Abstract
Although the cyanobacterium Microcystis is colonial during water blooms, isolated Microcystis strains become unicellular in culture. Studies indicate that heterotrophic bacteria can promote Microcystis colonies in culture, but little is known about the underlying mechanism or how widespread it is among bacteria. This study investigated the identity and colony-promoting effects of bacteria isolated from the 2014 Microcystis bloom in Lake Erie. Isolates were classified by their 16S rRNA gene sequences. Toxic and non-toxic cultures of M. aeruginosa were exposed to exudates of bacterial isolates, and their morphology, polysaccharide content, and reflectance spectra were compared to those of M. aeruginosa control cultures. Six isolates belonging to three genera enhanced the frequency or size of M. aeruginosa colonies in cultures where a dialysis barrier prevented direct contact between heterotrophic cells and M. aeruginosa cells. Toxic and non-toxic M. aeruginosa strains differed in how their morphology and optical properties responded to treatment. This study demonstrates that heterotrophic bacteria can promote colonial morphology in Microcystis without making physical contact with the Microcystis cells, as well as the first to indicate that toxic and non-toxic strains of the same morphospecies have different morphological and optical responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial harmful algal blooms (cyanoHABs) pose a threat to water quality and ecosystem health throughout the world (Paerl & Huisman, 2009; O’Neil et al., 2012; Paerl & Paul, 2012; Lürling et al., 2017). One of the most widespread bloom-forming cyanobacteria is Microcystis aeruginosa, characterized by large, buoyant colonies of cells embedded in extracellular polysaccharides (EPS) (Reynolds et al., 1981; Wu & Kong, 2009; Yamamoto et al., 2011; Xu et al., 2013; Zhu et al., 2014). M. aeruginosa typically loses colonial morphology when cultured, becoming predominantly unicellular (Bolch & Blackburn, 1996). Likewise, prior to the start of the bloom season, M. aeruginosa exists in lake water columns as small colonies and unicells (Wu & Kong, 2009; Zhu et al., 2014). Blooms begin to form as colonies increase in size and become more frequent relative to unicells (Yamamoto et al., 2011; Zhu et al., 2014). Larger colonies are more resistant to zooplankton predation (van Gremberghe et al., 2009; Yang & Kong, 2012) and more buoyant, maintaining position near the surface while smaller phytoplankton sink due to gravity or get pushed lower in the water column by wind-mixing (Wu & Kong, 2009; Yamamoto et al., 2011; Zhu et al., 2014; Li et al., 2016).
Multiple factors influence the size and frequency of colonies, including the hepatotoxin microcystin (MC) (Sedmak & Eleršek, 2006; Gan et al., 2012), chemical exudates from zooplankton (Burkert et al., 2001; Ha et al., 2004; Yang et al., 2005, 2008; van Gremberghe et al., 2009; Yang & Kong, 2012), low concentrations of antibiotics (Tan et al., 2018), and contact with heterotrophic bacteria (Shen et al., 2011; Wang et al., 2015). The process by which heterotrophic bacteria impact colony formation is not yet understood. Effects of heterotrophic bacteria on the morphology of Microcystis may be mediated by diffusible chemical signals, as is the case for certain colony-promoting zooplankton (Ha et al., 2004). Bacteria may release exudates such as quorum-sensing molecules (Xue et al., 2015; Passos da Silva et al., 2017; Zhai et al., 2012) or antimicrobial compounds (Tan et al., 2018) which cause cyanobacterial cells to modify their surface properties or mucilage, making them more adhesive. Bacterial exudates may promote colony formation by upregulating EPS production in Microcystis as MC amendments and zooplankton exudates do (Yang et al., 2008; Gan et al., 2012), or by inducing cyanobacterial cells to allocate more proteins to the cell surface or the extracellular mucilage (Tan et al., 2018).
Alternatively, Microcystis colonies might form as a result of highly adhesive heterotrophic cells attaching directly to the surfaces of any cells around them. Some bacteria isolated from Microcystis colonies have strong adhesive properties and form EPS-rich aggregates among themselves in the absence of Microcystis (Zhang et al., 2018). It is possible that these bacteria and the EPS they produce may bind aggregations of Microcystis and other bacteria together, rather than any properties of Microcystis itself or its own extracellular products.
Although previous studies showed that co-cultures of M. aeruginosa with colony-promoting bacteria were enriched in EPS compared to pure cultures of M. aeruginosa, it is not known if the excess EPS was produced by the cyanobacteria, the heterotrophs, or both (Shen et al., 2011; Wang et al., 2015). Co-cultures had a higher ratio of cell-bound EPS to soluble EPS than pure M. aeruginosa cultures (Wang et al., 2015), which could be attributed to the two species synthesizing different polysaccharides or to M. aeruginosa changing the composition of its endogenously produced polysaccharides in response to signals from bacterial neighbors. Furthermore, Wang et al. (2015) observed morphological changes prior to changes in EPS, indicating that EPS differences were not the immediate cause of colony induction.
While it has been shown that Microcystis strains belonging to different morphotypes form colonies in response to different bacterial neighbors (Wang et al., 2015), there is a lack of research comparing the responses of toxic and non-toxic strains belonging to a single morphotype to heterotrophic bacteria under controlled conditions. In laboratory studies, toxic and non-toxic Microcystis cultures have exhibited similar morphological responses to the addition exogenous MC (Sedmak & Eleršek, 2006; Gan et al., 2012). In the environment, non-toxic as well as toxic strains form large colonies, although colonies of toxic strains tend to be somewhat larger than those of non-toxic but otherwise similar strains (Kurmayer et al., 2003). It is possible that this is due to the availability of MC in the environment: toxic Microcystis strains form large colonies as a result of their own endogenously produced toxin, some of which diffuses through the water and induces other strains of Microcystis to form colonies of a size proportional to the presumably lower concentration of MC in their immediate vicinity. However, it is also possible that heterotrophic bacteria in the environment affect the formation of Microcystis colonies by mechanisms independent of MC. Such bacteria may induce large colony formation in non-toxic Microcystis even in the absence of MC-producing cyanobacteria. The reported trend of toxic strains forming larger colonies may result from MC enhancing an effect that colony-promoting bacteria have on all strains of Microcystis, or it may result from the strains having different metabolic responses to whatever bacteria are present.
