Abstract
Carbon dioxide released in the atmosphere and dissolved in water leads to acidification. Relatively few studies have focused on fresh waters, where biocalcifying species are more readily impacted by changes in pH. Sensitivity to pH of an endangered calcium-demanding organism, the crayfish Austropotamobius pallipes, was investigated in the Pinail nature reserve, a natural system with 3000 permanent ponds, some inhabited by the crayfish and others not, originally due to human introduction. From the 14 chemical parameters measured in this study, the main limiting factor preventing crayfish establishment appears to be water acidity (pH < 6.8), which affects calcification, molting, growth and reproduction. We predict that 20% of the Pinail populations will disappear by 2060 due to freshwater acidification with the present level of fossil fuel consumption. Ongoing and future restoration projects for conservation of this heritage crustacean must select hard water with the highest water pH (> 7).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemistry of freshwater is subject to local biotic and abiotic factors. Climate change is suspected to have significant impacts on freshwater chemistry and biology. The acidification of freshwater ecosystems may have multiple origins including the chemistry of carbon dioxide (CO2). As an example, it is now widely accepted that the ongoing ocean acidification is linked to atmospheric increase of carbon dioxide of anthropogenic origin (Caldeira & Wickett, 2003). Its effects on organisms are also highly documented for many taxa (Orr et al., 2005; Fabry et al., 2008; Kroeker et al., 2010, 2013). Nevertheless, acidification of fresh water due to anthropogenic CO2 release is relatively less studied and more difficult to assess because of the multiparameter regulation ability of such aquatic ecosystems (Carpenter et al., 1992; Heino et al., 2009; Hasler et al., 2018). In a general way, carbon dioxide reacts with water according to local temperature and ionic influence (Turley et al., 2006). The rising atmospheric CO2 may indirectly impact factors contributing to the pCO2 level (Hasler et al., 2016). In another way, factors such as limited nutrients, water connectivity, thermal changes, and desiccation may have additional roles with acidification to produce diverse altered physiological responses of biota, including physiological and phenological changes, migrations and extinctions. Therefore, generalizations about lakes, ponds, rivers or brooks, are almost impossible (each area locally subject to multiple environmental stressors potentially acting in synergy; Stumm & Morgan, 2012; Phillips et al., 2015).
Natural atmospheric carbon dioxide and CO2 from anthropogenic sources are dissolved in water and are involved in a series of reversible chemical reactions. CO2 leads to carbonic acid by combination with H2O molecules. Carbonic acid rapidly dissociates to form bicarbonate ions, which can themselves further dissociate to form carbonate ions. These last two reactions, which produce protons and induce local acidification, are enhanced by anthropogenic atmospheric CO2 elevation:
This disequilibrium can greatly affect the physiology of animals living in such acidified environment. For example, calcium and carbonate are vital ions forming CaCO3 in biomineralization processes occurring in many phyla from bacteria, algae (Krumbein, 1979), higher plants (Borowitzka, 1984) to animals. Most of the skeletons of non-chordate animals, such as corals, crustaceans, mollusks and echinoderms, comprise calcium carbonate, mainly found as calcite or aragonite, the two most stable polymorphs of calcium carbonate (Lowenstam & Weiner, 1989). Calcium carbonate can dissolve in water forming carbonate ions (CO32−), which react with carbon dioxide as follows:
An increase in CO2 lowers the pH and leads to diminution of carbonate ion (CO32−) levels for the production of bicarbonate ions (HCO3−) and reduction of the saturation states of the minerals aragonite and calcite. This lowers the bioavailability of vital calcium carbonate. It is particularly a concern for those synthesizing calcium carbonate skeletons or shells. Species-specific responses include stress, behavior changes, failure of reproduction and growth, reduced calcification (thinner and weaker calcium structures) and repair ability, and even sometimes dissolution of the skeleton of living organisms (Riebesell et al., 2000; Orr et al., 2005; Fabry et al., 2008; Doney et al., 2009; Caldwell et al., 2011).
The impact of natural and experimental pH variations (due for example to acid precipitation, detrital degradation, geology of the surroundings) has been monitored on freshwater organisms, especially crustaceans (Morgan & McMahon, 1982; France, 1984; Økland & Økland, 1985; Schindler, 1988; Renberg et al., 1993; Chen & Chen, 2003). More particularly, crayfish have been used as models to analyze water chemistry associated with their physiological responses, behavior, reproduction and survival (Mauro & Moore, 1987; Foster, 1995; Trouilhé et al., 2007; Favaro et al., 2010; Haddaway et al., 2013, 2015). Studies revealed that crayfish are absent in acid water with pH < 6.0 (France, 1993; Haddaway et al., 2013, 2015). This potential pH sensitivity may be linked with biocalcification biology in crayfish rather than to calcium concentration in water, which does not seem to be a limiting factor (Haddaway et al., 2015).
