Abstract
Eutrophication assessment is made widely using Carlson Trophic State indices (TSI) [e.g. secchi disc depth (TSISD)] or phytoplankton biomass. Recently, two Carlson type indices using rotifers (TSIROT) and crustaceans (TSICR) were developed from Polish lakes. In the present study, both indices were applied to zooplankton communities from 16 Greek lakes, covering the entire trophic state spectrum, in order to test their application in a different climatic zone, the Mediterranean. The evaluation of the indices (TSIROT and TSICR) was made comparing the trophic state of each sampling/lake based on TSISD and mean summer phytoplankton biomass. Both indices increased across the eutrophication gradient but misclassify the trophic state. We propose a new index, TSIZOO, the average of the formulae TSIROT and TSICR which are significantly correlated with the eutrophication proxies. All three zooplanktonic indices can efficiently detect low (oligotrophic–mesotrophic) and high (eutrophic–hypertrophic) trophic state using the boundaries < 45 for TSIROT and TSIZOO and < 50 for TSICR. All zooplanktonic indices are promising and effective tools for monitoring and assessment of eutrophication of Mediterranean lakes when mean values are used. Still, TSIZOO should be preferred as the best index that correlated with eutrophication which had the best estimations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increase of human population and the expansion of agricultural and industrial activities have led to anthropogenic eutrophication and deterioration of the health of freshwater systems worldwide (Callisto et al., 2014). One of the first and most widely used classification schemes developed for lake trophic state assessment is Carlson Trophic State Index (TSI) (Carlson, 1977) based on Secchi depth (SD), total phosphorus (TP) and the concentration of the phytoplankton pigment chlorophyll a (chl a). SD is an easy and low-cost variable of measuring water transparency, thus assessing lake water quality; still, it is influenced both by phytoplankton abundance and non-algal particulate matter (Carlson, 1977). Phytoplankton is considered one of the most appropriate elements for lake trophic state assessment due to its short generation times and direct responses to changing nutrient conditions (both total phosphorus and nitrogen) (Lyche-Solheim et al., 2013). Chl a as a proxy of phytoplankton biomass has been widely adopted in water quality assessments of national (e.g. Carlson, 1977; Wolfram et al., 2009), European (EC, 2008) and worldwide (OECD, 1982) lake monitoring and classification schemes. Nevertheless, its use as an indicator of biomass is questionable (Kruskopf & Flynn, 2006) because chl a concentration can vary considerably depending on algal composition, their physiological state and to a lesser extent on light (Reynolds, 1984; Moustaka-Gouni, 1989). Algal biomass estimation, being though a more laborious procedure that requires direct counts and measurements, is the most accurate and fundamental measurement of phytoplankton, the critical metric related to eutrophication and water quality (e.g. Katsiapi et al., 2016). Consequently, phytoplankton metrics can be successfully used in lake monitoring and trophic state assessments especially when eutrophication pressure is considered (Katsiapi et al., 2016). However, there are cases, as for example shallow macrophyte-dominated lakes, where top–down control by zooplankton grazers may limit phytoplankton biomass (Jeppesen et al., 1997), where other components of the lake food web should also be considered critical. Moreover, since both the structure and the function of the food web changes across the eutrophication gradient (Havens, 2014), there is a need for a holistic approach towards this direction. Even though zooplankton has been neglected as a biological quality element (BQE) in the Water Framework Directive 2000/60/EE, the Common Implementation Strategy (Guidance document 23) indicates “Zooplankton grazing (top–down control) which may be influenced by other anthropogenic activities” as an additional lake specific—supporting environmental factor featuring the eutrophication impact; zooplankton has also been included in the checklist for a holistic lake assessment (CIS, 2009). Recently, two Carlson type indices have been developed using zooplankton data from Polish lakes, TSIROT based on rotifer communities (Ejsmont-Karabin, 2012) and TSICR based on crustacean (cladocerans and copepods) communities (Ejsmont-Karabin & Karabin, 2013).
Zooplankton communities can respond quickly to changes resulting by trophic cascades either through bottom–up or top–down control (Carpenter et al., 1985). Thus, they have been used not only in trophic state assessment (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin 2013) but also in aquatic ecotoxicology (e.g. Sarma & Nandini, 2006; Snell & Joaquim-Justo, 2007; Kulkarni et al., 2013), and in providing information about water quality (Azémar et al., 2010; Haberman & Haldna, 2014). Crustaceans have even been included in recently developed multimetric indices for ecological water quality assessment (Moss et al., 2003; Kane et al., 2009). Regarding trophic state assessment, studies such as Pejler (1983) and Karabin (1985) have shown that zooplankton communities tend to have a higher abundance and biomass and increased contributions of bacterivorous rotifers and cyclopoids following an increase in trophic state. Moreover, some taxa begin to dominate the communities, while other decrease in average body weight. However, it should be taken into consideration that these patterns have been described mainly by the well-studied cold-temperate European lakes and might not apply in different climatic zones. Mediterranean lakes have been differentiated from temperate lakes by morphometric (basin size/lake size) and climatic characteristics (Alvarez Cobelas et al., 2005). Further biologically important differences are related to the increased availability of solar radiation in winter months leading to the continuous increase of phytoplankton mainly during late-autumn and winter months and to an extended period of fish reproduction resulting in more persistent fish predation on zooplankton (Moustaka-Gouni et al., 2014). Thus, patterns developed based on temperate lakes might be differentiated as the Plankton Ecology Group model (PEG-model) does (Moustaka-Gouni et al., 2014). Based on the Mediterranean PEG-model, it seems that zooplankton communities cannot effectively graze phytoplankton due to both high phytoplankton crops and fish predation. However, zooplankton communities still play an important role in lake assessment in the Mediterranean region, since rotifers have been used for discriminating anthropogenically disturbed lakes using taxonomic distinctness indices (Stamou et al., 2017) and crustaceans have been used for estimating the water quality of wetlands using QAELS index (Boix et al., 2005).
Based on the above differentiations of Mediterranean waters, we hypothesised that trophic state indices developed for temperate lakes, TSIROT and TSICR, might differentiate and may need adaptions when applied in a different climatic zone, in our case the Mediterranean. In order to test the above hypothesis, we evaluated their application in 16 Greek lakes, along the entire trophic spectrum, a wide range of altitude, surface area, mean and maximum depth. The evaluation of these indices was made using the trophic state estimated by the respective TSISD and mean summer phytoplankton biomass. Furthermore, we tested the dependance of each metric/formula of TSIROT and TSICR, on eutrophication and propose a new TSI index based on the whole zooplankton community, namely TSIZOO, combining, in part, both TSIROT and TSICR, as a zooplanktonic index for detecting eutrophication in Mediterranean lakes. Finally, we tested which of the three zooplanktonic indices (TSIROT, TSICR and TSIZOO) is more efficient in discriminating the trophic state in Mediterranean region.