Understanding how Microcystis morphology is determined is a necessary part of learning how Microcystis blooms begin and how to predict their onset. This is vitally important as changing climate patterns allow blooms to develop in environments from which they were previously absent (Paerl & Huisman, 2009; Paerl & Paul, 2012). Relatively little is known about how other members of the microbial community affect a key adaptive trait of Microcystis that makes the initiation and maintenance of blooms possible.
In this study, bacteria were isolated from the 2014 Microcystis bloom in Lake Erie (Francy et al., 2015) to determine if they enhanced the frequency and size of colonies in cultured M. aeruginosa. It was hypothesized that morphological effects of these bacteria would be mediated by chemical exudates that initiate changes in Microcystis mucilage and/or surface properties and would not depend on physical contact between the cyanobacteria and heterotrophic bacteria. It was further hypothesized that differences between toxic and non-toxic M. aeruginosa strains would be caused by microcystin enhancing the effects of colony-promoting exudates, either by promoting additional EPS production as in Gan et al. (2012) or by directly enhancing intercellular adhesion as in Kehr et al. (2006). Finally, it was hypothesized that any differences between toxic and non-toxic strains which could not be attributed to the aggregation-enhancing properties of microcystin would be associated with different strain-specific metabolic responses to colony-promoting bacteria, which could be observed in differences of reflectance spectra.
Methods
Isolation of bacteria
In August 2014, a toxic cyanobacterial bloom occurred in Maumee Bay (Lake Erie, Ohio, USA) during which Microcystis constituted more than 90% of total near shore cyanobacterial biovolume (Francy et al., 2015). To obtain bacteria associated with this bloom, samples were collected from the upper five centimeters of water in Maumee Bay on August 18, 2014. Cyanobacteria and their associated microorganisms were concentrated by filtering 100 ml samples through nitrocellulose membranes of 47 mm diameter and 3 μm pore size (Millipore, Darmstadt, Germany). Organisms collected on membranes were then resuspended in sterile deionized water to dissolve EPS mucilage (Plude et al., 1991). Aliquots of suspension were poured onto agar plates and spread evenly using sterilized metal spreaders. Three growth media were used: standard methods (plate count) agar, R2A agar (Reasoner & Geldreich, 1985) from Difco Laboratories (Detroit, MI, USA), and 0.9 g/l glucose and 1 g/l casamino acids with 15 g/l agar. Plates were incubated at room temperature for 4 weeks, and colonies were isolated by streak-plating.
16S rRNA gene sequencing of bacteria
To obtain DNA, isolates were incubated overnight at 37°C in nutrient broth. Subsequently, DNA was extracted using an UltraClean Microbial DNA purification kit (MoBio Laboratories, Carlsbad, CA, USA) (La Duc et al., 2009). DNA was amplified with bacterial primer 357f (Muyzer et al., 1993) and universal primer 1391r (Lane et al., 1985) targeting the 16S rRNA gene. PCR consisted of an initial denaturing step at 96°C for 5 min, followed by thirty cycles of 96°C for 50-s, 57°C for 1 min, and 72°C for 1 min, then a final extension at 72°C for 8 min. PCR products were cleaned with an ENZA DNA purification kit (Omega Bio-Tek, Norcross, GA, USA) and sent to the Ohio State University Molecular and Cellular Imaging Center (Wooster, OH, USA) for Sanger sequencing. Sequences were opened in Mega 7 (Kumar et al., 2004, 2016), and used as the template for a BLAST search of the NCBI database (Altschup et al., 1990). Isolate sequences and the closest matching sequences from the database were aligned with Clustal W (Thompson et al., 1994), and a maximum likelihood tree was constructed using 1,000 bootstrap iterations (Tamura et al., 2011). All isolate sequences were deposited in the NCBI database.
Preliminary isolate screening
Isolates were screened for the ability to enhance colony size and frequency in direct co-culture with M. aeruginosa. Two M. aeruginosa strains from the University of Texas Algal Culture Collection were used: toxic UTEX LB 2385 and non-toxic UTEX LB 2386. Bacterial isolates were cultured overnight in nutrient broth at 37°C prior to the experiment. M. aeruginosa was centrifuged at 2500×g for 15 min and rinsed with BG-11 medium three times, then resuspended in fresh BG-11. Each bacterial isolate was centrifuged for 20 min at 4500×g, rinsed with BG-11 medium three times, and added to M. aeruginosa suspension at a final density of 2 × 106 cells/ml for M. aeruginosa and 1.5 × 108 cells/ml for the bacterial isolate. Control cultures of M. aeruginosa contained 2 × 106 cells/ml with no added bacteria. All experiments were performed in triplicate. Cultures were incubated at 27°C under a 12H:12H light:dark cycle.
Microcystis aeruginosa cells were collected after 48 h and stained with Lugol’s iodine for observation. Cells were viewed on wet mount slides at 200× with an Olympus BX53 microscope (Olympus, Center Valley, PA, USA), and ten fields per slide were recorded with an Olympus SC100 digital camera. Colonies and unaggregated Microcystis cells were counted manually. Colony frequency was calculated as the ratio of colonies to unaggregated Microcystis cells. Surface area of each Microcystis colony was measured with MetaMorph version 7.7 (MetaMorph, Inc., Nashville, TN, USA). To determine colony size, equivalent spherical diameter (ESD) was calculated from visible surface area. Any replicate in which no colonies were found was recorded as having a frequency and colony ESD of zero. Any bacterial isolate that caused a significant increase in frequency or size of colonies in M. aeruginosa compared to control cultures was considered a colony-promoting isolate.
