Abstract
With the aim to expand the understanding of the feeding ecology of the swordfish, two supplementary techniques were used: analysis of stomach contents, including 244 samples; and analysis of stable carbon (δ13C) and nitrogen (δ15N) isotopes, with 128 samples of muscle tissues from animals collected in the western coast of Baja California. Samples were obtained from swordfish captured by the longline fleet that operates along the western coast of Baja California peninsula. Thirty-three different prey types were identified through the analysis of stomach contents. Based on the index of relative importance (IRI), cephalopods accounted for 98.91%, fish 0.88%, and pelagic crustaceans 0.21%. The stable isotope analysis showed δ15N values between 13.05‰ and 19.66‰, and δ13C values between − 19.32‰ and − 15.60‰, indicating that the swordfish feeds primarily on oceanic species. The stable isotope mixing model (SIAR) established that prey species that most contribute to the diet of Xiphias gladius were cephalopods, followed by the crustacean Pleuroncodes planipes, and the fish Lagocephalus lagocephalus. The SIBER test corroborates similar diets between sexes and sex-maturity groups, indicating that all swordfish analyzed essentially occupied the same trophic niche.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The swordfish (Xiphias gladius Linnaeus, 1758) is the most widely distributed billfish, and occurs worldwide from about 45°N to 45°S in all tropical, subtropical, and temperate seas (Palko et al., 1981). This species exhibits diel horizontal and vertical migrations, in which daylight hours are spent predominantly below the thermocline (300–1000 m), and night hours are spent above it (Abascal et al., 2010; Sepulveda et al., 2010; Dewar et al., 2011; Abascal et al., 2015). Swordfish typically forage in deep water during the day and stay in the mixed layer at night, where they can feed and, at the same time, recover from the thermal or oxygen debt acquired by day; apparently, the maximum nighttime depth is usually reached close to the full moon (Abascal et al., 2010). In the Eastern Pacific, particularly in the California Current—an area where the oxygen minimum layer is much shallower—swordfish show daytime basking movements (Dewar et al., 2011). This species is related to offshore seamounts and submarine canyons, particularly in the tip of Baja Peninsula where banks are common. In this area, swordfish display clear cycles of movement between deep waters and inshore banks; this onshore-offshore movements have been associated with feeding strategies (Carey & Robison, 1981; Sedberry & Loefer, 2001).
Swordfish show remarkable physiological versatility, inhabiting waters with sea surface temperatures (SSTs) ranging from 14 to 30°C, but tolerate both extremely low temperatures (4°C) and rapid and marked temperature changes (> 20°C) (Dewar et al., 2011). This species has been associated with frontal zones, where the presence, position, and strength of the convergent fronts play a key role with regard to swordfish catch rates (Seki et al., 2002; Hinton et al., 2005). Several of these fronts occur in the eastern Pacific Ocean (EPO), including areas off California and Baja California, Ecuador, Peru, and Chile, and in the equatorial Pacific (Hinton et al., 2005). Larger commercial catches have also been related to lower sea surface temperatures, higher chlorophyll concentrations, and higher latitudes. A latitudinal gradient in size composition has been also recorded, with juvenile swordfish associated with warmer conditions, higher salinity, and lower chlorophyll concentrations (Yañez et al., 2009).
Although different studies have analyzed the stock structure of swordfish in the Pacific Ocean, this is still not well known. Reeb et al. (2000) investigated the genetic structure of swordfish populations from the northwest and southwest Pacific, and found no differences among samples taken in the EPO. Alvarado-Bremer et al. (2006), with samples of Chile. Ecuador, Mexico, Hawaii, and eastern and western Australia, found a single stock in the southern EPO. The northeastern Pacific Ocean (from Ecuador to Mexico) differed from the north-central Pacific Ocean (Hawaii), which in turn was different from the southwestern Pacific Ocean (pooled eastern and western Australia). Hinton (2003), using fisheries data, concluded that there are northern and southern stocks of swordfish in the EPO, with the boundary between them at 5°S, and that some mixing of stocks from the central Pacific with the northeastern stock may occasionally take place.
Swordfish is a species of high economic value and supports commercial fisheries throughout much of its geographic range, with over 125 000 tons harvested worldwide annually (http://www.fao.org/fishery/species/2503/en). In the EPO, it is caught with large-scale and artisanal longline gear, gillnets, harpoons, and occasionally with recreational gear. During 2011-2013, the average longline catch of swordfish exceeded 22,000 tons (IATTC, 2015). Particularly along the western coast of Baja California peninsula within the Mexican Economic Exclusive Zone, swordfish is the only species reserved by law for sport-fishing with commercial fishing licenses. Commercial fishing is conducted outside of the fifty miles along the coast, and during 2010, recorded a catch of around 100 tons. In 2012, 39 commercial fishing licenses were operative for the same number of commercial vessels based at the ports of Ensenada BC and San Carlos, BCS (DOF, 2012). Longline fishery catch swordfish as well as other resources including shark, finfish, and demersal resources. Although the sport-fishing fleet in Baja California Sur also catches this species, it represents 0.1%, equivalent to 60 total swordfish per year (DOF, 2012). This low incidence of sport-fishing capture is due to its daytime migratory habits, which makes it hardly accessible to most sport-fishing activities, which are usually carried out during the daytime.
The swordfish and other species like sharks play a key role in the ecosystem as top predators, hence contributing to shape the structure of the trophic network through top-down trophic cascades (Kitchell et al., 2002).
Most studies on the feeding habits of swordfish have been conducted using stomach content analysis (SCA) in the Atlantic (Stillwell & Kohler, 1985; Azevedo, 1989; Clarke et al., 1995; Hernández-García, 1995; Barreto et al., 1996 Chancollon et al., 2006; Gorni et al., 2012) and the Pacific Oceans (Markaida & Sosa-Nishizaki, 1998; Ibáñez et al., 2004; Castillo et al., 2007; Letelier et al., 2009). Ménard et al. (2007) used stable isotopes analysis for Indian Ocean swordfish, and Young et al. (2006) used both stomach content and stable isotopes for this species caught off Eastern Australia. From these studies, those authors have considered that swordfish is an opportunistic species. Although some studies have found that swordfish feed mainly on fishes (Scott & Tibbo, 1986; Azevedo, 1989; Barreto et al., 1996; Potier et al., 2007), most of them have reported that this species feeds mainly on cephalopods and, to a lesser extent, on fish, with the minimum presence of crustaceans. Particularly for the western coast of Baja California, Markaida & Sosa-Nishizaki (1998) from the analysis of swordfish stomachs captured in two sampling zones (northern and southern Baja California) during 1992–1993 found differences in the diet depending on the area of capture, where swordfish caught in the north fed mainly on fish, while those captured in the south showed a preference for cephalopods, with the purpleback flying squid (Sthenoteuthis oualaniensis (Lesson, 1830)) as the most important prey.