Materials and methods
Data collection
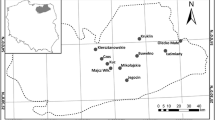
In our analysis, we used data from 16 Greek lakes; two of them, Kremasta and Tavropos, are reservoirs whereas the rest are natural lakes (Fig. 1). The 16 lakes encompass a wide range of altitude, surface area, mean and maximum depth (Table 1) and cover the entire trophic state spectrum. The dataset we used comprises published (from 1984 to 2016) and new data (from 2004 and 2016 to 2017) of the summer period (3 samplings during the period June to September) (Online Resource 1, Table I). The summer period was chosen because it was used for the development of TSIROT and TSICR but also because this is the period used for the lake ecological status assessment in Greece/Mediterranean region (e.g. Pahissa et al., 2015; Katsiapi et al., 2016; Petriki et al., 2017). The number of samples per lake ranged from 3 to 12 (Online Resource 1, Table I). The same sampling protocol for phytoplankton and zooplankton was followed for all lakes and it is well described by Mazaris et al. (2010) and Moustaka-Gouni et al. (2014). Secchi depth (SD) was also measured during the samplings.
Map of Greece showing the locations of the 16 lakes included in the study. Amv Amvrakia, Doi Doirani , Kas Kastorias , Kre Kremasta , MgP Megali Prespa , MkP Mikri Prespa , Oze Ozeros , Pam Pamvotis , Par Paralimni , Pet Petron , Tav Tavropos , Tri Trichonis , Veg Vegoritis , Vol Volvi, Vou Voulkaria , Yli Yliki
Zooplankton analysis
For zooplankton samples analysis, the lowest possible taxonomic level (genus or species) was identified using the taxonomic keys of Koste (1978), Nogrady et al. (1995), Segers (1995), Ricci & Melone (2000) and Nogrady & Segers (2002) for rotifers except Bdelloidea; of Amoros (1984), Korovchinsky (1992), Alonso (1996) and Benzie (2005) for cladocerans and of Dussart (1967a, b), Kiefer (1968, 1971), Reddy (1994), Einsle (1996) and Dussart & Defaye (2001) for copepods.
All lines of taxonomic information (i.e. spellings, valid names) were confirmed using the Rotifer World Catalog (Jersabek & Leitner, 2013) and the List of Available Names (LAN) part Rotifera (Segers et al., 2012; International Commission on Zoological Nomenclature: http://iczn.org/lan/rotifer), the cladoceran checklist (Kotov et al., 2013) and the World of Copepods database (Walter & Boxshall, 2018). Abundance estimation (expressed as ind/l) was performed following the method of Bottrell et al. (1976), Downing & Rigler (1984) and Taggart (1984). For each sample (total volume of 100 or 50 ml), five counts of 1 ml subsamples were made on a Sedgewick-Rafter cell. At least 300 individuals were counted or all the individuals were counted. For wet biomass estimations (expressed as mg/l), individual geometric formulae (Ruttner-Kolisko, 1977) or dry weight data and length–weight regressions were used (Dumont et al., 1975; Michaloudi, 2005); dry weight was consequently transformed to wet weight assuming that dry weight is 10% of wet (Dumont et al., 1975).
Phytoplankton analysis
Phytoplankton samples, live and preserved in Lugol’s solution, were examined in sedimentation chambers under a light inverted microscope; species were identified using appropriate taxonomic keys (e.g. Huber-Pestalozzi, 1938; Tikkanen, 1986).
Phytoplankton counts were performed using Utermöhl’s (1958) sedimentation method. For biovolume estimation, the dimensions of 30 individuals (cells, filaments or colonies) of each species were measured using tools of a digital microscope camera (Nikon DS-L1, software: DS camera control unit DS-L1) and the mean cell or filament volume estimates were calculated using appropriate geometric formulae (Hillebrand et al., 1999). Biovolume converted to biomass values using a density of 1 g/cm3.
Trophic state indices
For the assessment of lakes trophic state, we applied four trophic state indices. The TSI index based on Secchi depth (TSISD), the mean summer phytoplankton biomass and the TSI indices based on zooplankton communities, the Rotifer Trophic State index (TSIROT) and the Crustacean Trophic State index (TSICR).
TSISD was estimated based on the Secchi depth (SD) (Eq. 1) (Carlson, 1977) measured during each zooplankton–phytoplankton sampling; the classification was based on the modified boundaries of Carlson & Simpson (1996).
The mean summer phytoplankton biomass of the respective zooplankton–phytoplankton samplings was used to determine the lakes’ trophic state according to the following classification schemes: (a) Smith (2003) for natural oligotrophic lakes with mean depth > 15 m at altitude > 400 m and (b) Wetzel (2001) for the rest of the lakes.
Both TSIROT and TSICR are described based on data of Polish lakes as the average value of 6 formulae (Table 2). For both indices, the boundaries are set as TSI < 45 mesotrophic, 45 < TSI < 55 meso-eutrophic, 55 < TSI < 65 eutrophic and TSI > 65 hypertrophic (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013).
In the present study, we estimated TSIROT based on abundance and wet biomass data of rotifer communities of 16 Greek lakes, as the mean value of the TSIROT1, TSIROT2, TSIROT3 and TSIROT4 formulae (Eqs. 2–5, Table 2). We did not use the formula for the percentage of tecta form in the Keratella cochlearis (Gosse, 1851) population (Eq. 6, Table 2) since in our dataset the abundance of Keratella tecta (Gosse, 1851) was not always recorded separately from K. cochlearis since until recently K. tecta was considered a variation of K. cochlearis and not a separate species in the Keratella cochlearis species complex (Cieplinski et al., 2017). The TSIROT6 formula of the indicative of high trophic state rotifers (Eq. 7, Table 2) was not used since the indicator species are not appropriate for Greek lakes. For example, Ascomorpha ecuadis (Perty, 1850) and Gastropus stylifer (Imhof, 1891), which were used by Ejsmont-Karabin (2012) as indicators of low trophic state, were also found in eutrophic even hypertrophic Greek lakes (Yliki, Mikri Prespa, Trichonis) and Pompholyx sulcata (Hudson, 1885) an indicator of high trophic state, according to Ejsmont-Karabin (2012), was dominant in the oligotrophic lake Amvrakia (Online Resource 2). Based on the above data, it was evident that this formula could not be used for Greek lakes.
TSICR was also estimated based on abundance and wet biomass data of crustacean (cladocerans and copepods) communities of 16 Greek lakes, as the mean value of the TSICR1, TSICR2, TSICR3 and TSICR4 formulae (Eqs. 8–11, Table 2). We did not use the formula TSICR5 (Eq. 12, Table 2) for the ratio of cyclopoid to calanoid biomass since it was developed only for dimictic lakes and the studied lakes were polymictic and warm monomictic (Table 1). The TSICR6 formula (Eq. 13, Table 2) of the indicator crustaceans of high trophic state was also not used. The species Heterocope appendiculata (Sars G.O. 1863), Bosmina (Eubosmina) coregoni (Baird, 1857) and Bythotrephes longimanus (Leydig, 1860) which were proposed as indicators of low trophic state by Ejsmont-Karabin & Karabin (2013) have not been recorded in the studied Greek lakes while others, i.e. Daphnia (Daphnia) cucullata (Sars, 1862) (indicative of low trophic state) and Bosmina (Bosmina). longirostris (O. F. Müller, 1776) (indicative of high trophic state) have been recorded in the entire trophic spectrum in the studied Greek lakes indicating the unsuitability of the indicators species formula (Online Resource 2).