Assessment of colony-promoting effects of bacterial exudates
To investigate whether exudates of these bacteria could affect M. aeruginosa morphology in the absence of direct contact, additional experiments were performed. Cells were centrifuged and rinsed as described above. Cyanobacterial cells were resuspended at 2 × 106 cells/ml, and colony-promoting bacteria at 3 × 108 cells/ml. Thirty milliliters of each bacterial isolate suspension were loaded into a Slide-A-Lyzer dialysis cassette (Thermo Scientific, Waltham, MA, USA) with 10,000 Dalton molecular weight cut-off, and each cassette was submerged in M. aeruginosa suspension. Control cultures consisted of M. aeruginosa suspension with no amendment. Cultures were incubated as above for 48 h. All culture experiments were carried out in triplicate. At the end of the experiment, aliquots of M. aeruginosa suspension were stained and observed as described above.
EPS quantification
To separate soluble EPS from cells, aliquots of EPS suspension were centrifuged at 2500×g for 15 min (Xu et al., 2013). Supernatants, containing soluble extracellular polysaccharides (SL-EPS), were transferred to dialysis tubing with a cut-off 3,500 Da (Thermo Fisher Scientific, Waltham, MA). Pellets were resuspended in 5 ml of 0.05% NaCl solution, incubated in a water bath at 60°C for 40 min, and centrifuged at 4500×g for 60 min (Xu et al., 2013). Supernatants, containing bound extracellular polysaccharides (BD-EPS), were transferred to dialysis tubing as above. EPS fractions were dialyzed overnight and quantified by the phenol–sulfuric acid method using galactose as a standard (Dubois et al., 1956).
Microcystin analysis
To quantify microcystin (MC), cultures were filtered through GF/C membranes (Whatman, Maidstone, UK). Filtrates, containing extracellular MC, were purified according to the methods of Lawton et al. (1994). To extract intracellular MC, membranes were submerged for 1 h in methanol (Lawton et al., 1994). Supernatants were decanted, and the process was repeated twice. The methanol was evaporated, and extracts from each subsample were resuspended in methanol and recombined with extracellular MC from the same subsample. After final evaporation, extracts were resuspended in 500 μl methanol per subsample for quantification of MC by HPLC–UV following methods of Shamsollahi et al. (2015) using Shimadzu Prominence HPLC (Shimadzu, Kyoto, Japan) and standards from Cayman Chemical.
Reflectance readings
Spectral reflectance analysis was used to investigate changes in optical properties associated with enhanced colony frequency or size. M. aeruginosa suspension from each culture was filtered through a 47-mm diameter GF/F membrane (Whatman, Maidstone, UK). Filters were dried at 60°C for 24 h prior to freezing at − 80°C and subsequently dried for an additional 2 h at 60°C. For each filter, percent reflectance was measured for wavelengths 360–730 nm with a CM-2600d spectrophotometer (Konica Minolta, Tokyo, Japan), with an uncontaminated GF/F membrane as a blank. The data were denoised by taking first-order derivatives of blank-corrected reflectance readings at ten nm wavelength increments between 400 and 700 nm.
Statistical analysis
Statistical analyses were performed in R 3.6.1 (R Core Team,2016), augmented with the following packages: readr (Wickham et al., 2016), dunn.test (Dinno, 2017), StatMatch (D’Orazio, 2019), Hotelling (Curran, 2018). Because the culture experiments were performed in triplicate, the measured values for each dependent variable were averaged across the three replicates. Morphological and EPS data were tested for conformity to the assumptions of parametric ANOVA. Because Shapiro–Wilk testing (Shapiro & Wilk, 1965) confirmed that the dependent variables were non-normally distributed, the Kruskal–Wallis test was used as a non-parametric alternative to ANOVA. Data were ranked without regard to group assignment, and a test statistic based on ranks was compared to a χ2 probability distribution (Kruskal & Wallis, 1952). When treatment differences were detected at α < 0.05, Dunn’s post hoc test was used to analyze differences between individual treatment and control groups (Dunn, 1961). To explore correlations between morphology and EPS measurements, the strength of each relationship was summarized by an adjusted R-squared statistic and its significance was determined by F-test.
The strain-specific spectral response was calculated as the Mahalanobis distance between the average reflectance spectrum of three control cultures belonging to a single M. aeruginosa strain and the average reflectance spectrum of three cultures of the same strain under a particular bacterial isolate treatment. Mahalanobis distance was considered a suitable measure of dissimilarity between spectra because it accounts for the fact that reflectance readings at different wavelengths within the visible spectrum may be correlated (Mahalanobis, 1936; De Maesschalck et al., 2000). For each bacterial treatment, Hotelling’s T2 test (Hotelling, 1951) was then used to compare the strain-specific spectral responses of toxic and non-toxic M. aeruginosa.
Results
Thirty-two bacterial isolates were cultured and screened for colony-promoting effects. Six isolates induced significantly greater colony frequency and/or colony size in at least one M. aeruginosa strain (Table 1). Two of the colony-promoting isolates were affiliated with Gammaproteobacteria genus Pseudomonas, three with Firmicutes genus Exiguobacterium, and the last with Bacillus, a Firmicutes genus closely related to Exiguobacterium (Fig. 1). However, other isolates obtained also belonged to these genera yet did not have significant effects on the morphology of M. aeruginosa.
