levels of spatial beta-diversity, evidenced by lower rates of community turnover or ‘distance decay’ (Finlay, 2002; Shurin et al., 2009). For example, many freshwater algae have cosmopolitan distributions (Vanormelingen et al., 2008). Despite these general patterns, diatoms are known to have distributions ranging from global to highly localised, and constraints on taxonomic knowledge and cryptic lineages among microorganisms (Vanormelingen et al., 2008), influence observed community patterns, diversity and habitat associations. However, niche determinism may not be strong among closely related species, where phylogenetic conservatism should lead to similar habitat associations (Diamond & Case, 1986).
Our results suggest invertebrates were dispersal-limited to a greater extent, and exhibited greater niche determinism than algal communities. Thompson & Townsend (2006) observed that local diversity in stream invertebrates was also influenced by both dispersal processes and local environmental conditions. Our results support their findings, where both spatial gradients and local environmental context contributed strongly to invertebrate community composition (e.g. Fig. 4b). Moreover, many of the stream invertebrates recorded here were insects with active dispersal stages allowing adults to track environmental heterogeneity, and thus variation in spatial assembly cannot be considered truly neutral (Thompson & Townsend, 2006). Active dispersal is a key difference that contrasts with passively dispersed algal species. The findings of Thompson & Townsend (2006) identified patterns of assembly were dissimilar among invertebrates when differentiated by dispersal ability, where low and moderate dispersers were best explained by both environmental and spatial gradients. However, invertebrates characterised as high dispersers were freely accessing sites, and were not well predicted by either spatial or environmental gradients (Thompson & Townsend, 2006), similar to patterns observed for algae here, reinforcing dispersal is a key trait in determining patterns of community assembly.
Patterns relating to dispersal (and by proxy, neutral theory) are dependent on spatial scale (Chase, 2014), and our study was conducted over a much greater extent (e.g. South Island, NZ, ~ 150,000 km2) than that used by Thompson & Townsend (2006) or Farjalla et al. (2012). The latter study found little support for spatial structuring of aquatic communities associated with bromeliads (bacteria, zooplankton and macroinvertebrates) within Restinga de Jurubatiba National Park, Brazil (~ 150 km2). The broad spatial extent of our study may have predisposed it to detecting spatial decay in communities. River networks are also dendritic and spatially discrete, with frequent significant topographic barriers between catchments that are likely to strengthen spatial patterns. We expect that the scale of observation (Chase, 2014), combined with modes of dispersal, dispersal strength, barriers to dispersal (Soininen et al., 2007; Morán-Ordóñez et al., 2015) and the trophic level of organisms (Soininen, 2014) are likely to have influenced patterns in our study.
The role of generation and succession times may also influence niche and neutral process effects on algae and invertebrates as they sort along these gradients or fail to (Nekola & White, 1999). Where complex coexistence mechanisms interact with frequent perturbations, biological interactions may be weaker. This stochasticity in niche processes may preclude predictable patterns of assembly (Alonso et al., 2006). For example, founder control achieved through lottery effects could occur with either community examined here (Townsend, 1989). Despite this, differences observed here and in similar studies (Thompson & Townsend, 2006; Sweetman et al., 2010; Farjalla et al., 2012) reinforce that examining both spatial decay and environmental gradients can yield useful insight into processes that influence patterns of community assembly.