Statistical analysis
Linear regression and Analysis of Variance (ANOVA) were applied to test the dependence of each one of the TSIROT (TSIROT1 − TSIROT4) and TSICR (TSICR1 − TSICR4) formulae on eutrophication as estimated with the use of two proxies, TSISD and phytoplankton biomass.
Based on the above analyses, we propose a new index, the Zooplankton Trophic State Index (hereafter called TSIZOO) using only the mean value of the formulae, which were significantly correlated with eutrophication. The boundaries for TSIZOO used for lakes classification were the same used for the TSIROT and TSICR indices, i.e. TSI < 45 mesotrophic, 45 < TSI < 55 meso-eutrophic, 55 < TSI < 65 eutrophic and TSI > 65 hypertrophic.
Then, linear regression and ANOVA were applied to test the best-fitted trophic index based on zooplankton data, namely TSIROT (the average of TSIROT1 − TSIROT4), TSICR (the average of TSICR1 − TSICR4) and TSIZOO (the average of TSIROT1, TSIROT2, TSICR1 and TSICR2) compared to the two proxies of eutrophication. The same analyses were also applied to test the dependence of the TSIROT, TSICR and TSIZOO indices on the percentage contribution of cyanobacteria to total phytoplankton biomass since the Mediterranean lakes exhibit prolonged cyanobacterial blooms (Vardaka et al., 2005).
In order to evaluate the application of TSIROT, TSICR and TSIZOO indices in discriminating trophic categories, we used the lakes trophic category based only on phytoplankton biomass since it is a more reliable index compared to TSISD for Greek lakes (Katsiapi et al., 2016). Thus, the trophic category (oligotrophic, mesotrophic, eutrophic and hypertrophic) of each lake based on mean summer phytoplankton biomass (Table 3) was used and the cases characterized as “meso-eutrophic” and “eu-hypertrophic” were grouped to the mesotrophic and eutrophic category, respectively. ANOVA and Bonferroni correction were applied to reveal if the TSIROT, TSICR and TSIZOO differed among the four groups of trophic state. Weight cases for each parameter were used to reduce bias due to there being different number of lakes or samplings in each group. Furthermore, ANOVA and weight cases were also used to reveal if the TSIROT, TSICR and TSIZOO indices differed among the groups of low (oligotrophic and mesotrophic) and high (eutrophic and hypertrophic) trophic categories.
All statistical analyses were performed using IBM SPSS Statistics 25.
Results
The mean summer values (± standard deviation) of the trophic state indices are given in Table 3, while the values for each sampling are given in Online Resource 1 (Table II). The mean TSISD ranged from 24.46 ± 2.18 (Lake Trichonis) to 82.65 ± 9.15 (Lake Pamvotis). Mean summer phytoplankton biomass ranged from 0.16 ± 0.05 mg/l (Lake Amvrakia) to 42.24 ± 28.37 mg/l (Lake Voulkaria). The mean TSIROT ranged from 33.62 ± 2.36 (Lake Tavropos) to 54.06 ± 3.62 (Lake Yliki). In more detail, mean TSIROT1 ranged from 22.82 ± 5.38 (Lake Amvrakia) to 57.07 ± 3.20 (Lake Doirani); mean TSIROT2 ranged from 26.57 ± 3.37 (Lake Vegoritis, 1987) to 63.50 ± 4.49 (Lake Paralimni); mean TSIROT3 ranged from 44.30 ± 0 (Lake Ozeros) to 62.65 ± 1.50 (Lake Yliki) and mean TSIROT4 ranged from 24.72 ± 2.48 (Lake Tavropos) to 51.14 ± 9.56 (Lake Vegoritis, 1987). The mean TSICR ranged from 32.67 ± 18.77 (Lake Ozeros) to 59.81 ± 1.30 (Lake Volvi, 1986). In more detail, mean TSICR1 ranged from 37.88 ± 0.54 (Lake Tavropos) to 60.47 ± 2.40 (Lake Volvi, 1986); mean TSICR2 ranged from 24.85 ± 21.52 (Lake Ozeros) to 57.06 ± 5.04 (Lake Yliki); mean TSICR3 ranged from 25.29 ± 22.15 (Lake Ozeros) to 60.81 ± 0.80 (Lake Volvi, 1984) and mean TSICR4 ranged from 29.59 ± 25.59 (Lake Ozeros) to 66.18 ± 12.61 (Lake Kastorias, 2016).
The TSIROT formulae TSIROT1 and TSIROT2 were correlated significantly with both TSISD [R2 = 0.380, P < 0.001 and R2 = 0.277, P < 0.001, respectively (Table 4)] (Fig. 2) and phytoplankton biomass [R2 = 0.139, P = 0.002 and R2 = 0.085, P = 0.017, respectively (Table 4)] (Fig. 3). On the other hand, the formulae TSIROT3 and TSIROT4 were not correlated significantly with either TSISD [R2 = 0.039, P = 0.116 and R2 = 0.013, P = 0.374, respectively (Table 4)] (Fig. 2) or phytoplankton biomass [R2 = 0.007, P = 0.502 and R2 = 0.027, P = 0.182, respectively (Table 4)] (Fig. 3).
Scatter plots of TSIROT formulae a TSIROT1, b TSIROT2, c TSIROT3 and d TSIROT4 and TSICR formulae e TSICR1, f TSICR2, g TSICR3 and h TSICR4 against phytoplankton biomass (mg/l), the solid lines indicate the linear regression line and the dashed lines indicate the (95%) confidence and prediction limits of the model
The TSICR formulae TSICR1 and TSICR2 were correlated significantly with both TSISD [R2 = 0.361, P < 0.001 and R2 = 0.242, P < 0.001, respectively (Table 4)] (Fig. 2) and phytoplankton biomass [R2 = 0.134, P = 0.002 and R2 = 0.076, P = 0.024, respectively (Table 4)] (Fig. 3). On the other hand, the formulae TSICR3 and TSICR4 were correlated significantly only with the TSISD [R2 = 0.153, P = 0.001 and R2 = 0.060, P = 0.048, respectively (Table 4)] (Fig. 2)] and they were not correlated significantly with [or phytoplankton biomass [R2 = 0.047, P = 0.078 and R2 = 0.013, P = 0.355, respectively (Table 4)] (Fig. 3).