Phylogeny of colony-promoting isolates and selected other bacteria. Phylogenetic tree of heterotrophic bacterial isolates from the 2014 Microcystis bloom in Maumee Bay and their closest matches in the GenBank, based on 16S rDNA sequences, with bootstrap confidence levels greater than 50% are indicated at internodes. Asterisks indicate isolates that promoted increased size or frequency of colonies under standard laboratory conditions
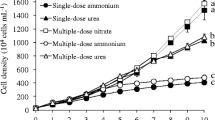
Subsequently, colony-promoting isolates were used in experiments to determine if exudates could promote colony formation without physical contact between the isolated bacterium and M. aeruginosa. Kruskal–Wallis revealed significant differences in colony frequency among bacterial exudate treatments (χ2 = 30.409, P = 0.004). Post hoc tests showed that three isolates (PI-2, PI-6, and EI-23) enhanced the frequency of toxic M. aeruginosa colonies relative to controls (Table 1, Fig. 2A). PI-6 demonstrated a similar effect on the frequency of non-toxic M. aeruginosa colonies, while EI-23 exudates had a marginally significant effect. In contrast, colonies occurred in non-toxic cultures treated with PI-2 at lower frequency than in control cultures.
Different bacterial treatments were associated with differences in mean colony size (χ2 = 28.615, P = 0.007) and maximum colony size (χ2 = 27.844, P = 0.001). Investigation of pairwise differences revealed that exudates from four bacterial isolates (PI-2, EI-12, EI-15, and BI-3) enhanced average colony size in non-toxic M. aeruginosa only (Table 1, Fig. 2B). The same four isolates all promoted greater maximum colony size in non-toxic M. aeruginosa compared to non-toxic controls, while only one isolate, PI-2, was associated with increased maximum colony size for toxic M. aeruginosa (Table 1, Fig. 2C).
Results suggest a tradeoff between colony frequency and colony size. In control cultures, toxic M. aeruginosa formed larger colonies than non-toxic M. aeruginosa, while colonies occurred more frequently in cultures of the non-toxic strain compared to the toxic strain (Fig. 2). Each treatment induced non-toxic M. aeruginosa to form colonies of either greater size or increased frequency compared to non-toxic controls, never both (Table 1, Fig. 2). Of three treatments that affected morphology of toxic M. aeruginosa, two promoted increased colony frequency compared to controls but smaller average colony size (Table 1, Fig. 2). There was a weak but statistically significant negative correlation between colony frequency and colony size across the entire dataset (Fig. 3A).
Correlations of colony frequency with colony size and EPS. Scatter plots of mean colony size (A Adjusted R2 = 0.078, F = 4.48, P = 0.04) and normalized EPS concentration (B, Adjusted R2 = 0.19, F = 10.59, P = 0.002) against colony frequency, where lower values indicate more unicells and higher values indicate more colonies
The amount of EPS normalized to estimated number of Microcystis cells differed among treatments (χ2 = 30.351, P = 0.004), largely due to non-toxic control cultures having higher EPS per cell than cultures treated with PI-2, BI-3, EI-12, or EI-15. Over the entire dataset, normalized EPS was positively correlated with colony frequency (Fig. 3B). When strains of M. aeruginosa were considered separately, the correlation was stronger among toxic cultures (Adjusted R2 = 0.391, F = 13.82, P = 0.002), whereas there was no significant correlation between frequency and normalized EPS values for non-toxic cultures. This indicates that the polysaccharide component of the mucilage may be important to colonial morphology in the toxic strain but not in the non-toxic strain. HPLC–UV analysis did not detect any MC, so different responses of toxic and non-toxic M. aeruginosa strains cannot be attributed to the presence of MC in toxic cultures at the time of the experiment.
Growth rates of M. aeruginosa cultures also varied among treatments (χ2 = 31.215, P = 0.003). Non-toxic M. aeruginosa cultures exposed to PI-2, EI-15, and BI-3 grew at significantly higher rates than did control cultures, whereas growth rates for toxic cultures exposed to these and other treatments were similar to growth rates of control cultures (Table 2). Furthermore, both M. aeruginosa strains exhibited higher growth rates in response to PI-2, EI-15, or BI-3 than in response to PI-6 or EI-23, the two isolates which enhanced the frequency of colonies with no effect on size.
Reflectance data showed that, even though the toxic M. aeruginosa strain had not produced detectable amounts of microcystin over the course of this experiment, its metabolic response to each treatment differed greatly from that of non-toxic M. aeruginosa (Table 3). Each of the six bacterial isolates had opposite or nearly opposite effects on the two M. aeruginosa strains over most of the visible spectrum (Fig. 4). At wavelengths where toxic M. aeruginosa under experimental treatment exhibited higher reflectance than the toxic control cultures, non-toxic M. aeruginosa exposed to the same treatment had lower reflectance relative to non-toxic controls, and vice versa.
Discussion
Six bacterial isolates from a cyanoHAB community in Lake Erie enhanced colony size or frequency in at least one strain of M. aeruginosa. Colony-promoting isolates were affiliated with Gammaproteobacteria genus Pseudomonas or with Firmicutes genera Bacillus and Exiguobacterium. Previously, Wang et al. (2015) isolated colony-promoting Bacillus cereus and Exiguobacterium acetylium from lakes in China. In this context, it is interesting that the colony-promoting bacteria isolated from Lake Erie included B. cereus and Exiguobacterium, a genus closely related to Bacillus (Wang & Sun, 2009). Other colony-promoting bacteria identified by Wang et al. (2015) were classified as Gammaproteobacteria, as were the remaining colony-promoting isolates in this study.