Although SCA provides key information including the identification of prey, this method involves some limitations, e.g., when the stomach is empty or biases in case of differences in the digestion rates between prey species (Reñones et al., 2002). In addition, this method does not account for long-term patterns of nutrient transfer (e.g., months or years) and instead provides only an instantaneous measure of the diet of an organism (Vander-Zanden et al., 1997). Currently, stable isotope analysis (δ13C and δ15N) has emerged as a valuable tool that supplements traditional SCA in feeding studies for providing a time-integrated dietary representation and characterization of trophic relationships over a scale of months or years (Buchheister & Latour, 2010).
The ratio of nitrogen isotopes (15N/14N, δ15N) is used to infer the trophic position (DeNiro & Epstein, 1981). In addition, δ15N values for marine predators have been used to determine their movements because there is a geographic variation in reference nitrogen values in the open ocean (Graham et al., 2010). On the other hand, the carbon isotopic composition (13C/12C, δ13C) is commonly used to infer the carbon source, and in marine ecosystems, it is used to evaluate high versus low latitudes, or to differentiate between nutrient sources, e.g., near the coast or in the open ocean (France, 1995).
Swordfish is a species of economic and ecological importance, and the knowledge about its feeding ecology in the western coast of Baja California is still scarce. Hence, the objective of this study was to determine the compositions of the diet and trophic positions of fishes caught in Baja California Sur (Mexico) through two supplementary techniques: the analysis of stomach contents to document the composition of the diet, and stable isotope analysis to derive long-term feeding information.
Materials and methods
Sampling
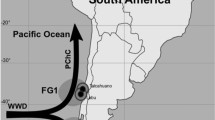
Samples of stomach contents (March 2011–December 2013) and dorsal muscle tissue (January–December 2013) were collected from swordfish caught by commercial longliners that operate in the western coast of Baja California (Fig. 1).
The date of capture, geographic position (latitude, longitude), sex, and lower jaw fork length (LJFL, cm) were recorded for each organism sampled. Muscles and stomachs were labeled and frozen (− 20°C) , and stored for later laboratory analyses.
Stomach content analysis
In the laboratory, stomachs were thawed and emptied, and prey types in the stomach were classified into major taxonomic categories such as fish, cephalopods, crustaceans, and unidentified organic matter (UOM). Prey items were identified to the lowest taxon possible according to the specialized literature or following specialized guidance, and in consideration of the degree of prey digestion. The number of items of each prey type in each stomach was recorded, as well as the wet weight of each individual item; in the case of cephalopods, mantle length was also noted. Fish were identified based on either axial and appendicular skeletons using the taxonomic keys proposed by Monod (1968) and Miller & Jorgensen (1973), or otoliths, by comparing them with the otolith collection of the Laboratory of Trophic Ecology. Cephalopods and their beaks were identified using Wolff (1984), Clarke (1986), and Iverson & Pinkas (1971). We identified crustaceans by their exoskeletons, or exoskeleton remains, according to Fischer et al. (1995).
To determine whether the number of samples analyzed was sufficient to accurately represent the swordfish diet, a randomized cumulative Shannon diversity curve was drawn using the software EstimateS v8 and Statistica 7.0, and calculating the coefficient of variation (CV) of these values. When an asymptotic stabilization of the curve was obtained (Cortés, 1997) and a CV below 0.05 was achieved, was considered that the minimum number of stomachs required to accurately describe the diet had been reached. The diversity curve was computed by mixing various levels of prey item identification (family, genus, and species).
The Index of Relative importance (IRI) was calculated as follows:
where %N is the number of individuals of each prey type, and %W is the wet weight of each prey type, expressed as percentage of the total number or weight of all prey items in the stomach contents, respectively.
The frequency of occurrence of each prey type (i.e., presence or absence) in all stomachs that contained food was recorded as percentage (%FO) (Cortés, 1997). To determine whether there were differences in the diet between sexes, size, or between years, similarity was analyzed using permutation randomization methods in a Bray–Curtis dissimilarity matrix (analysis of similarity [ANOSIM] in PRIMER 6 version 6.1.6). The global rank dissimilarity value, RANOSIM (0 ≤ RANOSIM≤ 1) is a useful comparative measure of the degree of separation. When RANOSIM approaches zero, the null hypothesis cannot be rejected, i.e., there is no separation between groups (Clarke & Warwick, 2001).
The breadth of the swordfish trophic niche was evaluated by Levin’s standardized index, Bi (Krebs, 1999). Bi ranges from 0 to 1, with low values (< 0.6) indicating a diet dominated by a few prey types (i.e., a specialist predator) and higher values (≥ 0.6) indicating a generalist predator.
where Bi is Levin’s index for predator j; ∑P2ij is the numerical proportion of the ith prey type in predator j’s diet; and n is the number of prey categories.
Differences in swordfish diet between sizes were explored through an analysis of similarity [ANOSIM]. To this end, animals were grouped into immature or mature. DeMartini et al. (2000) estimated that L50 was 102 cm eye-fork length (EFL) for males and 144 cm for females. These measurements were transformed into LJFL using the morphometric relationships established by Setyadji et al. (2016), resulting in L50 values of 118 cm for males and 160 cm for females. Considering sex and size at maturity, swordfish were grouped into group I (immature females < 160 cm LJFL); group II (mature males ≥ 118 cm LJFL); and group III (mature female ≥ 160 cm LJFL). Since the number of immature males (< 118 cm LJFL) was low (only two), these were pooled with unidentified organisms in group IV.
The trophic position (TP) based on stomach content analysis was calculated using the equation proposed by Christensen & Pauly (1992):
where DCij is the diet composition by weight in terms of the proportion of prey type j in the diet of predator i; TPj is the trophic position of prey type j; and n is the number of prey types in the diet.
Isotopic analysis
For the isotopic analyses, samples were placed in vials fitted with Teflon caps and dried for 24 h in a Labconco lyophilizer at − 45°C and 0.120 millibars. Afterward, samples were ground in an agate mortar. Since lipids can be inferred from the ecological interpretation of δ13C signals (Post et al., 2007), these were removed from samples prior to analysis following the protocol of Kim & Koch (2011). Subsamples (1 mg) were weighed using an Ohaus analytical scale (± 0.0001 g) and stored in tin capsules (8 × 5 mm). Carbon isotope (δ13C) and nitrogen isotope (δ15N) compositions were determined at the Stable Isotope Laboratory, at CICIMAR-IPN, using an isotope-ratio mass spectrometer (20-20 mass spectrometer; PDZ Europa Scientific, Sandbach, UK) with a precision of 0.02‰. The C:N ratio was calculated from values obtained with an elemental analyzer coupled to the isotope-ratio mass spectrometer (EMRI 20–20, PDZ Europa, Sandbach, UK).