Based on these formulae that significantly correlated with both eutrophication proxies (Figs. 2 and 3, Table 4), we propose a new index, TSIZOO, as the average of the TSIROT1 (rotifers abundance/numbers), TSIROT2 (rotifers wet biomass), TSICR1 (crustaceans abundance) and TSICR2 (cyclopoids wet biomass). The mean TSIZOO ranged from 33.04 ± 1.74 (Lake Vegoritis, 1987) to 58.65 ± 0.42 (Lake Doirani) (Table 3).
Linear regressions were also applied to test the dependence of the three zooplanktonic TSI indices, i.e. TSIROT (the average of TSIROT1 − TSIROT4), TSICR (the average of TSICR1 − TSICR4) and TSIZOO (the average of TSIROT1, TSIROT2, TSICR1 and TSICR2), on eutrophication proxies TSISD and phytoplankton biomass as it is shown in Fig. 4. All indices were significantly correlated (P < 0.05) with both eutrophication proxies and the fitted relationships are shown in Table 5. The best-fitted index compared to TSISD is TSIZOO (F = 51.29, R2 = 0.449) while compared to phytoplankton biomass both TSIROT and TSIZOO are almost equally well fitted (F = 11.86, R2 = 0.154 and F = 11.50, R2 = 0.150, respectively).
The three zooplanktonic TSI indices—TSIROT, TSICR and TSIZOO—were also correlated with the percentage of cyanobacteria to total phytoplankton biomass (Fig. 5). Even though these correlations were not statistical significant (P > 0.05), the most influenced index was the TSIROT (F = 3.77, R2 = 0.055, P = 0.056) (Table 5).
When the trophic state of each sampling was estimated based on all trophic indices (mean summer phytoplankton biomass, TSISD, TSIROT, TSICR and TSIZOO), it was observed that they did not identify the same trophic category in all cases (Online Resource 1, Table III). However, when the mean values for the whole summer period were used for each zooplanktonic index (Table 6), using the proposed boundaries by Ejsmont-Karabin (2012) and Ejsmont-Karabin & Karabin (2013) there were some cases identifying the same trophic state [e.g. Trichonis, Vegoritis (1987) and Volvi (1985) (Table 6)]. Still the three zooplanktonic TSI indices (TSIROT, TSICR and TSIZOO) only identified mesotrophic and eutrophic states.
When TSIROT, TSICR and TSIZOO were evaluated based on only the phytoplankton index, they detected different trophic states. Each index was significantly differentiated between the trophic state categories indicated by mean summer phytoplankton biomass (ANOVA, P < 0.0001); however, the pairwise test revealed different potentials among the three indices to detect each trophic state. TSIROT detected different trophic states (ANOVA, F = 11.628, P < 0.0001) and differences between the oligotrophic lakes and the rest of the trophic states were significant (Bonferoni, P < 0.05) (Fig. 6). TSICR detected different trophic states (ANOVA, F = 7.055, P < 0.0001) discriminating oligotrophic lakes from eutrophic and hypertrophic lakes but not from mesotrophic lakes (Bonferoni, P < 0.05) (Fig. 6). TSIZOO also differentiated significantly between the trophic state categories (ANOVA, F = 13.688, P < 0.0001) and the pairwise test indicated significant differences between oligotrophic–mesotrophic lakes and eutrophic–hypertrophic lakes (Bonferoni, P < 0.05) (Fig. 6).
Based on the Bonferoni tests, we consider that a classification of only two trophic states—the low (oligotrophic and mesotrophic lakes) and the high (eutrophic and hypertrophic lakes)—might be more appropriate (Fig. 7). Thus, we propose that the boundaries for TSIROT and TSIZOO should be set at 45 in order to detect low and high trophic state and for TSICR the boundary should be set at 50. Using these boundaries, the mean values of the summer period TSIROT could detect correctly 78% of the lakes trophic state; TSICR could detect correctly 83% of the lakes trophic state and TSIZOO could detect correctly 87% of the trophic state according to Table 7. Moreover, all zooplanktonic TSI indices could detect the two trophic categories (low and high) as defined by mean summer phytoplankton biomass (ANOVA TSIROT: F = 7.316, P = 0.011, TSICR: F = 5.787, P = 0.022 and TSIZOO: F = 15.514, P < 0.0001).
Box plots of a TSIROT, b TSICR and c TSIZOO based on data of zooplankton communities from the 16 Greek lakes grouped into low (oligotrophic–mesotrophic) and high (eutrophic–hypertrophic) trophic groups (indicated by mean summer phytoplankton biomass). The dashed line indicates the boundary proposed in the present study for each index. *, ** Significant differences (Bonferoni procedure)
Discussion
Rotifer Trophic State Index (TSIROT) and Crustacean Trophic State Index (TSICR) are two recently developed indices based on data from zooplankton communities from Polish lakes (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013) and thus their original forms have been mainly applied in Poland, with TSIROT being used in more studies (e.g. Gutkowska et al., 2013; Jekatierynczuk-Rudczyk et al., 2014; Dembowska et al., 2015; Marszelewski et al., 2017) as compared to TSICR (Ochocka & Pasztaleniec, 2016; Dunalska et al., 2018). In different regions, only some of the proposed formulae have been used for TSIROT; more specifically TSIROT1 has been used in Mexico (Gutiérrez et al., 2017; Moreno-Gutiérrez et al., 2018) and Nigeria (Bolawa et al., 2018), while Haberman & Haldna (2014) used both TSIROT1 and TSIROT4 in the Estonian Lake Vortsjärv. TSICR has also been partially used in Nigeria (TSICR5) (Bolawa et al., 2018) and in Poland (TSICR3, TSICR4 and TSICR6) by Jekatierynczuk-Rudczyk et al. (2014). In the Mediterranean zone, only TSIROT1 has been applied in Portugal (Geraldes & Pasupuleti, 2016). The present work is the first application of both TSIROT and TSICR for Greek lakes.
We applied TSIROT and TSICR as the average of 4 out of the 6 proposed formulae for each index due to data limitations and differences in the trophic state indicator species. Despite the fact that in our attempt to evaluate the TSIROT index for the Mediterranean lakes, we did not use the formula TSIROT5 (the percentage of tecta form in the K. cochlearis population) this formula might be suitable for eutrophication assessment, as in the case of the Neva Estuary (Baltic Sea) (Gopko & Telesh, 2013). The same stands for the TSIROT6 and TSICR6 formulae of the indicator species which we did not use because the proposed indicator species do not follow the suggested pattern of dominating only oligotrophic or eutrophic lakes when the Greek lakes were examined. However, they are considered as a suitable tool for trophic state assessment and even as a metric for water quality in Poland (Ochocka & Pasztaleniec, 2016). Still, in order to use these formulae of the indicator species, further research should be done in order for indicator species to be identified for each region in general as well as the Mediterranean. This is further supported by the different patterns of dominance for the same species recorded throughout the literature, e.g. for rotifers the genus Trichocerca Lamarck, 1801, is thought to be an indicator of oligotrophic systems by Sládecek (1983); while Trichocerca capucina (Wierzejski & Zacharias, 1893) and Trichocerca pusilla (Jennings, 1903) are even considered typical of eutrophic conditions (Gulati, 1983); while D. cucullata is considered both an indicator of oligotrophic lakes (Karabin, 1985) and typical of eutrophication condition (Pejler, 1983).