Conclusions
Didymosphenia geminata strongly influenced invertebrate and algal assemblage composition, decreasing beta-diversity and homogenising communities. However, the negative effects of D. geminata were contingent on the formation of proliferations, which were restricted to oligotrophic, hydrologically stable lotic ecosystems (Bray et al., 2016). Our results further support that any perceived dichotomy between deterministic and neutral theories may be irrelevant where aspects of both processes influence diversity and community structure. Contrasting strengths of these processes between community types were likely reflective of trait commonalities, and could be explained by hypotheses related to body size. The ‘size-plasticity’ hypothesis of Farjalla et al. (2012) was supported here as niche filtering was strongest within macroinvertebrate assemblages. However, the influence of D. geminata on invertebrates was proportionally weaker than that observed for algal communities. Our data also suggested that invertebrate communities were more dispersal-limited than algal communities. Taken in isolation, aspects of the ‘everything is everywhere, but the environment selects’ hypothesis were supported where algae exhibited low spatial structuring and niche filtering predominated. However, we suggest an amendment may be added to the axiom ‘everything (small) is everywhere, but the environment selects’, where compared to larger-bodied organisms the environment may weakly select smaller organisms. Regardless, proliferations of D. geminata appeared to have a strong influence on stream algal and macroinvertebrate communities. Our study further highlights the negative impacts that an invasive, habitat-modifying organism such as D. geminata can have on freshwater ecosystems.
Data availability
Data will be made available upon request.
References
Allen, C. R., A. S. Garmestani, T. D. Havlicek, P. A. Marquet, G. D. Peterson, C. Restrepo, C. A. Stow & B. E. Weeks, 2006. Patterns in body mass distributions: sifting among alternative hypotheses. Ecology Letters 9(5): 630–643.
Alonso, D., R. S. Etienne & A. J. McKane, 2006. The merits of neutral theory. Trends in Ecology & Evolution 21(8): 451–457.
Anderson, M. J., K. E. Ellingsen & B. H. McArdle, 2006. Multivariate dispersion as a measure of beta diversity. Ecology Letters 9(6): 683–693.
Anderson, M. J., T. O. Crist, J. M. Chase, M. Vellend, B. D. Inouye, A. L. Freestone, N. J. Sanders, H. V. Cornell, L. S. Comita, K. F. Davies, S. P. Harrison, N. J. B. Kraft, J. C. Stegen & N. G. Swenson, 2011. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters 14(1): 19–28.
Biggs, B. J. F. & C. Kilroy, 2000. Stream periphyton monitoring manual. NIWA, Christchurch.
Biggs, B. J. F. & G. M. Price, 1987. A survey of filamentous algal proliferations in New Zealand rivers. New Zealand Joural of Marine and Freshwater Research 21: 175–191.
Bonnett, M. L., D. J. Jellyman & J. Sykes, 2008. Impacts of Didymosphenia geminata on the distribution of and abundance of native fish. NIWA Client Report CHC2008-063.
Borcard, D. & P. Legendre, 1994. Environmental control and spatial structure in ecological communities: an example using oribatid mites (Acari, Oribatei). Environmental and Ecological Statistics 1(1): 37–61.
Borcard, D. & P. Legendre, 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling 153: 51–68.
Bothwell, M. L., B. W. Taylor & C. Kilroy, 2014. The Didymo story: the role of low dissolved phosphorus in the formation of Didymosphenia geminata blooms. Diatom research 29: 229–236.
Bray, J., 2014. The invasion ecology of Didymosphenia geminata. A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy University of Canterbury.
Bray, J., J. S. Harding, C. Kilroy, P. Broady & P. Gerbeaux, 2016. Physicochemical predictors of the invasive diatom Didymosphenia geminata at multiple spatial scales in New Zealand rivers. Aquatic Ecology 50: 1–14.
Bray, J., C. Kilroy, P. Gerbeaux & J. S. Harding, 2017a. Ecological eustress? Nutrient supply, bloom stimulation and competition determine dominance of the diatom Didymosphenia geminata. Freshwater Biology 62(8): 1433.
Bray, J., J. O’Brien & J. S. Harding, 2017b. Production of phosphatase and extracellular stalks as adaptations to phosphorus limitation in Didymosphenia geminata (Bacillariophyceae). Hydrobiologia 784(1): 51–63.
Brown, J. H. & A. Kodric-Brown, 1977. Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58: 445–449.
Chapin, S. F., E. S. Zavaleta, V. T. Eviner, R. L. Naylor, P. M. Vitousek, H. L. Reynolds, D. U. Hooper, S. Lavorel, O. E. Sala, S. E. Hobbie, M. C. Mack & S. Díaz, 2000. Consequences of changing biodiversity. Nature 405(6783): 234–242.