As with all arthropods, crayfish molt regularly to replace their rigid exoskeleton allowing growth to occur. A peculiarity of most crustaceans is that this cuticle is calcified, mainly as calcium carbonate and, to a lesser extent, calcium phosphate (Roer & Dillaman, 1984; Greenaway, 1985; Luquet, 2012; Kunkel, 2013). Consequently, these animals have to find sufficient calcium and carbonate ions in each molting cycle. For all the aquatic species, water is the main source of these ions. Nevertheless, terrestrial species, as well as some aquatic species, develop calcium storage structures during each intermolt period (Greenaway, 1985; Luquet, 2012). Crayfish, like terrestrial crabs, lobsters and Norway lobsters, synthesize such storage structures, called gastroliths, in their stomach wall (Travis, 1960). After molting, these gastroliths are released to the stomach and the ions are reabsorbed and used to start hardening the soft new cuticle. Nevertheless, the calcium ions stored as gastroliths represent a very low proportion of the amount required. Food and, more particularly, postmolt calcium uptake are necessary for the complete calcification of the new cuticle (France, 1987; Hessen et al., 1991; Luquet, 2012).
In this paper, the studied model is an endangered species of heritage interest: the white-clawed crayfish Austropotamobius pallipes (Lereboullet, 1858), a crustacean that provides an important ecological service of water cleaning by feeding on dead material. The native white-clawed crayfish, A. pallipes, is commonly found in brooks and rivers of the Poitou–Charentes region and populations also inhabit ponds of the National Natural Reserve of the Pinail (Beaune et al., 2017) where it is found as the only recorded French population of A. pallipes living in ponds, surrounded by an ecosystem dominated by heather cover (Beaune et al., 2017). The surrounding populations in lotic systems of the region are in serious danger of extinction or are already extirpated from streams and brooks by the highly contagious crayfish plague (Aphanomyces astaci, Schikora 1906) transported by exotic invasive crayfish (Grandjean et al., 2000). Thus, the ecological service of cleaning water is compromised in such habitats and several conservation programs are underway in suitable conservation conditions (Füreder et al., 2010; UICN, 2014; Beaune et al., 2017). Isolated lentic systems like the Pinail ponds now constitute important conservation reservoirs (also called Ark sites in other projects; Kozák et al., 2011).
Here we explore the possible reason(s) why the white-clawed crayfish, a calcifying freshwater organism, is absent or present in ponds close to each other in an apparently stable environment, the Pinail nature reserve. A group of 27 ponds with similar water supply conditions (no surrounding runoff, rain as 100% water source) was analyzed. First, chemical and physical parameters of ponds with or without crayfish were recorded and analyzed to identify correlations between parameters and the presence or absence of crayfish. Having identified pH as the main variable parameter of the 27 analyzed ponds, we considered the possible impacts of reduced pH on the physiology and survival of A. pallipes. Finally, we predicted future pH changes with atmospheric CO2 increase in the studied ponds, assessed future risks to A. pallipes in the Pinail and recommended an approach to reduce risks in planning management of crayfish in the reserve and for other sites.
Methods
Study area and study species
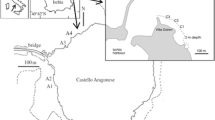
The national nature reserve of the Pinail (Réserve naturelle nationale du Pinail, www.reserve-pinail.org) is a unique concentration of 5000 ponds, of which 3000 have permanent water and are scattered across 135 ha (Fig. 1). Historically ponds were created over ten centuries for millstone extraction. The resulting thousands of waterholes were used by people as fishponds (Préau et al., 2017) where Austropotamobius pallipes crayfish were probably introduced and maintained as a delicacy in few of them where survival persisted. After WWII, exploitation stopped, ponds were not used any more, and crayfish populations survived or became extinguished due to unsuitable conditions. This allowed an unexpected natural experiment of habitat suitability for crayfish.
The Pinail nature reserve is surrounded by 4166 ha of forest mainly classified within Natura 2000 status (ZSC: special zone of preservation and ZPS: zone of special protection), ZICO (zone of Europe community interest for birds; European Nature Protection area network) and ZNIEFF of 1 and 2 types: natural Zone of Ecological Interest Fauna and Flora (French natural inventory of biodiversity). The reserve is covered with diversified Erica moors on acid and oligotrophic ground (podzol) resulting from management of the land as pasture, with periodic burning to promote new growth. Geology of the site is sedimentary from the Oxfordian to Ludian age (163–42 Myr). The uppermost geological layer is a gray and silty clay from the Pliocene–Quaternary (0.5–3 m thickness); some ponds may be in contact with other layers (marl, sandy clay, limestone), leading to subtle physical and chemical variations.