In our attempt to find the more efficient formulae for the assessment of Mediterranean systems, TSIROT and TSICR formulae were correlated with the eutrophication proxies TSISD and phytoplankton biomass. Based on the fact that only TSIROT1, TSIROT2, TSICR1 and TSICR2 were significantly correlated both with TSISD and phytoplankton biomass, we propose a new index of the whole zooplankton community called TSIZOO. This new index is the average of these formulae, which had the best-fitted model when the three indices used in the present study (TSIROT, TSICR and TSIZOO) were correlated with the two eutrophication proxies. Hence, TSIZOO seems to be the most efficient of the three zooplanktonic indices in discriminating eutrophication. Further research could be done in order to relate these indices with other commonly used eutrophication proxies such as nutrients (i.e. total phosphorous) or other TSI indices (i.e. TSICHL).
For the evaluation of the indices used in the present study, we assessed the trophic state of the same lake/sampling using all trophic state indices. The classification of lakes was dissimilar for many cases when all indices or when only the zooplanktonic indices were used. Such discordances among different trophic indices have been recorded both in reservoirs and natural lakes (e.g. Duggan et al., 2001; García-Chicote et al., 2018). Differences between the trophic indices were smoothened when the mean values of the summer period were used, e.g. Lake Volvi in 1985 was characterised as mesotrophic up to eutrophic using monthly values of all three zooplanktonic TSI indices while only as meso-eutrophic when mean values were used, thus, we propose the trophic state estimation to be done based on the mean values of the zooplanktonic indices.
So far all studies that have applied the TSIROT and TSICR indices in order to evaluate them only compared their values with the trophic state as indicated by TSI indices or even by other indices based on zooplankton (e.g. Jekatierynczuk-Rudczyk et al., 2014; Gutiérrez et al., 2017), except Dembowska et al. (2015) who correlated TSIROT with phytoplankton indices. In the present study, acknowledging that phytoplankton is the most reliable element for assessing trophic state (e.g. Katsiapi et al., 2016), we evaluated all indices based on the trophic state as indicated by mean summer phytoplankton biomass. We found that TSISD overestimated the trophic state of Lake Ozeros due to increased detritus as well as the reservoirs (Tavropos and Kremasta), since riverine systems have increased organic material (total suspended solids or allochthonous-dissolved organic matter) lowering transparency (e.g. Mash et al., 2004; Bolgrien et al., 2009). TSISD also underestimated the trophic state of the deep lakes Trichonis and Vegoritis. Despite these differentiations, Katsiapi et al. (2016) propose the use of TSISD for practical large-scale and long-term monitoring purposes of Mediterranean lakes when combined with low-frequency high-quality phytoplankton data during the warm period (June to October). However, it should not be used for the calibration of other eutrophication indices, thus the rest of our analyses were made using only the phytoplankton biomass as a eutrophication proxy.
Regarding the zooplanktonic TSI indices and their proposed limits (Ejsmont-Karabin, 2012; Ejsmont-Karabin & Karabin, 2013), oligotrophic lakes were overestimated since all lakes with TSI < 45 were grouped as mesotrophic and hypertrophic lakes were underestimated since none of the three indices took values over 65. TSIROT underestimated also the majority of the studied eutrophic lakes. TSIROT had lower values compared to TSICR leading in estimation of different categories for many cases. This was expected due to the increased impact of fish predation on crustacean (Ejsmont-Karabin & Karabin, 2013). However, TSICR had very low values in the samplings of July of lakes Trichonis and Ozeros because cyclopoid copepods were not recorded in crustacean’s abundance resulting in zero values for the TSICR2, TSICR3 and TSICR4 formulae, indicating that TSICR should not be used in lakes without cyclopoids or when no cyclopoids are recorded. TSIZOO being the average of the two indices had better results estimating the trophic state, strengthening the result of being the index most depended on eutrophication.
Estimations of different trophic states using different indices, especially for TSIROT, have been recorded elsewhere as well, e.g. in Madín Reservoir in Mexico (Moreno-Gutiérrez et al., 2018). Even in Polish lakes in some cases TSIROT underestimates the trophic state for eutrophic and hypertrophic lakes (Jekatierynczuk-Rudczyk et al., 2014). Cyanobacterial blooms have been identified as one possible reason for these misclassifications when TSIROT is applied (Dembowska et al., 2015). Mediterranean freshwater systems can exhibit prolonged cyanobacterial blooms (up to 8 months) compared to temperate lakes, and their phytoplankton community can even be dominated by cyanobacteria up to more than 90% in eutrophic lakes (Vardaka et al., 2005). This was also the case in the present study, where cyanobacteria contributed to the total biomass in all lakes, except Kremasta Reservoir, and were the dominant group in terms of biomass in lakes of the entire trophic spectrum [i.e. their contribution reached up to 75.98% in the oligotrophic lake Amvrakia cyanobacteria, 99.96% in the deep lake Trichonis and up to 100% in the hypertrophic lake Pamvotis (Online Resource 1, Table II)]. Nevertheless, despite the increased cyanobacteria contribution, no statistically significant relationships with the zooplanktonic TSI indices used in the present study were recorded.
Despite discrepancies in trophic state estimations, all zooplanktonic indices (TSIROT, TSICR, TSIZOO) were significantly differentiated among the groups of trophic state indicated by mean summer phytoplankton biomass. It is of interest that TSIROT differentiated significantly only for the oligotrophic state. Even though the dataset used for TSIROT development included lakes covering the mesotrophic to the hypertrophic trophic spectrum (Ejsmont-Karabin, 2012), oligotrophic lakes might be differentiated from mesotrophic even in temperate lakes. However, further research is needed based on a bigger dataset of temperate lakes in order to clarify this, and to propose, if possible, a boundary between oligotrophic and mesotrophic lakes. TSICR on the other hand differentiated significantly the oligotrophic category from the eutrophic and hypertrophic categories and TSIZOO differentiated oligotrophic and mesotrophic lakes from eutrophic and hypertrophic lakes. Based on the above, there is a need to modify the boundaries proposed by Ejsmont-Karabin (2012) and Ejsmont-Karabin & Karabin (2013); however, value overlaps do not show a clear pattern. Based on the fact that there is no clear differentiation among the trophic categories, we propose that zooplanktonic indices should be used in order to differentiate two trophic groups: one of the low trophic state that includes oligotrophic and mesotrophic lakes, and one of high trophic state that includes eutrophic and hypertrophic lakes. When this categorisation was used also for the trophic state defined by the phytoplankton biomass, all three zooplanktonic indices (TSIROT, TSICR, TSIZOO) significantly differentiated the two categories. The boundaries for discriminating the categories of low and high trophic state for TSIROT and TSIZOO could remain TSI = 45 as already proposed by Ejsmont-Karabin (2012) (TSI < 45 mesotrophic or low trophic state) but for TSICR, we propose the boundary to be TSICR < 50, a bit higher than the proposed boundary by Ejsmont-Karabin & Karabin (2013). When the above boundaries were used, TSIZOO classified correctly the majority of the studied lakes, except Lake Trichonis which was underestimated possibly due to the increased fish predation by Atherina boyeri Risso, 1810 (Chrisafi et al., 2007) and the shallow lakes Ozeros and Paralimni which were overestimated; Lake Ozeros had increased abundance of detritus indicating a possible higher trophic state and Lake Paralimni had increased rotifers abundance and domination (> 70%).