Chase, J. M., 2014. Spatial scale resolves the niche versus neutral theory debate. Journal of Vegetation Science 25(2): 319–322.
Chin, W. W., 2001. PLS-graph user’s guide, Version 3.0, February, 2001 edition ©1993–2001. Soft Modeling Inc.
Diamond, J. & T. J. Case, 1986. Community ecology. HarperCollins, Cambridge.
Didham, R. K., J. M. Tylianakis, N. J. Gemmell, T. A. Rand & R. M. Ewers, 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends in Ecology and Evolution 27: 489–496.
Dudley, T. L., S. D. Cooper & N. Hemphill, 1986. Effects of macroalgae on a stream invertebrate community. Journal of the North American Benthological Society 5(2): 93–106.
ESRI, E. S. R. I., 2012. ArcGIS Release 10.1. Redlands.
Falk-Petersen, J., T. Bøhn & O. Sandlund, 2006. On the numerous concepts of invasion biology. Biological Invasions 8: 1409–1424.
Farjalla, V. F., D. S. Srivastava, N. A. C. Marino, F. D. Azevedo, V. Dib, P. M. Lopes, A. S. Rosado, R. L. Bozelli & F. A. Esteves, 2012. Ecological determinism increases with organism size. Ecology 93(7): 1752–1759.
Finlay, B. J., 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063.
Fontaneto, D., 2011. Biogeography of microscopic organisms: is everything small everywhere?. Cambridge University Press, Cambridge.
Gillis, C. A. & I. Lavoie, 2014. A preliminary assessment of the effects of Didymosphenia geminata nuisance growths on the structure and diversity of diatom assemblages of the Restigouche River basin, Quebec, Canada. Diatom Research 29(3): 281–292.
Graham, I. E., 2009. Continent on the move: New Zealand geoscience into the 21st century.
Harding, J., J. Clapcott, J. Quinn, J. Hayes, M. Joy, R. Storey, H. Greig, J. Hay, T. James, M. Beech, R. Ozane, A. Meredith & I. Boothroyd, 2009. Stream habitat assessment protocols for wadeable rivers and streams of New Zealand.
Hillebrand, H., C.-D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calcumation for pelagic and benthic microalgae. Journal of Phycology 35(2): 403–424.
Hubbell, S. P., 2001. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32), Vol. 32. Princeton University Press, Princeton.
Hutchinson, G. E., 1957. Concluding remarks. Cold spring harbor symposium. Quantitative Biology 22: 415–427.
Jellyman, P. G. & J. S. Harding, 2016. Disentangling the stream community impacts of Didymosphenia geminata: how are higher trophic levels affected? Biological Invasions 18(12): 3419–3435.
John, D. M., B. A. Whitton & A. J. Brook, 2002. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae. Cambridge University Press, Cambridge.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69(3): 373–386.
Jowett, I. G. & J. Richardson, 1990. Microhabitat preferences of benthic invertebrates in a New Zealand river and the development of in-stream flow-habitat models for Deleatidium spp. New Zealand Journal of Marine and Freshwater Research 24(1): 19–30.
Kilroy, C. & M. Unwin, 2011. The arrival and spread of the bloom-forming, freshwater diatom, Didymosphenia geminata, New Zealand. Aquatic invasions 6(3): 249–262.
Kilroy, C., B. J. F. Biggs, N. Blair, P. Lambert, B. Jarvie, K. Dey, K. Robinson & D. Smale, 2006. Ecological studies on Didymosphenia geminata. NIWA Client Report: CHC2005-123 September 2005.
Kilroy, C., S. T. Larned & B. J. F. Biggs, 2009. The non-indigenous diatom Didymosphenia geminata alters benthic communities in New Zealand rivers. Freshwater Biology 54(9): 1990–2002.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds.), Süsswasserflora von Mitteleuropa, Band 2/2. VEB Gustav Fischer Verlag, Jena: 596.