Isotopic ratios were expressed as δ (‰) following the equation:
where Rsample is the ratio between isotopes (15N/14N o 13C/12C, respectively). The standards used were Pee Dee Belemnite limestone (PDB) for δ13C, and atmospheric nitrogen (AIR) for δ15N (Hansson et al., 1997). The standard deviation of δ13C and δ15N values in the certified laboratory material was ~ 0.2‰.
The Shapiro–Wilk test was performed to assess the data normality of isotopic δ15N and δ13C values, while the homogeneity of variances was evaluated with the Levene´s test. All analyses were performed using the R statistical programming language, with a significance level of 0.05 (R Core Team, 2017).
The TP based on stable isotope values was estimated using the equation by Post (2002):
where λ is the trophic position for particulate organic material (POM), which is equal to 1; Δn is the theoretical value of 15N enrichment for each trophic position (3.4‰, Post, 2002); δ15NPredator is the isotopic value for X. gladius; and δ15Nbase is the value for POM (White et al., 2007; POM = 5.7‰).
SIAR model
The relative contribution of the various food sources was obtained using a Bayesian stable-isotope mixing model (SIAR; Parnell et al., 2008), which allows for the inclusion of isotopic signatures, elemental concentrations and fractionation, together with the uncertainty of these values within the model. Isotopic fractionation is the difference between each trophic position, being 3.5‰ for δ15N is (DeNiro & Epstein, 1981), and 1‰ for δ13C (Rau et al., 1983). Since no isotope analysis of prey items was performed, these were obtained from previous investigations conducted in the same study area (Ochoa-Díaz, 2009; Torres-Rojas et al., 2013; Tripp-Valdez et al., 2015).
Niche width and the potential overlap of the trophic niche between sexes and across size intervals were determined through a test using Stable Isotope Bayesian Ellipses in R (SIBER) (Jackson et al., 2011) from the SIAR package (Parnell & Jackson, 2013). This analysis uses metrics based on ellipses and provides the corrected standard ellipse area (SEAc) used as trophic niche breadth and overlap between ellipses, when the overlapping area between the ellipses reaches a proportion value close to 1, representing a high degree of trophic overlap.
Results
Stomach content analysis
In total, 244 swordfish ranging in size from 98 to 300 cm LJFL and composed of 165 females and 58 males, and 21 swordfish of unknown sex were examined for stomach content (Table 1). From the stomachs analyzed, 65% (n = 159) contained food and 35% (n = 85) were empty. The size frequency distribution of males, females and animals of unknown sex with stomach content is shown in Fig. 2.
Cumulative prey-curve predictions indicated that the sample size was sufficient to characterize the swordfish diet according to the groups established (CV < 0.05) (Fig. 3). Group IV was considered to avoid missing information, but the small sample size made it impossible to obtain information without a bias in the conclusion derived from it.
Cumulative prey curve diversity of stomach content analysis for: A Immature females, B Mature females and C Mature males showing that the diet is well represented where the curve becomes asymptotic and the coefficient of variation (CV) reaches a value lower than 0.05. Vertical lines represent standard deviation
The diet of swordfish was composed of 33 prey items, including 15 cephalopods, 14 fishes, 3 crustaceans, and unidentified organic matter (UOM) (Table 2). Cephalopods were the most important prey item (98.91% IRI). The jumbo squid (Dosidicus gigas (Orbigny, 1835)) (95.07% IRI) was the main prey item, followed by other cephalopods such as Gonatus spp. (1.15% IRI) and Argonauta spp. (1.07% IRI). The fish prey item with the highest percentage of importance in the diet was Scomber japonicus Hottuyn, 1872 (0.59% IRI) (Table 2).
The highest variability in the diet was found in group I (immature females < 160 cm). In this category, D. gigas (78.7%), Pleuroncodes planipes Stimpson, 1860 (5.6%), and S. japonicus (4.75%) were the most important prey species. The cephalopod D. gigas was the dominant prey item (IRI > 98%) in the diet of larger swordfish (groups II & III) (Table 3). No significant differences were found between the three groups (RANOSIM = − 0.015, P > 0.05).
The analysis of the interannual variation in the diet (2011–2013) revealed that cephalopods were dominant in terms of frequency, abundance, and biomass. Although both fish and crustaceans were present in the swordfish diet, the interannual variability and similarity analyses did not show significant differences in diet composition during the study period (RANOSIM = 0.079, P > 0.05).
The estimated trophic niche breadth (Bi = 0.03) was below 0.6, indicating a strong selective behavior. The TP calculated using stomach content data was 3.9 (± 0.34).
Stable isotope analysis (SIA)
A total of 128 muscle samples collected in 2013 were analyzed. The size of swordfish analyzed ranged between 90 and 287 cm LJFL. δ15N values ranged from 13.05‰ to 19.66‰ (17.42 ± 1.26‰), and δ13C values ranged from − 19.32‰ to − 15.60‰ (− 17.50 ± 0.54‰). The carbon–nitrogen ratios (C:N ratio) ranged between 2.6 and 3.5, with an average of 3.2.
δ13C and δ15N data were normally distributed (W = 0.93, P > 0.05 and W = 0.88, P > 0.05 respectively) and with homogenous variances (F = 0.37, P > 0.05 and F = 0.15, P > 0.05 respectively). No statistical differences were found between groups, either for δ13C values (F = 0.036, P > 0.05) nor for δ15N (F = 0.001, P > 0.05) (Table 4, Fig. 4).
Mean ± standard deviation δ 15N and δ 13C for the sex-maturity groups for swordfish captured off the southern Baja California peninsula: I: immature female (< 160 cm lower jaw fork length); II mature males (≥ 118 cm LJFL); III (mature females ≥ 160 cm LJFL) and IV (immature males and unidentified organisms)
The estimated isotopic trophic position was 4.6 (± 0.36). Mean values ranged from 3.98 (± 0.42) for size group I to 4.80 (± 0.27) for size group III, as shown in Table 4.
Stable isotope mixing models (SIAR)
The isotopic contribution of each main prey item was determined using the SIAR model, based on the prey consumed by swordfish. The mixing model was constructed using isotopic values of the main prey item reported in previous studies: D. gigas (Torres-Rojas et al., 2013), Gonatus spp. (Ochoa-Díaz, 2009), S. japonicus, Ancistrocheirus lesueurii (d’Orbigny [in Férussac & d’Orbigny], 1842), P. planipes (Tripp-Valdez et al., 2015), and Thysanoteuthis rhombus Troschel, 1857 (Ochoa-Díaz, 2009). All cephalopods were pooled into a single category, as isotopic values were very similar for these organisms; other prey items were analyzed at the species level (no pooling). The most important prey group consumed by this predator was cephalopods, with a mean contribution to the diet of 37%, followed by P. planipes with 21% and Lagocephalus. lagocephalus (Linnaeus, 1758) with 17.6%; the other two species accounted for the remaining 24% (Fig. 5).