In conclusion, we have shown that the Rotifer Trophic State Index (TSIROT) and the Crustacean Trophic State Index (TSICR) applied to the zooplankton communities from 16 Greek lakes are increasing across eutrophication in Mediterranean lakes. Furthermore, a new index TSIZOO, the Zooplankton Trophic State Index, was proposed as the average of the formulae which were significantly correlated with the eutrophication proxies (TSISD and phytoplankton biomass). The evaluation of the indices indicated that they can detect efficiently two groups of low (oligotrophic–mesotrophic) trophic and high (eutrophic–hypertrophic) trophic state in the Mediterranean region. Moreover, despite the range overlaps, we propose the boundaries to be < 45 for the low category for TSIROT and TSIZOO and < 50 for TSICR when applied in the Mediterranean region. TSIROT and TSICR can be promising and useful tools for trophic state estimation because they do not require species-level identifications and are relatively easy and inexpensive to measure; however, TSICR should not be used in lakes without cyclopoid copepods. TSIZOO, which represents the entire zooplankton community, was better related to eutrophication and had better estimations of the trophic state especially when the mean summer value was used. Thus, we propose TSIZOO as a promising and effective tool for monitoring and assessment of eutrophication of Mediterranean lakes since it can be an easy and cost-effective tool in large-scale and long-term monitoring programmes and it could be considered as a parameter of a multimetric index of ecological water quality, based on zooplankton.
References
Alonso, M., 1996. Crustacea, Branchiopoda. In Ramos, M. A., et al. (eds), Fauna Iberica, Vol. 7. Museo Nacional de Ciencias Naturales Consejo Superior de Investigaciones Cientificas, Madrid.
Alvarez Cobelas, M., C. Rojo & D. G. Angeler, 2005. Mediterranean limnology: current status, gaps and the future. Journal of Limnology 64: 13–29.
Amoros, C., 1984. Introduction pratique à la systématique des organismes des eaux continentales françaises -5- Crustacés Cladocères. Bulletin de la Société Linnéenne de Lyon 53: 71–145.
Azémar, F., T. Maris, B. Mialet, H. Segers, S. Van Damme, P. Meire & M. Tagkx, 2010. Rotifers in the Schelde estuary (Belgium): a test of taxonomic relevance. Journal of Plankton Research 32: 981–997.
Benzie, J. A. H., 2005. The genus Daphnia (including Daphniopsis) (Anomopoda: Daphniidae). In Dumont, H. J. (ed.), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 21. Kenobi Productions, Ghent & Backhuys Publishers, Leiden.
Boix, D., S. Gascón, J. Sala, M. Martinoy, J. Gifre & X. D. Quintana, 2005. A new index of water quality assessment in Mediterranean wetlands based on crustacean and insect assemblages: the case of Catalunya (NE Iberian Peninsula). Aquatic Conservation: Marine and Freshwater Ecosystems 15: 635–651.
Bolawa, O. P., A. A. Adedej & Y. F. Taiwo, 2018. Temporal and Spatial Variations in Abundance and Diversity of Zooplankton Fauna of Opa Reservoir, Obafemi Awolowo University, Ile-Ife, Southwest Nigeria. Notulae Scientia Biologicae 10: 265–274.
Bolgrien, D. W., J. V. Scharold, T. R. Angradi, T. D. Corry, E. W. Schwieger & J. R. Kelly, 2009. Trophic status of three large Missouri river reservoirs. Lake Reservoir Manage 25: 176–190.
Bottrell, H. H., A. Duncan, Z. M. Gliwicz, E. Grygierek, A. Herzig, Α. Hillbright-Ilkowska, H. Kurasawa, P. Larsson & T. Weglenska, 1976. A review of some problems in zooplankton production studies. Norwegian Journal οf Zoology 24: 419–456.
Callisto, M., J. Molozzi & J. L. E. Bardosa, 2014. In Ansari, A. A. & S. S. Gill (eds), Eutrophication: Causes, Consequences and Control. Springer, Dordrecht: 55–73.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369.
Carlson, R. E. & J. Simpson, 1996. A Coordinator’s Guide to Volunteer Lake Monitoring Methods. North American Lake Management Society, Madison.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639.
Chrisafi, E., P. Kaspiris & G. Katselis, 2007. Feeding habits of sand smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). Journal of Applied Ichthyology 23: 209–214.
Cieplinski, A., T. Weisse & U. Obertegger, 2017. High diversity in Keratella cochlearis (Rotifera, Monogononta): morphological and genetic evidence. Hydrobiologia 796: 145–159.
CIS, 2009. Guidance document on eutrophication assessment in the context of european water policies. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Guidance Document No 23. Luxembourg.
Dembowska, E., P. Napiórkowski, T. Mieszczankin & S. Józefowicz, 2015. Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecological Indicators 56: 15–22.
Downing, J. A. & F. H. Rigler, 1984. A manual on methods for the assessment of secondary productivity in fresh waters. Blackwell Scientific, Oxford, UK.
Duggan, I. C., J. D. Green & K. Thomasson, 2001. Do rotifers have potential as bioindicators of lake trophic state? Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 27: 3497–3502.
Dumont, H. J., I. Van de Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97.
Dunalska, J. A., A. Napiórkowska-Krzebietke, A. Ławniczak-Malińska, E. Bogacka-Kapusta & G. Wiśniwski, 2018. Restoraton of flow-through lakes – Theory and practice. Ecology & Hydrobiology. https://doi.org/10.1016/j.ecohyd.2018.06.009.
Dussart, B. H., 1967a. Les copépodes des eaux continentales d’ Europe occidentale. Tome I: calanoïds et Harpacticoïdes. N. Boublée & Cie. Eds., Paris.
Dussart, B. H., 1967b. Les Copépodes des Eaux continentales d’ Europe occidentale. Tome II: Cyclopoïdes et Biologie. N. Boublée & Cie. Eds, Paris.
Dussart, B. H. & D. Defaye, 2001. Introduction to the Copepoda. In Dumont, H. J. F. (ed.), Guides to the Identification of the Macroinvertebrates of the Continental Waters of the World 16. Backhuys Publishers, Leiden.