Krammer, K. & H. Lange-Bertalot, 1991a. Süßwasserflora von Mitteleuropa. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae.
Krammer, K. & H. Lange-Bertalot, 1991b. Süßwasserflora von Mitteleuropa. Bacillariophyceae 4. Teil: Achnanthaceae.
Krammer, K. & H. Lange-Bertalot, 1995. Süsswasserflora von Mitteleuropa: Bacillariophyceae. 1. Teil: Naviculaceae. Süßwasserflora von Mitteleuropa. Band 2/1: 876, VEB Gustav Fischer Verlag, Jena. Fischer Verlag, Stuttgart.
Larned, S. T., D. Arscott, N. Blair, W. Jarvie, D. Jellyman, K. Lister, M. Schallenberg, S. Sutherland, K. Vopel & R. Wilcock, 2007. Ecological studies of Didymosphenia geminata in New Zealand, 2006–2007. NIWA Client Report: CHC2007-070 For MAF Biosecurity New Zealand:120.
Leathwick, J. R., D. West, P. Gerbeaux, D. Kelly, H. Robertson, D. Brown, W. L. Chadderton & A. G. Ausseil, 2010. Freshwater ecosystems of New Zealand (FENZ) geodatabase. Version one—August 2010 User guide Department of Conservation.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law & D. Tilman, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7(7): 601–613.
Morán-Ordóñez, A., A. Pavlova, A. M. Pinder, L. Sim, P. Sunnucks, R. M. Thompson, J. Davis & J. Diez, 2015. Aquatic communities in arid landscapes: local conditions, dispersal traits and landscape configuration determine local biodiversity. Diversity and Distributions 21(10): 1230–1241.
Nekola, J. C. & P. S. White, 1999. The distance decay of similarity in biogeography and ecology. Journal of Biogeography 26(4): 867–878.
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, M. H. H. Stevens, M. J. Maintainer Jari Oksanen, MASS Suggests, 2008. The vegan package. Community ecology package: 1–255.
Opsahl, R., T. Wellnitz & N. LeRoy Poff, 2003. Current velocity and invertebrate grazing regulate stream algae: results of an in situ electrical exclusion. Hydrobiologia 499(1–3): 135–145.
Parker, I. M., D. Simberloff, W. M. Lonsdale, K. Goodell, M. Wonham, P. M. Kareiva, M. H. Williamson, B. Von Holle, P. B. Moyle, J. E. Byers & L. Goldwasser, 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions 1(1): 3–19.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87(10): 2614–2625.
Pfankuch, D. J., 1975. Stream Reach Inventory and Channel Stability Evaluation. USDA Forest Service, Missoula.
Power, M. E., W. J. Matthews & A. J. Stewart, 1985. Grazing minnows, piscivorous bass, and stream algae: dynamics of a strong interaction. Ecology 66(5): 1448–1456.
R Development Core Team, 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Reid, B. & R. Torres, 2014. Didymosphenia geminata invasion in South America: ecosystem impacts and potential biogeochemical state change in Patagonian rivers. Acta Oecologica 54: 101–109.
Rosindell, J., S. P. Hubbell, F. He, L. J. Harmon & R. S. Etienne, 2012. The case for ecological neutral theory. Trends in Ecology & Evolution 27(4): 203–208.
Rost, A. L., C. H. Fritsen, J. Memmott, C. Davis & E. Wirthlin, 2008. Environmental controls and potential foodweb impacts of Didymosphenia geminata, a comparative ecosystem study. In: Bothwell, M. L. & S. A. Spaulding (eds), Proceedings of the 2007 International Workshop on Didymosphenia geminata. Canadian Technical Report on Fisheries and Aquatic Sciences 2795: 41–44.
RStudio Team, 2015. RStudio: Integrated Development for R. RStudio, Inc., Boston, MA http://www.rstudio.com/.
Sarnelle, O. & R. A. Knapp, 2004. Zooplankton recovery after fish removal: limitations of the egg bank. Limnology and Oceanography 49(4): 1382–1392.