Microcystis colonies observed by Wang et al. (2015) and Shen et al. (2011) may have been driven not by changes in the Microcystis cells but by their contact with highly adhesive co-cultured bacteria. Some heterotrophic bacteria that attach to Microcystis in the environment exhibit strong adhesive properties and a tendency to autoaggregate, leading some authors to speculate that they may play a role in holding together the large colonial assemblages found in blooms (Zhang et al., 2018). However, those bacteria were not tested for the ability to restore colonial morphology to any predominantly unicellular strains of Microcystis. The bacteria that demonstrated autoaggregation belonged to a wider variety of phyla and classes than the known colony-promoting isolates, representing Actinobacteria, Bacteroidetes, and Alphaproteobacteria in addition to Firmicutes and Gammaproteobacteria (Zhang et al., 2018). Although none of the autoaggregating bacteria were affiliated with the same genera as the known colony-promoters, one of the bacteria with the strongest propensity for autoaggregation and general adhesiveness was Staphylococcus caprae, belonging to Bacillales, the same order as Bacillus and Exiguobacterium.
Our study shows that exudates from heterotrophic bacteria enhance colony formation in Microcystis even in the absence of physical contact between cyanobacterial and heterotrophic cells. Genera that exhibited colony-promoting capabilities are known to exude a variety of secondary metabolites, including antimicrobial compounds (Raaijmakers et al., 2002; Haas & Défago, 2005; Molohon et al., 2011; Pathak et al., 2013) and quorum-sensing molecules (Winson et al., 1995; Jones & Blaser, 2003; Lombardía et al., 2006; Biswa & Doble, 2013; Lee & Zhang, 2015; Fernandes et al., 2018). Some antimicrobials enhance aggregation of Microcystis cells when added to cultures in low doses (Tan et al., 2018). Quorum-sensing molecules such as acyl homoserine lactones (AHLs) produced by some Pseudomonas (Juhas et al., 2005; Case et al., 2008) and Exiguobacterium (Biswa & Doble, 2013) and autoinducer 2 (AI-2) produced by B. cereus (Auger et al., 2006; Fernandes et al., 2018) take part in regulating EPS production and/or cell aggregation (Keller & Surette, 2006; Rickard et al., 2006; Ryan & Dow, 2008; Zhai et al., 2012; Xue et al., 2015). The above types of molecules can diffuse through a liquid medium to be taken up by receptive cells that need not have direct contact with the cells that synthesized the molecules. In this study, the dialysis barrier that separated M. aeruginosa from heterotrophic bacteria ensured that the diffusion of cellular exudates was the only means by which the heterotrophs could have influenced morphology of the cyanobacteria. Further research is needed to identify the colony-promoting compounds and the concentrations at which they induce changes in Microcystis morphology. This will advance understanding not only of the mechanisms by which Microcystis strains form and maintain colonies but also of how likely environmental concentrations of colony-promoting exudates and environmental abundances of the bacteria that produce them are to induce ecologically significant changes to Microcystis populations with which they have contact.
Colony size is important to Microcystis success. Colonies that are large (80–100 μm) can escape consumption by zooplankton grazers (Jarvis et al., 1987) and zebra mussels (White et al., 2011; White & Sarnelle, 2014). Larger colonies tend to be more resistant to sinking than smaller ones, and colonies with a diameter of at least 100 μm maintain or quickly return to position near the surface when wind-mixing carries smaller colonies deeper into the water column (Wu & Kong, 2009; Zhu et al., 2014). In this study, colony size of non-toxic M. aeruginosa increased from approximately 30 to 100 μm within 48 h of exposure to PI-2 and EI-15, suggesting that inter-relationships with bacteria may impact bloom formation. Because Microcystis blooms are initiated by rapid accumulation of colonies near the water surface (Wu & Kong, 2009; Yamamoto et al., 2011; Zhu et al., 2014) and facilitated by consumers that reject Microcystis while feeding on its phytoplankton competitors (Vanderploeg et al., 2001; Wang et al., 2010; Leitão et al., 2018), these bacteria may contribute to the ability of a Microcystis strain to initiate a bloom.
Some isolates had notably different effects on two M. aeruginosa strains, which may have wider ecological implications. For example, exudates of EI-15 induced non-toxic M. aeruginosa to form larger colonies than toxic M. aeruginosa (Fig. 4), a difference that could make the non-toxic strain less vulnerable than its toxic conspecific to light limitation (Wu & Kong, 2009; Yamamoto et al., 2011; Zhu et al., 2014) and consumption (Jarvis et al., 1987; White et al., 2011; White & Sarnelle, 2014). Toxic and non-toxic Microcystis typically co-occur in the environment and MC concentration is often correlated with the ratio of toxic to total Microcystis (Kurmayer et al., 2003; Kardinaal, 2007; Davis et al., 2009; Joung et al., 2011). The morphological responses observed in this study imply that interactions with heterotrophic bacteria may influence which Microcystis strains predominate within a bloom.
The prediction that colony-promoting bacteria would promote increased production of EPS was only tentatively supported for toxic M. aeruginosa and not at all supported for non-toxic M. aeruginosa. For toxic M. aeruginosa cultures, colony frequency was correlated with EPS concentration, but treatment differences in EPS concentration were not statistically significant. For non-toxic M. aeruginosa, colony frequency was unrelated to EPS, while colony size was greater in cultures that contained less EPS per cell than was found in non-toxic controls. Therefore, the mechanism by which the bacterial isolates in this study promoted the formation and growth of non-toxic M. aeruginosa colonies must not depend upon the adhesive properties of EPS. Variations in reflectance readings indicate that exudates of each bacterial isolate were associated with strain-specific differences in the production or breakdown of cellular metabolites of M. aeruginosa. Perhaps colony-promoting bacteria induced non-toxic M. aeruginosa cells to increase their adhesive potential with cell surface proteins (Kehr et al., 2006; Zilliges et al., 2008). Alternatively, chemical signals from these bacteria may have induced M. aeruginosa to exude more extracellular proteins, thus increasing the adhesiveness of the mucilage without increasing polysaccharide content (Tan et al., 2018).