Isotopic niche breadth and overlap (SIBER test)
The ellipses generated for the different sex and maturity groups shows that group I has the bigger isotopic niche with a SEAc of 9.7, while the other groups possess similar values (group II, SEAc = 2.9; group III, SEAc = 3.3). The overlap values for the different groups were also relatively high, with a proportion of Bayesian overlap > 0.5 (Fig. 6).
Discussion
D. gigas was the dominant prey in the swordfish diet, during the 3 years of sampling (2011-2013), so that swordfish can be classified as a teutophagus predator. These findings are consistent with those reported in previous studies (Clarke et al., 1995; Chancollon et al., 2006; Young et al., 2006). However, Azevedo (1989) for Portuguese continental waters, Barreto et al. (1996) for the Caribbean Sea and Portier et al. (2007) for western Indian Ocean have reported fish as the dominant food type. These variations are common in fish. Food preferences of predatory fishes are highly complex, being influenced by various factors, such as prey accessibility and mobility, prey abundance, ontogenetic changes, prey energy content, and prey size selection, as well as seasonal and spatial changes (Ibáñez et al., 2004). Changes in the diet between areas have been reported in previous studies (Carey & Robison, 1981; Hernández-García, 1995), particularly in the study area, Markaida & Sosa-Nishizaki (1998) found differences even in two adjacent areas; while in northern of Baja California swordfish fed mainly on fish, in southern areas it preferred cephalopods, although, in this case, the most important prey was the squid S. oualaniensis.
In general, the swordfish diet is associated with food availability in the area, which tends to change with time (Markaida & Sosa-Nishizaki, 1998; Ibáñez et al., 2004; Ménard et al., 2007; Mejuto et al., 2012). This could be the reason why D. gigas was the main species in the swordfish diet in this study, as this cephalopod was abundant in northwestern Mexico where the average catch exceeded 30,000 tons during 2011–2012 (DOF, 2014). The high abundances of this squid in the region recorded since 1994 could be the cause of the difference in swordfish diet observed previously in the study area, with samples taken in 1992–1993, when the cephalopod S. oualaniensis was the main species (Markaida & Sosa, 1998). In addition, during 1992–1993, when a moderate El Niño event was recorded (Strub & James, 2002), it has been reported that D. gigas changes its distribution range during events of this type (Taipe et al., 2001), so D. gigas was likely not available at that time, being replaced by S. oualaniensis, a species that prefers warmer waters. Although interannual diet variations could be closely related to the predominant prey in the area, this study found no significant differences between years, although the presence of fish and some crustaceans (P. planipes) were recorded in 2012 and 2013.
In recent studies on the feeding habits of top predators, D. gigas has frequently been reported in the diet of different species, such as Coryphaena hippurus Linnaeus, 1758, Kajikia audax (Philippi, 1887), Makaira nigricans Lacepède, 1802, etc. (Torres-Rojas et al., 2013; Tripp-Valdez et al., 2015; Ortega-García et al., 2017), and in particular in X. gladius (Castillo et al., 2007; Letelier et al., 2009). D. gigas is itself an active predator with an important impact on local food webs in areas where it is abundant (Markaida & Sosa-Nishizaki, 2003). This cephalopod, in addition to being one of the most abundant squid species in the Pacific Ocean (Nigmatullin et al., 2001), carries out regular diel vertical migrations over distances of 300 m with oxygen levels of 9.18 ± 3.2 μM during daytime to near-surface water at night (Gilly et al., 2006; Bazzino et al., 2010). Swordfish typically forage in deep water during the day and stay in the mixed layer at night (Abascal et al., 2010); thus, this species and the jumbo squid co-occur in time and space. This relationship has been established in other studies, suggesting that the stomach content of pelagic fish such as swordfish directly reflects the pelagic cephalopod fauna (Tsuchiya et al., 1998). To note, the presence of bathypelagic species such as Vampyroteuthis infernalis Chun, 1903 indicate that swordfish are capable of feeding at great depths and that this species is physiologically adapted to function even at extreme temperature and oxygen conditions (Carey & Robison, 1981; Van den Burg et al., 2005; Abecassis et al., 2012). The presence of V. infernalis has been reported in the swordfish stomach content in others studies (Markaida & Sosa-Nishizaki, 1998; Markaida & Hochberg, 2005; Young et al., 2006; Letelier et al., 2009).
Ontogenetic shifts in the diet are known in fish, closely linked to maturity stages, since energy requirements vary throughout in the life history of the predator (Wootton, 1990). According to Nikolsky (1963), the changes in feeding behavior may be influenced by morphological changes, with juveniles foraging on small prey, which does not involve a high energy demand. Although no significant differences were found between males and females, coinciding with the results of previous studies (Barreto et al., 1996; Markaida & Sosa-Nishizaki, 1998; Young et al., 2006; Letelier et al., 2009), a slight variation was observed in the diet between sex-maturity groups. Swordfish in group I (immature females < 160 cm LJFL) consumed a greater variety of prey types, including crustaceans, which are common in the diet of smaller organisms. Similar results have also been reported by Letelier et al. (2009), with females showing a greater diversity of fish and being the only group in which crustaceans were found; for their part, Young et al. (2006) did not find significant difference in prey mass between males and females, but reported that smaller females (< 150 cm OFL) fed relatively more fish than cephalopods, whereas smaller males fed relatively more cephalopods. In this study, although fish did not dominate the diet of immature females (< 160 cm LJLF), these were present in the diet along with crustaceans, although in small proportion. Group II (mature males ≥ 118 cm LJFL) and group III (mature females > 160 cm LJFL) fed almost exclusively on cephalopods. Swordfish larvae feed on copepods, whereas juveniles and adults feed preferentially on fish and cephalopods, according to the season and habitat. In this sense, prey diversity in the diet of this species is frequently associated with different feeding areas rather than life stages (juvenile–adult). As swordfish is a migratory species, its feeding strategy depends on local prey availability, and when performing extensive migratory movements (Abecassis et al., 2012; Abascal et al., 2015), they change their habitat constantly, and this is likely reflected in a changing diet.