This site forms a very rich ecological complex where 2500 species (fauna, flora, fungi) have been recorded. Water originates from rainfall, no runoffs occurring on this elevated land.
Physical and chemical water analyses
Twenty-seven ponds were sampled twice, on 9 May 2009 and on 19 June 2012 between 8 am and 1 pm. Among these 27 ponds, 10 ponds with crayfish were compared with 17 other uninhabited ponds physically similar, in the same area and habitat (mean depth of 2 m, distance < 800 m between two ponds, no riparian vegetation > 3 m, same substrate) but empty of A. pallipes. It should be noted that no significant differences were found between the two samplings performed in 2009 and 2012. Only the results obtained in 2012 are presented here.
Samples of water were taken in 2 L polyethylene bottles and stored at 4°C, for the measurement of physical and chemical parameters linked to organic matter (turbidity, total organic carbon (TOC), and UV absorbance (at 254 nm) and dissolved oxygen. In situ measurements of dissolved oxygen (O2), percent O2 saturation, pH (at 20°C in May and June for comparison) and conductivity (an indication of the hardness of water) were recorded using a digital meter (WTW) with the appropriate probes (model Hanna HI9828). Water turbidity was measured using a HACH Pocket Turbidimeter. On unfiltered samples, total organic carbon (TOC) was analyzed using a Shimadzu TOC 5000A analyzer. UV absorbance (indicators of photosynthetic activity) at 254 nm was measured with a UV–Vis spectrophotometer SAFAS DES (Double Energy System) 190. Chlorides (Cl), nitrates (NO3) and sulfates (SO4) were measured by ionic chromatography using a Vydac column (302 IC 4.6).
Statistical analysis
First, multivariate analyses using principal component analyses (PCA) were used to discriminate factors (continuous) affecting the presence of A. pallipes (binary). The importance of each chemical parameter was estimated by a Generalized Linear Model analysis (GLM) with a Gaussian distribution. The discriminatory factors revealed by GLM with and without crayfish were tested by ad hoc parametric Student’s t test or nonparametric Wilcoxon rank sum test according to normality tests (Shapiro–Wilk normality and graphic tests). The power analysis of the tests is specified when a difference is detected. Analyses were performed using R 2.11R (R Development Core Team 2011).
Modeling pH change
The pH depends on CO2 partial pressure, composition of the water and temperature. The following formula gives an estimate for modeling pH unit volume of water (Stumm & Morgan, 2012):
with [Alk] for alkalinity, Ka1 as the first dissociation constant of carbonic acid, KH as the Henry’s law constant for CO2, and pCO2 as the partial pressure in atmosphere. While other parameters may change as well as temperature in the future, we kept them as constant and increased only pCO2 with the R statistical software to indicate the effect of this variable alone.
The average gas (atmospheric air) pressure at 120 m and 15°C in 2012 (corresponding to the mean altitude and temperature of the Pinail) was 99.89 kPa. For instance, the partial pressure of CO2 in 2012 was 39.25 kPa using current atmospheric levels at 393 ppm CO2.
Results
The PCA showed that the Eigenvalues of the first two components represented up to 60.96% of the total variance of the observations (PC1 37.14%, PC2 23.82%; Fig. 2a).
a Projection of the 14 experimentally measured parameters with variable factors mapped in the main plane from the 27 ponds of the Pinail Nature Reserve. The two dimensions of this plane presented here explain 60.96% of the variability in the data. b Ponds with and without A. pallipes are clearly separated
Crayfish presence was correlated with pH, Cl (chlorine), inorganic carbon and oxygen among other assessed factors. These variables contributed mainly to the construction of component axis 1 (Dimension 1) and corresponded to the scores of the ponds with A. pallipes plotted in the left area of the individual factor map (Fig. 2b). pH of the water seems to be a limiting factor for A. pallipes (Fig. 3). According to the GLM analysis, only pH is a significant predictor of crayfish presence (t = 5.66, p = 0.00001; eta.sq = 0.15; eta.sq.part = 0.72).
a Mean survival curve of A. pallipes according to pH in 33 ponds of the Pinail (blue dots). Crayfish are not present with pH < 6.8 (right vertical dotted line). 6.6 is the highest pH recorded from the analyzed uninhabited ponds (left vertical dotted line). b Mean pHs in ponds with (left box plot, N = 10) and without (right box plot, N = 23) living white-clawed crayfish, Austropotamobius pallipes, are significantly different (W = 209, p value < 0.001)
pH of the ten inhabited ponds ranged from 6.80 to 7.84 with a mean of 7.43 ± 0.33, resulting in a highly significant difference within inhabited ponds (in June: t = − 8.4, df = 21, p < 0.001; in May: t = − 13.307, df = 10.239, p < 0.001; power analysis > 88%). The uninhabited ponds are more acidic, with a mean pH of 6.06 ± 0.27 (range 5.75–6.61).