Conclusions
Six bacterial isolates from a Microcystis bloom in Lake Erie enhanced frequency or size of colonies in toxic and/or non-toxic strains of Microcystis aeruginosa. Four of the isolates induced non-toxic M. aeruginosa to produce colonies sufficiently large to escape from predation and wind-mixing. This demonstrates that bacteria which occur in association with Microcystis blooms can influence the physical characteristics of Microcystis strains in ways that potentially contribute to the initiation and maintenance of blooms or to succession of strains within a bloom. Furthermore, bacteria were able to affect the morphology of M. aeruginosa in the absence of direct contact with M. aeruginosa cells, supporting the hypothesis that heterotrophic bacteria influence Microcystis morphology via a mechanism mediated by diffusible chemical signals.
However, the hypothesis that colony-promoting bacteria would enhance both frequency and size of colonies by inducing any strain of M. aeruginosa to increase production of extracellular polysaccharides was not supported. Different responses of toxic and non-toxic M. aeruginosa strains, low EPS concentrations in cultures that exhibited increased colony size, and an overall negative correlation between colony frequency and colony size all indicate that the mechanisms by which heterotrophic bacteria promote Microcystis colony formation are complex and diverse. Morphological responses of the non-toxic strain to colony-promoting bacteria were independent of the adhesive properties of extracellular polysaccharides. Additional study is needed to clarify the mechanisms by which heterotrophic bacteria contribute to Microcystis colony formation and to explore how these interactions play out in complex communities.
References
Altschup, S. F., W. Gish, W. Miller, E. W. Myers & D. J. Lipman, 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410.
Auger, S., E. Krin, S. Aymerich & M. Gohar, 2006. Autoinducer 2 affects biofilm formation by Bacillus cereus. Applied and Environmental Microbiology 72: 937–941.
Biswa, P. & M. Doble, 2013. Production of acylated homoserine lactone by Gram-positive bacteria isolated from marine water. FEMS Microbiology Letters 343: 34–41.
Bolch, C. J. S. & S. I. Blackburn, 1996. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kutz. Journal of Applied Phycology 8: 5–13.
Burkert, U., P. Hyenstrand, S. Drakare & P. Blomqvist, 2001. Effects of the mixotrophic flagellate Ochromonas sp. on colony formation in Microcystis aeruginosa. Aquatic Ecology 35: 11–17.
Case, R. J., M. Labbate & S. Kjelleberg, 2008. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME Journal 2: 345.
Curran, J., 2018. Hotelling: hotelling’s T^2 test and variants. R package version 1.0-5. https://CRAN.R-project.org/package=Hotelling.
Davis, T. W., D. L. Berry, G. L. Boyer & C. J. Gobler, 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8: 715–725.
De Maesschalck, R., D. Jouan-Rimbaud & D. L. Massart, 2000. The mahalanobis distance. Chemometrics and Intelligent Laboratory Systems 50: 1–18.
Dinno, A., 2017. dunn.test: Dunn’s test of multiple comparisons using rank sums. R package version 1.3.5. https://CRAN.R-project.org/package=dunn.test.
D’Orazio, M., 2019. StatMatch: statistical matching or data fusion. R package version 1.3.0. https://CRAN.R-project.org/package=StatMatch.
Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Roberts & F. Smith, 1956. Phenol sulphuric acid method for carbohydrate determination. Annals of Chemistry 28: 350–359.
Dunn, O. J., 1961. Multiple comparisons among means. Journal of the American Statistical Association 56: 52–64.
Fernandes, M. D. S., L. M. R. Esper, D. Y. Kabuki & A. Y. Kuaye, 2018. Quorum sensing in Enterococcus faecium, Enterococcus faecalis and Bacillus cereus strains isolated from ricotta processing. Ciênc Rural. https://doi.org/10.1590/0103-8478cr20161111.
Francy, D. S., J. L. Graham, E. A. Stelzer, C. D. Ecker, A. M. G. Brady, P. Struffolino & K. A. Loftin, 2015. Water quality, cyanobacteria, and environmental factors and their relations to microcystin concentrations for use in predictive models at Ohio Lake Erie and inland lake recreational sites, 2013–14: U.S. Geological Survey Scientific Investigations Report 2015–5120, 58 p. http://dx.doi.org/10.3133/sir20155120.
Gan, N., Y. Xiao, L. Zhu, Z. Wu, J. Liu, C. Hu & L. Song, 2012. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environmental Microbiology 14: 730–742.
Gantt, E., 1975. Phycobilisomes: light-harvesting pigment complexes. Bioscience 25: 781–788.
Ha, K., M. H. Jang & N. Takamura, 2004. Colony formation in planktonic algae induced by zooplankton culture media filtrate. Journal of Freshwater Ecology 19: 9–16.
Haas, D. & G. Défago, 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology 3: 307.
Hotelling, H., 1951. A generalized T test and measure of multivariate dispersion. In Proceedings of the Second Berkeley Symposium on Mathematical Statistics and Probability. The Regents of the University of California.
Jarvis, A. C., R. C. Hart & S. Combrink, 1987. Zooplankton feeding on size fractionated Microcystis colonies and Chlorella in a hypertrophic lake (Hartbeespoort Dam, South Africa): implications to resource utilization and zooplankton succession. Journal of Plankton Research 9: 1231–1249.
Johnson, S. G., 2016. The NLopt nonlinear-optimization package, http://abinitio.mit.edu/nlopt.
Jones, M. B. & M. J. Blaser, 2003. Detection of a luxS-signaling molecule in Bacillus anthracis. Infection and Immunity 71: 3914–3919.