Compared with other top predators in the study area, C. hippurus 86 prey items (Tripp-Valdez et al., 2015), K. audax 41prey items (Ortega-Garcia et al., 2017), Thunnus albacares (Bonnaterre, 1788) 21 prey items (Alatorre-Ramirez et al., 2017), swordfish has a narrow variety of food types in their diet (33 food items), with cephalopods accounting for 98.91% IRI, with D. gigas as the main prey item. Although the dominance of D. gigas was reflected in the narrow trophic breadth obtained (Bi = 0.03), X. gladius can be best described as an opportunistic predator, assuming that prey items are consumed in proportion to their abundance and availability. When prey species are abundant in the region, predators can select certain food types to maximize their consumption and energy use. In contrast, when food is scarce, predators cannot afford this selectivity and feed on any available prey (Wetherbee et al., 1990). For example, the presence of crustaceans such as P. planipes is indicative of an opportunistic predator: although swordfish consume this prey type when it is abundant, providing a large amount of energy, it is not part of its preferred diet. Swordfish include prey species that are not important in the diet, suggesting an opportunistic foraging behavior; however, the results from the SCA and stable isotopes support that the swordfish has a marked preference for squid; consequently, it was classified as a teutophagous predator. Similar results were reported by Letelier et al. (2009) off the coast of central and northern Chile. In the study area, the presence of the high abundances of D. gigas has led to lower niche breadth values, resulting in the classifying of some top predators as specialists (i.e., striped marlin Ortega-Garcia et al., 2017).
Pelagic fishes like tuna, sharks and billfishes have been identified as top predators in marine ecosystems (Young et al., 2006). Since swordfish consume mainly other carnivorous organisms (i.e., cephalopods), it occupies a high trophic position (TP). In this case, the estimated TP using SCA was 3.9, which was similar to the TP calculated by Markaida & Sosa-Nishizaki (1998) (TP = 3.97) for the western coast of Baja California. In the study area, similar results have been estimated for T. albacares (TP = 3.9) and Katsuwonus pelamis (Linnaeus, 1758) (TP = 3.5) (Alatorre-Ramirez et al., 2017). However, Froese & Pauly (2015) reported a TP of 4.5 for swordfish based on diet studies in areas where fish were the dominant prey. Therefore, in areas where X. gladius fed on cephalopods, the TP was lower relative to areas where this species fed on fish.
δ13C and δ15N stable isotope analysis
The stable isotope (δ13C and δ15N) technique is currently used in trophic ecology as a tool that supplements traditional methods to infer the sources and types of food consumed by organisms during a longer period, obtaining a wider picture on their trophic ecology. Stable isotope analysis (δ13C and δ15N) has been used for other pelagic predators such as C. hippurus (Tripp-Valdez et al., 2015) and billfish (M. nigricans, K. audax, Torres-Rojas et al., 2013); however, only Young et al. (2006) and Ménard et al. (2007) have applied stable isotope analysis for swordfish from Australia and the Indian Ocean. The study reported here is the first focusing on the trophic ecology of swordfish in Mexican waters using SIA, as well as the first estimation of its trophic position.
The δ13C and δ15N values found in this study (δ15N = 17.42 ± 0.26‰, range: 13.05—19.66‰; δ13C = − 17.50 ± 0.54‰, range: − 19.32 to − 15.60‰) contrasted with those observed by Ménard et al. (2007), whose values ranged from − 17.4 to − 15.0‰ for δ13C, and from 11.8 to 16.2‰ for δ15N. Young et al. (2006) reported a mean δ15N value of 12.91‰ for X. gladius in Australian waters. Although part of the variation in δ13C and δ15N values found in this study vs. those authors could be due to spatial and temporal differences that determine prey availability, as well as to the variability of the isotopic signature of each prey, we need to take into account that the main variability is due to the baseline values, as these differ between and within ocean basins.
It is possible that the range in δ13C values observed for X. gladius (− 19.32 to − 15.60‰) reflects horizontal movements (coastal and oceanic migrations). More positive δ13C values (− 15.60‰) mean that swordfish are more enriched in carbon, and hence were feeding in coastal areas; conversely, less positive δ13C values (− 19.32‰) mean that swordfish were feeding in more oceanic areas. This variability could be attributed to onshore-offshore movements that have been reported particularly in waters surrounding the tip of the Baja Peninsula where swordfish display clear cycles of movement between deep water and an inshore bank (Carey & Robison, 1981).
Isotopic comparison between swordfish groups
Body size plays a crucial role in predator–prey interactions. As organisms attain larger sizes, they tend to consume larger prey items with higher biomass, possibly at higher trophic positions (Cury et al., 2003). Young et al. (2006) reported that δ15N values varied with size in X. gladius. These authors assumed that smaller organisms fed preferentially on fish such as myctophids, which belong to lower trophic levels, compared with larger individuals that consumed mostly cephalopods, had higher δ15N values, and belonged to a higher trophic position. Ménard et al. (2007) used prediction models that reflected the change in δ15N and δ13C values along longitudinal gradients in swordfish body size (68–225 cm LJFL), observing that the δ15N isotopic difference between small and large organisms was approximately 2.3‰, and that δ13C values showed the same size reduction pattern, but with smaller isotopic differences (0.8‰). In this study, no clear trend regarding ontogenetic changes was evident. Some larger organisms (266.1–310 cm LJFL) showed isotopic values corresponding to swordfish feeding closer to the coast (coastal prey), relative to smaller organisms that showed more oceanic carbon values. The less negative carbon isotopic values of large organisms suggest that these were feeding in areas either close to the coast, or in more productive areas where prey abundance is higher, which was likely the case due to their migration capacity. These prey types have relatively lower isotopic values (more negative δ13C values) because they usually feed on primary sources from lower trophic levels (primary producers).
The TP value obtained using SIA was higher (TP = 4.5) than the one based on SCA (TP = 3.9). This difference may be due to the extensive migrations of swordfish to feed on larger cephalopods, which would result in higher isotopic enrichment with the consequent high trophic position. Cephalopods have a marked ontogenetic shift in the diet, reflecting variations in their trophic position (Pauly et al., 1998). Slight differences were observed in X. gladius relative to other predators, i.e., M. nigricans (TP = 4.47), K. audax (TP = 4.29), Makaira indica (Cuvier, 1832) (TP = 4.5), C. hippurus (TP = 4.4) (Torres-Rojas et al., 2013; Froese & Pauly, 2015); these are related to differences in food types and preferences. Overall, based on stomach contents and stable isotopes, X. gladius could be considered as a tertiary predator.
Isotopic contribution: Bayesian mixing model (SIAR)
Based on the SIAR mixing model, the contribution of some prey types to swordfish muscle tissue was assessed. There was a strong influence of cephalopods on the isotopic values of the predator; this result is consistent with the findings observed with traditional SCA. The other important prey species, P. planipes, accounted for an important percentage of the diet and is seemingly consumed in a larger proportion than the one reflected in SCA. A possible explanation is that SCA tends to overestimate some prey types such as cephalopods, as beaks tend to remain in the stomach over long periods of time (Santos et al., 1999). In this way, isotopes are helpful in determining the importance of this prey type. No studies are currently available on the relative contribution of the different prey types to the swordfish diet; therefore, this is the first study where both the SIA-based trophic position and the contribution of each prey to the predator (X. gladius) are reported.