Shearer, K. A., J. Hay & J. W. Hayes, 2007. Interim report: invertebrate drift and trout growth potential in a Didymo (Didymosphenia geminata) affected reach of the Mararoa River. Prepared for Biosecurity New Zealand. Cawthron Report No. 1178, p 34.
Shurin, J. B., K. Cottenie & H. Hillebrand, 2009. Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159(1): 151–159.
Sivarajah, B., J. Kurek, K. M. Ruhland & J. P. Smol, 2015. Effects of Didymosphenia geminata blooms on benthic diatom assemblages in the Restigouche River Watershed, eastern Canada. Botany 95(5): 317–323.
Snelder, T. H. & B. J. F. Biggs, 2002. Multiscale river environment classification for water resources management. JAWRA Journal of the American Water Resources Association 38(5): 1225–1239.
Soininen, J., 2014. A quantitative analysis of species sorting across organisms and ecosystems. Ecology 95(12): 3284–3292.
Soininen, J., R. McDonald & H. Hillebrand, 2007. The distance decay of similarity in ecological communities. Ecography 30(1): 3–12.
Sweetman, J., N. S. Jon, M. R. Kathleen & P. S. John, 2010. Environmental and spatial factors influencing the distribution of cladocerans in lakes across the central Canadian Arctic treeline region. Journal of limnology 69(1): 76–87.
Taylor, B., 2012. Tritrophic effects of nuisance algal blooms on top predators in rivers. Conference proceedings: Ecological Society of America 2012.
Thompson, R. & C. Townsend, 2006. A truce with neutral theory: local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. Journal of Animal Ecology 75(2): 476–484.
Townsend, C. R., 1989. The patch dynamics concept of stream community ecology. Journal of the North American Benthological Society 8(1): 36–50.
Vanormelingen, P., E. Verleyen & W. Vyverman, 2008. The diversity and distribution of diatoms: from cosmopolitanism to narrow endemism. Biodiversity and Conservation 17(2): 393–405.
Vellend, M., 2010. Conceptual synthesis in community ecology. The Quarterly Review of Biology 85(2): 183–206.
Vellend, M., D. S. Srivastava, K. M. Anderson, C. D. Brown, J. E. Jankowski, E. J. Kleynhans, N. J. Kraft, A. D. Letaw, A. A. M. Macdonald & J. E. Maclean, 2014. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 123(12): 1420–1430.
Vincent, C., D. Mouillot, M. Lauret, T. D. Chi, M. Troussellier & C. Aliaume, 2006. Contribution of exotic species, environmental factors and spatial components to the macrophyte assemblages in a Mediterranean lagoon (Thau lagoon, Southern France). Ecological Modelling 193(1–2): 119–131.
Vitousek, P. M., H. A. Mooney, J. Lubchenco & J. M. Melillo, 1997. Human domination of Earth’s ecosystems. Science 277: 494–499.
Winterbourn, M. J., K. L. D. Gregson & C. H. Dolphin, 2000. Guide to the aquatic insects of New Zealand. Bulletin of the Entomological Society of New Zealand No 13.
Acknowledgements
The Department of Conservation, Ministry for Primary Industries, and The Miss E. L. Hellaby Indigenous Grasslands Research Trust helped fund this research. We appreciate Hayley Devlin’s assistance with invertebrate samples, and the support provided by the FERG research group.
Author information
Authors and Affiliations
Contributions
JPB, CK, JSH and PG, designed the study. JPB carried out the study. JPB analysed the data and wrote the manuscript with input from FJB. FJB and JSH provided editorial advice.
Corresponding author
Additional information
Handling editor: David Philip Hamilton
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bray, J.P., Kilroy, C., Gerbeaux, P. et al. Ecological processes mediate the effects of the invasive bloom-forming diatom Didymosphenia geminata on stream algal and invertebrate assemblages. Hydrobiologia 847, 177–190 (2020). https://doi.org/10.1007/s10750-019-04080-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04080-5