Joung, S. H., H. M. Oh, S. R. Ko & C. Y. Ahn, 2011. Correlations between environmental factors and toxic and non-toxic Microcystis dynamics during bloom in Daechung Reservoir, Korea. Harmful Algae 10: 188–193.
Juhas, M., L. Eberl & B. Tümmler, 2005. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environmental Microbiology 7: 459–471.
Kardinaal, W. E. A., I. Janse, M. P. Kamst-van Agterveld, M. Meima, J. Snoek, L. R. Mur, J. Huisman, G. Zwart & P. M. Visser, 2007. Microcystis genotype succession in relation to microcystin concentrations in freshwater lakes. Aquatic Microbial Ecology 48: 1–12.
Kehr, J. C., Y. Zilliges, A. Springer, M. D. Disney, D. D. Ratner, C. Bouchier, P. H. Seeberger, N. Tandeau de Marsac & E. Dittmann, 2006. A mannan binding lectin is involved in cell–cell attachment in a toxic strain of Microcystis aeruginosa. Molecular Microbiology 9: 893–906.
Keller, L. & M. G. Surette, 2006. Communication in bacteria: an ecological and evolutionary perspective. Nature Reviews Microbiology 4: 249.
Kruskal, W. H. & W. A. Wallis, 1952. Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association 47: 583–621.
Kumar, S., K. Tamura & M. Nei, 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefs in Bioinformatics 5: 150–163.
Kumar, S., G. Stecher & K. Tamura, 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874.
Kurmayer, R., G. Christiansen & I. Chorus, 2003. The abundance of microcystin-producing genotypes correlates positively with colony size in Microcystis sp. and determines its microcystin net production in Lake Wannsee. Applied and Environmental Microbiology 69: 787–795.
La Duc, M. T., S. Osman, P. Vaishampayan, Y. Piceno, G. Andersen, J. A. Spry & K. Venkateswaran, 2009. Comprehensive census of bacteria in clean rooms by using DNA microarray and cloning methods. Applied and Environmental Microbiology 75: 6559–6567.
Lane, D. J., B. Pace, G. J. Olsen, D. A. Stahl, M. L. Sogin & N. R. Pace, 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proceedings of the National Academy of Sciences 82: 6955–6959.
Lawton, L. A., C. Edwards & G. A. Codd, 1994. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 119: 1525–1530.
Lee, J., & L. Zhang, 2015. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein and Cell 6: 26–41.
Leitão, E., K. A. Ger & R. Panosso, 2018. Selective grazing by a tropical copepod (Notodiaptomus iheringi) facilitates Microcystis dominance. Frontiers in Microbiology 9: 301.
Li, M., W. Zhu, L. Guo, J. Hu, H. Chen & M. Xiao, 2016. To increase size or decrease density? Different Microcystis species has different choice to form blooms. Scientific Reports 6: 37056.
Lombardía, E., A. J. Rovetto, A. L. Arabolaza & R. R. Grau, 2006. A LuxS-dependent cell-to-cell language regulates social behavior and development in Bacillus subtilis. Journal of Bacteriology 188: 4442–4452.
Lürling, M., F. Van Oosterhout & E. Faassen, 2017. Eutrophication and warming boost cyanobacterial biomass and microcystins. Toxins 9: 64.
Mahalanobis, P. C., 1936. On the generalised distance in statistics. Proceedings of the National Institute of Science of India 12: 49–55.
Molohon, K. J., J. O. Melby, J. Lee, B. S. Evans, K. L. Dunbar, S. B. Bumpus, N. L. Kelleher & D. A. Mitchell, 2011. Structure determination and interception of biosynthetic intermediates for the plantazolicin class of highly discriminating antibiotics. ACS Chemical Biolology 6: 1307–1313.
Muyzer, G., E. C. De Waal & A. G. Uitterlinden, 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology 59: 695–700.
O’Neil, J. M., T. W. Davis, M. A. Burford & C. J. Gobler, 2012. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14: 313–334.
Paerl, H. W. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports 1: 27–37.
Paerl, H. W. & V. J. Paul, 2012. Climate change: links to global expansion of harmful cyanobacteria. Water Research 46: 1349–1363.
Passos da Silva, D., M. C. Schofield, M. R. Parsek & B. S. Tseng, 2017. An update on the sociomicrobiology of quorum sensing in gram-negative biofilm development. Pathogens 6: 51.
Pathak, R., R. Kumar & H. K. Gautam, 2013. Cross-species induction and enhancement of antimicrobial properties in response to gamma irradiation in Exiguobacterium sp. HKG 126. Indian Journal of Microbiology 53: 130–136.
Plude, J. L., D. L. Parker, O. J. Schommer, R. J. Timmerman, S. A. Hagstrom, J. M. Joers & R. Hnasko, 1991. Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flos-aquae C3-40. Applied and Environmental Microbiology 57: 1696–1700.
R Core Team, 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Raaijmakers, J. M., M. Vlami & J. T. De Souza, 2002. Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 81: 537.
Reasoner, D. J. & E. E. Geldreich, 1985. A new medium for the enumeration and subculture of bacteria from potable water. Applied and Environmental Microbiology 49: 1–7.
Reynolds, C. S., G. H. M. Jaworski, H. A. Cmiech & G. F. Leedale, 1981. On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz. emend. Elenkin. Philosophical Transactions of the Royal Society of London Series B 293: 419–477.
Rickard, A. H., R. J. Paler, D. S. Blehert, S. R. Campagna, M. F. Semmelhack, P. G. Egland, B. L. Bassler & P. E. Kolenbrander, 2006. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Molecular Microbiology 60: 1446–1456.
Ryan, R. P. & J. M. Dow, 2008. Diffusible signals and interspecies communication in bacteria. Microbiology 154: 1845–1858.
Sedmak, B. & T. Eleršek, 2006. Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microbial Ecology 51: 508–515.