EC, 2008. Commission Decision of 30 October 2008 establishing, pursuant to Directive 2000/60/EC of the European Parliament and the Council, the values of the Member State monitoring system classifications as a result of the intercalibration exercise 2008/915/EC. Official Journal of the European Communities, L332/20. European Commission, Brussels.
Einsle, U., 1996. Copepoda: Cyclopoida Cyclops, Megacyclops, Acanthocyclops. In Dumont, H. J. F. (ed), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 10. SPB Academic Publishing bv, Amsterdam.
Ejsmont-Karabin, J., 2012. The usefulness of zooplankton as lake ecosystem indicators: rotifer trophic state index. Polish Journal of Ecology 60: 339–350.
Ejsmont-Karabin, J. & A. Karabin, 2013. The suitability of zooplankton as lake ecosystem indicators: crustacean trophic state index. Polish Journal of Ecology 61: 561–573.
García-Chicote, J., X. Armengol & C. Rojo, 2018. Zooplankton abundance: a neglected key element in the evaluation of reservoir water quality. Limnologica 69: 46–54.
Geraldes, A. M. & R. Pasupuleti, 2016. Zooplankton: a valuable environmental indicator tool in reservoir ecological management? Asian Journal of Environment & Ecology 1: 1–9.
Gopko, M. & I. V. Telesh, 2013. Estuarine trophic state assessment: new plankton index based on morphology of Keratella rotifers. Estuarine, Coastal and Shelf Science 130: 222–230.
Gulati, R. D., 1983. Zooplankton and its grazing as indicators of trophic status in Dutch lakes. Environmental Monitoring and Assessment 3: 343–354.
Gutiérrez, S. G., S. S. S. Sarma & S. Nandini, 2017. Seasonal variations of rotifers from a high altitude urban shallow waterbody, La Cantera Oriente (Mexico City, Mexico). Chinese Journal of Oceanology and Limnology 35: 1387–1397.
Gutkowska, A., E. Paturej & E. Kowalska, 2013. Rotifer trophic state indices as ecosystem indicators in brackish coastal waters. Oceanologia 55: 887–899.
Haberman, J. & M. Haldna, 2014. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: long term study of Lake Võrtsjärv. Journal of Limnology 73: 263–273.
Havens, K. E., 2014. Lake eutrophication and plankton food webs. In Ansari, A. A. & S. S. Gill (eds), Eutrophication: Causes, Consequences and Control, Vol. 2. Springer, Dordrecht: 73–80.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalge. Journal of Phycology 35: 403–424.
Huber-Pestalozzi, G., 1938. Das Phytoplankton des Süsswassers. Systematik und Biologie. In: Thienemann A, ed Die Binn Eugewässer. Stuttgart.
International Commission on Zoological Nomenclature. [available on internet at http://iczn.org/lan/rotifer] accessed June 2018.
Jekatierynczuk-Rudczyk, E., P. Zieliński, M. Grabowska, J. Ejsmont-Karabin, M. Karpowicz & A. Więcko, 2014. The trophic status of Suwałki Landscape Park lakes based on selected parameters (NE Poland). Environmental Monitoring and Assessment 186: 5101–5121.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. L. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164.
Jersabek, C. D. & M. F. Leitner, 2013. The Rotifer World Catalog. World Wide Web electronic Publication. [available on internet at http://www.rotifera.hausdernatur.at/], accessed June 2018.
Kane, D. D., S. I. D. Gordon, M. Munawar, M. N. Charlton & D. A. Culver, 2009. The Planktonic Index of Biotic Integrity (P-IBI): an approach for assessing lake ecosystem health. Ecological Indicators 9: 1234–1247.
Karabin, A., 1985. Pelagic zooplankton (Rotatoria + Crustacea) variation in the process of lake eutrophication. I. Structural and quantitative features. Ekologia Polska 33: 567–616.
Katsiapi, M., M. Moustaka-Gouni & U. Sommer, 2016. Assessing ecological water quality of freshwaters: PhyCoI – a new phytoplankton community index. Ecological Informatics 31: 22–29.
Kiefer, F., 1968. Versuch einer revision der gattung Eudiaptomus Kiefer (Copepoda Calanoida). Memorie dell’ Istulo Italiano di Idrobiologia 24: 9–160.
Kiefer, F., 1971. Revision der Bacillifer - gruppe der gattung Arctodiaptomus Kiefer (Crustacea Copepoda: Calanoida). Memorie dell’ Istulo Italiano di Idrobiologia 27: 113–267.
Korovchinsky, N. M., 1992. Sididae and Holopedidae (Crustacea: Daphniformes). Guides to the Identification of the Microinvertebrate of the Continental Waters of the World. SPB Academics, The Hague.
Koste, W., 1978. Rotatoria, die Rãdertiere Mitteleuropas. Gebrüder Borntraeger, Berlin.
Kotov, A., L. Forró, N. M. Korovchinsky & A. Petrusek, 2013. World checklist of freshwater Cladocera species. World Wide Web electronic publication. [available on internet at http://fada.biodiversity.be/group/show/17], accessed September 2018.
Kruskopf, M. & K. J. Flynn, 2006. Chlorophyll content and fluorescence responses cannot be used to gauge reliably phytoplankton biomass, nutrient status or growth rate. New Phytologist 169: 841–842.
Kulkarini, D., A. Gergs, U. Hommen, H. T. Ratte & T. G. Preuss, 2013. A plea for the use of copepods in freshwater ecotoxicology. Environmental Science and Pollution Research 20: 75–85.
Lyche-Solheim, A., C. K. Feld, S. Birk, G. Phillips, L. Carvalho, G. Morabito, U. Mischke, N. Willby, M. Søndergaard, S. Hellsten, A. Kolada, M. Mjelde, J. Böhmer, O. Miler, M. T. Pusch, C. Argillier, E. Jeppesen, T. L. Lauridsen & S. Poikane, 2013. Ecological status assessment of European lakes: a comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 704: 57–74.
Marszelewski, W., E. A. Dembowska, P. Napiórkowski & A. Solarczyk, 2017. Understanding abiotic and biotic bonditions in Post-Mining Pit lakes for efficient management: a case study (Poland). Hydrobiologia 36: 418–428.
Mash, H., P. K. Westerhoff, L. A. Baker, R. A. Nieman & M.-L. Nguyen, 2004. Dissolved organic matter in Arizona reservoirs: assessment of carbonaceous sources. Organic Chemistry 35: 831–843.
Mazaris, A. D., M. Moustaka-Gouni, E. Michaloudi & D. C. Bobori, 2010. Biogeographical patterns of freshwater micro- and macroorganisms: a comparison between phytoplankton, zooplankton and fish in the eastern Mediterranean. Journal of Biogeography 37: 1341–1351.
Michaloudi, E., 2005. Dry weights of the zooplankton of Lake Mikri Prespa (Macedonia, Greece). Belgian Journal of Zoology 135: 223–227.