SIBER
This test confirmed our findings using stomach content analysis, i.e., the sex-maturity groups occupy similar trophic niches, with both using the same resources. This suggests that, in the study area there is no apparent feeding segregation, between sex and maturity groups; therefore, mature males and immature–mature females seemingly do not require different resources to meet their particular energy demands. In addition, this may be because cephalopods were highly abundant in the study area during the analyzed period (DOF, 2012), hence being accessible to swordfish of all sizes and sexes.
Conclusions
The results of this study confirmed that swordfish are mainly teutophagus predators, although can consume other resources when these are abundant in the habitat. No ontogenetic changes in the diet were detected during the study period, although the presence of some fish and crustaceans was recorded in the stomach of immature females. The high variability found in δ13C reflects horizontal migrations that could be related to onshore–offshore movements due the great number of banks in the study area. In this sense and from the mixing model SIAR, the group of prey with the greatest relative contribution to the swordfish diet was cephalopods. In turn, both the stomach content analysis and the isotopic methods showed that X. gladius belongs to the group of tertiary predators because of the high trophic level value obtained.
References
Abascal, F. J., J. Mejuto, M. Quintans & A. Ramos-Cartelle, 2010. Horizontal and vertical movements of swordfish in the Southeast Pacific. ICES Journal of Marine Science 67: 466–474.
Abascal, F. J., J. Mejuto, M. Quintans, B. García-Cortés & A. Ramos-Cartelle, 2015. Tracking of the broadbill swordfish, Xiphias gladius, in the central and eastern North Atlantic. Fisheries Research 162: 20–28.
Abecassis, M., H. Dewar, D. Hawn & J. Polovina, 2012. Modeling swordfish daytime vertical habitat in the North Pacific Ocean from pop-up archival tags. Marine Ecology Progress Series 452: 219–236.
Alatorre-Ramirez, Galvan-Magaña, F., Y. E., Torres-Rojas, & R.J. Olson, 2017. Trophic segregation of mixed schools of yellowfin tuna (Thunnus albacares) and skipjack tuna (Katsuwonus pelamis) caught in the eastern tropical Pacific Ocean. Fishery Bulletin 115: 252–268.
Alvarado-Bremer, J. R., M. G. Hinton & T. W. Greig, 2006. Evidence of spatial genetic heterogeneity in Pacific swordfish (Xiphias gladius) revealed by the analysis of LDH—A sequences. Bulletin of Marine Science 79: 493–503.
Azevedo, M., 1989. Information on the swordfish fishery in the Portuguese Continental EEZ. ICCAT Collective Volume of Scientific Papers 32(2): 282–286.
Barreto, M. A., L. A. Marcano, J. J. Alio, X. Gutiérrez & A. Zerpa, 1996. Alimentación del pez espada (Xiphias gladius), en el área del Caribe Venezolano. Collective Volume of Scientific Paper ICCAT 45: 337–342.
Bazzino, G., W. F. Gilly, U. Markaida, C. A. Salinas-Zavala & J. Ramos- Castillejos, 2010. Horizontal movements, vertical-habitat utilization and diet of the Humboldt squid (Dosidicus gigas) in the Pacific Ocean off Baja California Sur, Mexico. Progress in Oceanography 86(1–2): 59–71.
Buchheister, A. & R. J. Latour, 2010. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Canadian Journal of Fisheries and Aquatic Sciences 67: 445–461.
Carey, F. G. & B. H. Robison, 1981. Daily patterns in the activities of swordfish, (Xiphias gladius) observed by acoustic telemetry. Fisheries Bulletin 79: 277–292.
Castillo, K., C. M. Ibañez, C. González & J. Chong, 2007. Dieta del pez espada Xiphias gladius Linnaeus, 1758, en distintas zonas de pesca frente a Chile central durante el otoño de 2004. Revista de Biología Marina y Oceanografía 42: 149–156.
Chancollon, O., C. Pusineri & V. Ridoux, 2006. Food and feeding ecology of Northeast Atlantic swordfish (Xiphias gladius) off the Bay of Biscay. ICES Journal of Marine Science 63: 1075–1085.
Christensen, V. & D. Pauly, 1992. ECOPATH II-A software for balancing steady-state ecosystem models and calculating network characteristics. Ecological Modelling 61: 169–185.
Clarke, M. R., 1986. A handbook for the identification of cephalopod beaks. Oxford University Press, Oxford.
Clarke, M. R., D. C. Clarke, H. R. Martins & H. M. Silva, 1995. The diet of swordfish (Xiphias gladius) in Azorean waters. Arquipélago. Life and Marine Science 13: 53–69.
Clarke, K. R. & R. M. Warwick, 2001. Change in marine communities: an approach to statistical analysis and interpretation, 2nd ed. Primer-E Ltd, Plymouth.
Cortés, E., 1997. A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences 54: 726–738.
Cury, P., L. J. Shannon & Y. J. Shin, 2003. The functioning of marine ecosystems: a fisheries perspective. In Sinclair, M. & G. Valdimarsson (eds), Responsible fisheries in the marine ecosystem. FAO and CABI publishing, Rome, Wallingford, UK: 103–123.
DeMartini, E. E., J. H. Uchiyama & H. A. Williams, 2000. Sexual maturity, sex ratio, and size composition of swordfish, Xiphias gladius, caught by the Hawaii-based pelagic longline fishery. U.S. Fishery Bulletin 98: 489–506.
DeNiro, M. J. & S. Epstein, 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45: 341–351.
Dewar, H., E. D. Prince, M. K. Musyl, R. W. Brill, C. Sepulveda, J. Luo, D. Foley, E. S. Orbesen, M. L. Domeier, N. Nasby-Lucas, D. Snodgrass, R. M. Laurs, J. P. Hoolihan, B. A. Block & L. M. Mcnaughton, 2011. Movements and behaviours of swordfish in the Atlantic and Pacific Oceans examined using pop-up satellite archival tags. Fisheries Oceanography 2: 219–241.
DOF, 2012. Diario Oficial de la Federación (DOF). Carta Nacional Pesquera, Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA), México.
DOF, 2014. Diario Oficial de la Federación (DOF). Plan de Manejo Pesquero de Calamar Gigante (Dosidicus gigas). Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA), México.
Fischer W, Krupp F, Schneider W, Sommer C, Carpenter K. 1995. Guia FAO para la identificación de especies para los fines de pesca. In: Fischer W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem VH, (eds) Pacifico centro-oriental FAO Roma. 648–1652.
France, R. L., 1995. Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Marine Ecology Progress Series 124: 307–312.
Froese, R. & D. Pauly, 2015. FishBase. World Wide Web electronic publication. www.fishbase.org, version 02/2015.
Gilly, W. F., U. Markaida, C. H. Baxter, B. A. Block, A. Boustany, L. Zeidberg, K. Reisenbichler, B. Robinson, G. Bazzino & C. Salinas, 2006. Vertical and horizontal migrations by jumbo squid, Dosidicus gigas, revealed by electronic tagging. Marine Ecology Progress Series 324: 1–17.