Shamsollahi, H. R., M. Alimohammadi, R. Nabizadeh, S. Nazmara, & A. H. Mahvi, 2015. Measurement of microcystin-LR in water samples using improved HPLC method. Global Journal of Health Science 7: 66
Shapiro, S. S. & M. B. Wilk, 1965. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611.
Shen, H., Y. Niu, P. Xie, M. I. N. Tao & X. I. Yang, 2011. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshwater Biology 56: 1065–1080.
Tamura, K., D. Peterson, N. Peterson, G. Stecher, M. Nei & S. Kumar, 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739.
Tan, L. R., P. F. Xia, R. J. Zeng, Q. Li, X. F. Sun & S. G. Wang, 2018. Low-level concentrations of aminoglycoside antibiotics induce the aggregation of cyanobacteria. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-018-1894-5.
Thompson, J. D., D. G. Higgins & T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680.
Van Gremberghe, I., P. Vanormelingen, B. Vanelslander, K. Van der Gucht, S. D’hondt, L. De Meester & W. Vyverman, 2009. Genotype-dependent interactions among sympatric Microcystis strains mediated by Daphnia grazing. Oikos 118: 1647–1658.
Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel & T. F. Nalepa, 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Science 58: 1208–1221.
Wang, W. & M. Sun, 2009. Phylogenetic relationships between Bacillus species and related genera inferred from 16 s rDNA sequences. Brazilian Journal of Microbiology 40: 505–521.
Wang, X., B. Qin, G. Gao & H. W. Paerl, 2010. Nutrient enrichment and selective predation by zooplankton promote Microcystis (Cyanobacteria) bloom formation. Journal of Plankton Research 32: 457–470.
Wang, W., H. Shen, P. Shi, J. Chen, L. Ni & P. Xie, 2015. Experimental evidence for the role of heterotrophic bacteria in the formation of Microcystis colonies. Journal of Applied Phycolog. https://doi.org/10.1007/s10811-015-0659-5.
White, J. D. & O. Sarnelle, 2014. Size-structured vulnerability of the colonial cyanobacterium, Microcystis aeruginosa, to grazing by zebra mussels (Dreissena polymorpha). Freshwater Biology 59: 514–525.
White, J. D., R. B. Kaul, L. B. Knoll, A. E. Wilson & O. Sarnelle, 2011. Large variation in vulnerability to grazing within a population of the colonial phytoplankter, Microcystis aeruginosa. Limnology and Oceanography 56: 1714–1724.
Wickham, H., J. Hester & R. Francois, 2016. readr: Read Tabular Data. R package version 1.0.0. https://CRAN.R-project.org/package=readr.
Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. C. Salmonds, B. W. Bycroft, A. Lazdunski, G. S. A. B. Stewart & P. Williams, 1995. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences 92: 9427–9431.
Wu, X. & F. Kong, 2009. Effects of light and wind speed on the vertical distribution of Microcystis aeruginosa colonies of different sizes during a summer bloom. International Review of Hydrobiology 94: 258–266.
Xu, H., G. Yu & H. Jiang, 2013. Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 93: 75–81.
Xue, T., J. Ni, F. Shang, X. Chen & M. Zhang, 2015. Autoinducer-2 increases biofilm formation via an ica-and bhp-dependent manner in Staphylococcus epidermidis RP62A. Microbes and Infection 17: 345–352.
Yamamoto, Y., F. K. Shiah & Y. L. Chen, 2011. Importance of large colony formation in bloom-forming cyanobacteria to dominate in eutrophic ponds. Annals de Limnologie-International Journal of Limnology 7: 167–173.
Yang, Z. & F. Kong, 2012. Formation of large colonies: a defense mechanism of Microcystis aeruginosa under continuous grazing pressure by flagellate Ochromonas sp. Journal of Limnology 71: 5.
Yang, Z., F. X. Kong, H. S. Cao & X. L. Shi, 2005. Observation on colony formation of Microcystis aeruginosa induced by filtered lake water under laboratory conditions. Annals de Limnologie-International Journal of Limnology 41: 169–173.
Yang, Z., F. Kong, X. Shi, M. Zhang, P. Xing & H. Cao, 2008. Changes in the morphology and polysaccharide content of Microcystis aeruginosa (cyanobacteria) during flagellate grazing. Journal of Phycology 44: 716–720.
Zhai, C., P. Zhang, F. Shen, C. Zhou & C. Liu, 2012. Does Microcystis aeruginosa have quorum sensing? FEMS Microbiology Letters 336: 38–44.
Zhang, P., M. Chen, Y. Zhang, Y. Li, S. Lu & P. Li, 2018. Autoaggregation and adhesion abilities in bacteria associated with colonies of Microcystis. Hydrobiologia 832: 205–216.
Zhu, W., M. Li, Y. Luo, X. Dai, L. Guo, M. Xiao, J. Huang & X. Tan, 2014. Vertical distribution of Microcystis colony size in Lake Taihu: its role in algal blooms. Journal of Great Lakes Research 40: 949–955.
Zilliges, Y., J. C. Kehr, S. Mikkat, C. Bouchier, N. T. de Marsac, T. Börner & E. Dittmann, 2008. An extracellular glycoprotein is implicated in cell-cell contacts in the toxic cyanobacterium Microcystis aeruginosa PCC 7806. Journal of Bacteriology 190: 2871–2879.
Funding
This research was funded in part by the Art and Margaret Herrick Memorial Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Stefano Amalfitano
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akins, L., Ortiz, J. & Leff, L.G. Strain-specific responses of toxic and non-toxic Microcystis aeruginosa to exudates of heterotrophic bacteria. Hydrobiologia 847, 75–89 (2020). https://doi.org/10.1007/s10750-019-04073-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04073-4