Moreno-Gutiérrez, R. M., S. S. S. Sarma, A. S. Sobrino-Figueroa & S. Nandini, 2018. Population growth potential of rotifers from a high altitude eutrophic waterbody, Madín reservoir (State of Mexico, Mexico): the importance of seasonal sampling. Journal of Limnology. https://doi.org/10.4081/jlimnol.2018.1823.
Moss, B., D. Stephen, C. Alvarez, E. Becares, W. van de Bund, S. E. Collings, E. van Donk, E. de Eyto, T. Feldmann, C. Fernández-Aláez, M. Fernández-Aláez, R. J. M. Franken, F. García-Criado, E. M. Gross, M. Gyllström, L. A. Hansson, K. Irvine, A. Järvalt, J. P. Jensen, E. Jeppesen, T. Kairesalo, R. Kornijów, T. Krause, H. Künnap, A. Laas, E. Lill, B. Lorens, H. Luup, M. R. Miracle, P. Nõges, T. Nõges, M. Nykänen, I. Ott, W. Peczula, E. T. H. M. Peeters, G. Phillips, S. Romo, V. Russell, J. Salujõe, M. Scheffer, K. Siewertsen, H. Smal, C. Tesch, H. Timm, L. Tuvikene, I. Tonno, T. Virro, E. Vicente & D. Wilson, 2003. The determination of ecological status in shallow lakes – a tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquatic Conservation: Marine Freshwater Ecosystem 13: 507–549.
Moustaka-Gouni, M., 1989. Temporal and spatial distribution of chlorophyll a in lake Volvi, Greece. Archiv für Hydrobiologie Supplement 82: 475–485.
Moustaka-Gouni, M., E. Michaloudi & U. Sommer, 2014. Modifying the PEG model for Mediterranean lakes – no biological winter and strong fish predation. Freshwater Biology 59: 1136–1144.
Nogrady, T. & H. Segers, 2002. Rotifera Volume 6: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 18. Backhuys Publishers, Leiden.
Nogrady, T., R. Pourriot & H. Segers, 1995. The Notommatidae and the Scaridiidae. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 8. SPB Academic Publishing BV, Amsterdam.
Ochocka, A. & A. Pasztaleniec, 2016. Sensitivity of plankton indices to lake trophic conditions. Environmental Monitoring and Assessment 188: 622.
OECD, Organisation for Economic Co-operation and Development, 1982. Eutrophication of Waters: Monitoring, Assessment and Control. OECD, Paris.
Pahissa, J., J. Catalan, G. Morabito, G. Dörflinger, J. Ferreira, C. Laplace-Treyture, R. Gîrbea, A. Marchetto, P. Polykarpou & C. de Hoyos, 2015. Benefits and limitations of an intercalibration of phytoplankton assessment methods based on the Mediterranean GIG reservoir experience. Science of the Total Environment 538: 169–179.
Pejler, B., 1983. Zooplanktic indicators of trophy and their food. Hydrobiologia 101: 111–114.
Petriki, O., M. Lazaridou & D. Bobori, 2017. A fish-based index for the assessment of the ecological quality of temperate lakes. Ecological Indicators 78: 556–565.
Reddy, Y. R., 1994. Copepoda: Calanoida: Diaptomidae Key to the genera Heliodiaptomus, Allodiaptomus, Neodiaptomus, Phyllodiaptomus, Eodiaptomus, Arctodiaptomus and Sinodiaptomus. SPB Academic Publishing, The Hague.
Reynolds, C. S., 1984. The Ecology of Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Ricci, C. & G. Melone, 2000. Key to the identification of the genera of bdelloid rotifers. Hydrobiologia 418: 73–80.
Ruttner-Kolisko, A., 1977. Suggestions for biomass calculations of plankton rotifers. Archiv für Hydrobiologie-Beiheft Ergebnisse der Limnologie 8: 71–76.
Sarma, S. S. & S. Nandini, 2006. Review of Recent Ecotoxicological Studies on Cladocerans. Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes 41: 1417–1430.
Segers, H., 1995. Rotifera. Vol. 2: The Lecanidae (Monogononta). In Dumont, H. J. F. (ed), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. SPB Academic Publishing BV, The Hague.
Segers, H., W. H. De Smet, C. Fischer, D. Fontaneto, E. Michaloudi, R. L. Wallace & C. D. Jersabek, 2012. Towards a list of available names in zoology, partim Phylum Rotifera. Zootaxa 3179: 61–68.
Sládecek, V., 1983. Rotifers as indicators of water quality. Hydrobiologia 100: 169–201.
Smith, V. H., 2003. Eutrophication of freshwater and coastal ecosystems: a global problem. Environmental Science and Pollution Research 10: 126–139.
Snell, T. W. & C. Joaquim-Justo, 2007. Workshop on rotifers in ecotoxicology. Hydrobiologia 593: 227–232.
Stamou, G., C. Polyzou, A. Karagianni & E. Michaloudi, 2017. Taxonomic distinctness indices for discriminating patterns in freshwater rotifer assemblages. Hydrobiologia 796: 319–331.
Taggart, C. T., 1984. Hypolimnetic aeration and zooplankton distribution: a possible limitation to the restoration of cold-water fish production. Canadian Journal of Fisheries and Aquatic Sciences 41: 191–198.
Tikkanen, T., 1986. Kasviplanktonopas. Suomen Luonnonsuojelun Tuki Oy, Helsinki.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitative Phytoplanktonmethodik. Mitteilungen Internationale Vereinigung Theorie Angewandte Limnologie 9: 1–38.
Vardaka, E., M. Moustaka-Gouni, C. M. Cook & T. Lanaras, 2005. Cyanobacterial blooms and water quality in Greek waterbodies. Journal of Applied Phycology 17: 391–401.
Walter, T. C. & G. Boxshall, 2018. World of Copepods database. [available on internet at http://www.marinespecies.org/], accessed September 2018.
Wetzel, R. G., 2001. Limnology, 3rd ed Academic Press, San Diego, CA.
Wolfram, G., C. Argillier, J. De Bortoli, G. Buzzi, M. T. Dokulil, E. Hoehn, A. Marchetto, P. J. Martinez, G. Morabito, M. Reichmann, S. Remec-Rekar, U. Riedmüller, C. Rioury, J. Schaumburg, L. Schulz & G. Urbanic, 2009. Reference conditions and WFD compliant class boundaries for phytoplankton biomass and chlorophyll-a in Alpine lakes. Hydrobiologia 633: 45–58.
Acknowledgements
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme « Human Resources Development, Education and Lifelong Learning » in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research” (MIS-5000432), implemented by the State Scholarships Foundation (ΙΚΥ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Steven A. J. Declerck, Diego Fontaneto, Rick Hochberg & Terry W. Snell / Crossing Disciplinary Borders in Rotifer Research
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stamou, G., Katsiapi, M., Moustaka-Gouni, M. et al. Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia 844, 83–103 (2019). https://doi.org/10.1007/s10750-018-3880-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3880-9