Gorni, G. R., S. Loibel, R. Goitein & A. F. Amorim, 2012. Stomach contents analysis of swordfish (Xiphias gladius) caught off southern Brazil: a bayesian analysis. Collective Volume of Scientific Papers ICCAT 68: 1594–1600.
Graham, B. S., P. L. Koch, S. D. Newsome, K. W. Mcmahon & D. Aurioles, 2010. Using isoscapes to trace the movements and foraging behavior of top predator in oceanic ecosystems. In West, J. B., G. Bowen, T. Dawson & K. Tu (eds), Isoscapes: Understanding movement, pattern, and process on Earth through isotope mapping. Springer, Berlin: 299–318.
Hansson, A., W. G. Hobbie, R. Elmgreen, U. Larsson, B. Fry & S. Johansson, 1997. The stable nitrogen isotope ratio as marker of food web interactions and fish migration. Ecology 78: 2249–2257.
Hernández-García, V., 1995. The diet of the swordfish (Xiphias gladius Linnaeus, 1758) in the central East Atlantic, with emphasis on the role of cephalopods. Fisheries Bulletin 93(2): 403–411.
Hinton, M. G., 2003. Status of swordfish stocks in the eastern Pacific Ocean estimated using data from Japanese tuna longline fisheries. Marine and Freshwater Research 54: 393–399.
Hinton, M. G., W. H. Bayliff & J. Suter, 2005. Assessment of swordfish in the eastern Pacific Ocean. Inter-American Tropical Tuna Commission Stock Assessment Report 5: 291–326.
IATTC, 2015. Tunas, billfishes and other pelagic species in the eastern pacific ocean in 201. Fishery Status Report Inter-American Tropical Tuna Commission, La Jolla, CA: 199.
Ibáñez, C. M., C. González & L. Cubillos, 2004. Dieta del pez espada Xiphias gladius Linnaeus, 1758, en aguas oceánicas de Chile central en invierno de 2003. Investigaciones marinas 32: 113–120.
Iverson, L. K. & L. Pinkas, 1971. A pictorial guide to beak of certain eastern Pacific cephalopods. California Fish and Game 2: 83–105.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80: 595–602.
Kim, S. L. & P. L. Koch, 2011. Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environmental Biology of Fishes 95: 53–63.
Kitchell, J. F., T. E. Essington, C. H. Boggs, D. E. Schindler & C. J. Walters, 2002. The role of sharks and longline fisheries in a pelagic ecosystem of the central Pacific. Ecosystems 5: 202–216.
Krebs, C. J., 1999. Ecological methodology, 2nd ed. Menlo Park California, Addison Wesley Longman.
Letelier, S., R. Meléndez, E. Carreño, S. López & P. Barría, 2009. Alimentación y relaciones tróficas del pez espada (Xiphias gladius Linneaus, 1758) frente a Chile centro-norte, durante el 2005. Latin American Journal of Aquatic Research 37: 107–119.
Markaida, U. & O. Sosa-Nishizaki, 1998. Food and feeding habitats of swordfish, Xiphias gladius, off western Baja California. In Barret I, Sosa-Nishizaki O, Bartoo N (eds) Biology and fisheries of swordfish, Xiphias gladius. Papers from the International Symposium of Pacific Swordfish, Ensenada, Mexico, 11-14 December 1994. NOAA Technical Report NMFS 142: 245-259.
Markaida, U. & O. Sosa-Nishizaki, 2003. Food and feeding habits of jumbo squid (Dosidicus gigas) (Cephalopoda:Ommastrephidae) from the Gulf of California, México. Journal of the Marine Biological Association of the United Kingdom 83: 507–522.
Markaida, U. & F. G. Hochberg, 2005. Cephalopods in the diet of swordfish (Xiphias gladius) caught off thewest coast of Baja California, Mexico. Pacific Science 59: 25–41.
Mejuto, J., B. García & A. M. Ramos, 2012. Sinopsis sobre la Biología y el comportamiento del pez espada (Xiphias gladius). [Available on internet at http://www.co.ieo.es/tunidos/esp/FLOTAesp/SWO.pdf].
Ménard, F., A. Lorrain, M. Poiter & F. Marsac, 2007. Isotopic evidence of distinct feeding ecologies and movement patterns in two migratory predators (yellowfin tuna and swordfish) of the western Indian Ocean. Marine Biology 153: 141–152.
Miller, D. J. & S. C. Jorgensen, 1973. Meristic characters of some marine fishes of the western Atlantic Ocean. California Department. Fishery Bulletin 1: 301–312.
Monod, T., 1968. Le complexe urophore des poissons Téléostéens. Memories de Linnstitute Fundamental D´ Affrique Noire 81: 1–705.
Nigmatullin C. M., Nesis K. N., & A. I. Arkhipkin, 2001. Biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fisheries Research 54:9–19.
Nikolsky, G. V., 1963. The Ecology of fishes. USA, Academic Press, New York.
Ochoa-Díaz, M. R., 2009, Espectro trófico del tiburón martillo Sphyrna zygaena (Linnaeus, 1758) en Baja California Sur: Aplicación de d13C y d15N. Master degree thesis. Centro Interdisciplinario de Ciencas Marinas (CICIMAR-IPN), La Paz, Baja California Sur, México.
Ortega-Garcia, S., Arizmendi-Rodriguez D. I., & M. S. Zuñiga-Flores, 2017. Striped marlin (Kajikia audax) diet variability off Cabo San Lucas, B.C.S., Mexico during El Niño-La Niña events. Journal of the Marine Biological Association of the United Kingdom. https://doi.org/10.1017/S0025315417000595
Palko, B. J., G. L. Beardsley & W. J. Richards, 1981. Synopsis of the biology of the Swordfish, Xiphias gladius Linnaeus. FAO Fisheries Synopsis 127: 1–21.
Parnell, A. & A. Jackson, 2013. SIAR: Stable isotope analysis in R. R package version 4.2. [Available on internet at http://CRAN.R-project.org/package=siar].
Parnell, A. C., J. Haslett, J. R. Allen, C. E. Buck & B. Huntley, 2008. A flexible approach to assessing synchroneity of past events using Bayesian reconstructions of sedimentation history. Quaternary Science Reviews 27: 1872–1885.
Pauly, D., A. W. Trites, E. Capuli & V. Christensen, 1998. Diet composition and trophic levels of marine mammals. ICES Journal of Marine Science 55: 467–481.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83: 703–718.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montaña, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189.
Potier, M., F. Marsac, Y. Cherel, V. Lucas, R. Sabatié, O. Maury & F. Ménard, 2007. Forage fauna in the diet of three large pelagic fishes (lancetfish, swordfish and yellowfin tuna) in the western equatorial Indian Ocean. Fisheries Research 83: 60–72.
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Viena, Austria. [Available on internet at https://www.R-project.org/].
Rau, G., A. J. Meams, D. R. Young, R. J. Olson, H. A. Schafer & I. Kaplan, 1983. Animal 13C/12C Correlates with trophic level in pelagic food webs. Ecology 64: 1314–1318.
Reñones, O., N. V. C. Polunin & R. Goni, 2002. Size related dietary shifts of Epinephelus marginatus in a western Mediterranean littoral ecosystem: an isotope and stomach content analysis. Journal of Fish Biology 61: 122–137.
Reeb, C., L. Arcangeli & B. Block, 2000. Structure and migration corridors in Pacific populations of the swordfish, Xiphias gladius, as inferred through analysis of mitochondrial DNA. Marine Biology 136: 1123–1131.
Santos, B. M., G. J. Pierce, P. R. Boyle, R. J. Reid, H. M. Ross, I. A. P. Patterson, C. C. Kinse, S. Tougaard, R. Lick, U. Piatkowski & V. Hernández-Garcia, 1999. Stomach contents of sperm whales Physeter macrocephalus stranded in the North Sea 1990-1996. Marine Ecology Progress series 183: 281–294.
Scott, W. B. & S. N. Tibbo, 1986. Food and feeding habits of swordfish, Xiphias gladius in the western North Atlantic. Journal of the Fisheries Research Board of Canada 25: 903–919.
Sedberry, G. R. & J. K. Loefer, 2001. Satellite telemetry tracking of swordfish, Xiphias gladius, off the eastern United States. Marine Biology 139: 355–360.
Seki, M. P., J. J. Polovina, R. Kobayashi, R. R. Bidigare & G. T. Mitchum, 2002. An oceanographic characterization of swordfish (Xiphias gladius) longline fishing grounds in the springtime subtropical North Pacific. Fisheries Oceanography 11: 251–266.
Sepulveda, C., A. Knight, N. Nasby-Lucas & M. L. Domeier, 2010. Fine-scale movements of the swordfish Xiphias gladius in the Southern California Bight. Fisheries Oceanography 19: 279–289.
Seryadji, B., I. Jatmiko & A. Wujdi, 2016. Modelling several morphometric relationships of swordfish (Xiphias gladius), black marlin (Makaira indica) and blue marlin (Makaira nigricans) caught from Indonesian longliners in the eastern Indian Ocean. Journal of Fisheries 4(2): 371–376.
Stillwell, C. E. & N. E. Kohler, 1985. Food and feeding ecology of the swordfish Xiphias gladius in the North Atlantic Ocean with estimates of daily ration. Marine Ecology Progress Series 22: 239–247.
Strub, P. T. & C. James, 2002. The 1997–1998 oceanic El Niño signal along the southeast and northeast Pacific boundaries an altimetric view. Progress in Oceanography 54: 439–458.
Taipe, A., C. Yamashiro, L. Mariátegui, P. Rojas & C. Roque, 2001. Distribution and concentrations of jumbo flying squid (Dosidicus gigas) off the Peruvian coast between 1991 and 1999. Fisheries Research 54: 21–32.
Torres-Rojas, Y., A. Hernandez-Herrera, S. Ortega-Garcia & M. Domeier, 2013. Stable isotope differences between blue marlin (Makaira nigricans) and striped marlin (Kajikia audax) in the Southern Gulf of California, Mexico. Bulletin of Marine Science 89: 421–436.
Tripp-Valdez, A., F. Galván-Magaña & S. Ortega-García, 2015. Food sources of common dolphinfish (Coryphaena hippurus) based on stomach content and stable isotopes analyses. Journal of the Marine Biological Association of the United Kingdom 95: 1–13.
Van den Burg, E. H., R. R. Peeters, M. Verhoye, J. Meek, G. Flik & A. Van der Linden, 2005. Brain responses to ambient temperature fluctuations in fish: reduction of blood volume and initiation of a whole-body stress response. Journal of Neurophysiology 93: 2849–2855.
Vander-Zanden, M. J., G. Cabana & J. B. Rasmussen, 1997. Comparing the trophic position of littoral fish estimated using stable nitrogen isotopes (δ15N) and dietary data. Canadian Journal of Fisheries and Aquatic Sciences 54: 1142–1158.
Wetherbee, B. M., S. H. Gruber & E. Cortés, 1990. Diet, feeding habits, digestion and consumption in sharks, with special reference to the lemon shark, Negaprion brevirostris. In: Pratt HL, Gruber SH, Taniuchi T (eds) Elasmobranchs as living resources: advances in the Biology, Ecology, Systematics and the status of the Fisheries. NOAA Tech Rep 90, Seattle: 29–47.
White, A. E., F. G. Prahl, R. M. Letelier & B. N. Popp, 2007. Summer surface waters in the Gulf of California: Prime habitat for biological N2 fixation. Global Biogeochemical Cycles 21: 1–11.
Wolff, C. A., 1984. Identification and estimation of size from the beaks of eighteen species of cephalopods from the Pacific Ocean. NOAA Technical Report NMFS 17: 1–50.
Wootton, R. J., 1990. Ecology of teleost fishes. Chapman & Hall, London: 404.
Yáñez, E., C. Silva, M. Barbieri1, A. Órdenes & R. Vega, 2009. Environmental conditions associated with swordfish size compositions and catches off the Chilean coast. Latin American Journal of Aquatic Research 37(1): 71–81.
Young, J., M. Lansdell, S. Riddoch & A. Revill, 2006. Feeding ecology of broadbill swordfish, Xiphias gladius, off eastern Australia in relation to physical and environmental variables. Bulletin of marine science 79: 793–809.
Acknowledgements
This study was developed in the projects: SIP20141071- SIP20150861 supported by Instituto Politécnico Nacional, and the project, “From the Bay to the Banks: understanding food web links between Magdalena Bay and the offshore pelagic fish community,” in collaboration with the Rutgers University and supported by the Billfish foundation (SIP2012/19). We owe our gratitude to the staff of the Fish Ecology and Marine Chemistry Laboratories at the Centro Interdisciplinario de Ciencias Marinas (CICIMAR), where our samples were analyzed. The corresponding author is a scholar of EDI and COFAA, IPN. We offer our special thanks to Carolina Minte, Marlon H. Roman Verdesoto, and María Elena Sánchez-Salazar, who contributed to the English edition of the manuscript. Our thanks go to the anonymous reviewers for their constructive comments on an earlier version of this manuscript that helped improve it.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Iacopo Bertocci
Rights and permissions
About this article
Cite this article
Trujillo-Olvera, A., Ortega-García, S., Tripp-Valdez, A. et al. Feeding habits of the swordfish (Xiphias gladius Linnaeus, 1758) in the subtropical northeast Pacific. Hydrobiologia 822, 173–188 (2018). https://doi.org/10.1007/s10750-018-3680-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3680-2