If the current pCO2 evolution is maintained, pH will continue decreasing with atmospheric CO2 increase (Fig. 4). With the IPCC “Intergovernmental Panel on Climate Change” ‘business as usual’ scenario, the Earth’s atmosphere is predicted to reach 610 ppm of CO2 in 2060. At the 610 ppm level, pH would decrease by 0.2 pH in all ponds (Fig. 4), which means that the two more acidic of the ten Pinail ponds with current A. pallipes populations would be likely to reach pH 6.8, the current lower limit described in this paper for the presence of crayfish. Consequently, 20% of the existing A. pallipes habitats within the site might cease to be suitable, leading to the expected loss of their populations.
pH variation with atmospheric CO2 level in ponds of the Pinail National Nature Reserve (France). This prediction starts at CO2 = 393 ppm (mean value in January 2012). The solid blue line is the mean pH in the ten ponds inhabited by Austropotamobius pallipes. Dotted blue lines are maximum and minimum pH recorded. With the IPCC “business as usual” scenario, atmospheric [CO2] might reach 610 ppm around 2060. The predicted pH would decrease by 0.2. pH 6.8 is the current pH limit of survival of A. pallipes in the Pinail Nature Reserve ponds
Discussion
Our study provides an example of acidic exclusion for an endangered species, the white-clawed crayfish, A. pallipes. At the Pinail, this crayfish is restricted to 10 ponds among more than 3000. The striking difference lies in the pH value: crayfish are excluded from ponds with pH < 6.8. The high pH ponds are in contact with a limestone layer that buffers water acidity (with higher conductivity, Table 1), while water origins and the surrounding ecosystem are similar.
Our findings are in accordance with previous studies. For example, Jay & Holdich (1977, 1981) reported that A. pallipes only inhabits British Isles waters within a pH range of 6.8–8.2, with calcium levels > 5 mg/ml. They also demonstrated that they can survive only a few hours at pH 1.5, 7 days at pH 5.0 and not many more at pH 6.0 in laboratory conditions. France (1993) observed that Orconectes sp. crayfish are excluded from Canadian Shield lakes with pH < 6.0. Haddaway and collaborators (2015) reviewed 23 studies linked with A. pallipes and found that this species is absent below pH 6.0. Moreover, under stable conditions (ex situ), Haddaway et al. (2013) have shown that crayfish growth and survival are compromised in water of pH 6.5. One of the main differences for explaining small discrepancies between all these results (particularly when they are obtained from the same species) relates to size (surface/volume) and nature of the studied water areas. In limited size and volume ecosystems, such as the Pinail ponds, crayfish cannot possibly find variable chemical environmental conditions in different locations and/or at different times, unlike populations living in running water or in bigger lentic water bodies.
One of the possible explanations of the absence of crayfish in low pH lies in the poor availability of bicarbonate ions regularly needed for these molting organisms, which are cyclically subject to an important calcium turnover. Indeed, crustaceans need to quickly harden their regularly renewed exoskeleton after ecdysis to protect against cannibalism, predation and other threats (Scalici & Gibertini, 2009; Luquet, 2012). As calcium uptake is dependent on other ions, especially bicarbonate, low pH conditions can inhibit or greatly reduce postmolt calcium uptake (Malley, 1980; France, 1993; France & Collins, 1993). In the same way, it was shown that Ca-poor lakes (< 5 mg/ml) have crayfish exoskeleton mineralization greatly reduced (France, 1987; Taugbøl et al., 1997) as well as reproduction and early stage development greatly impacted (Rukke, 2002; Hammond et al., 2006; Cairns & Yan, 2009). As crayfish regularly develop not only an outer calcified exoskeleton but also inner calcium carbonate storage structures of great importance in the first steps of calcification, we cannot exclude this storage process from impact by environmental low pH conditions, as they are in water of low Ca-concentration, resulting in absence of molting (Hessen et al., 1991). Changes in pH can also affect intracellular H+ concentration and thus metabolism, protein synthesis, iono-regulation, oxygen supply (decrease of oxygen affinity with respiratory pigments) and ultimately survival of crustaceans (Morgan & McMahon, 1982; Appelberg, 1985; Taylor & Whiteley, 1989; Carpenter et al., 1992; Whiteley & Taylor 1992; Chen & Chen, 2003; Whiteley, 2011).
Finally, a decrease in pH could also have an impact on reproduction and development (Lowery, 1988; DiStefano et al., 1991; Reynolds, 2002; Chen & Chen, 2003; Bechmann et al., 2011; Caldwell et al., 2011; Long et al., 2013). Indeed, it was observed that in artificial as well as in natural conditions, crustaceans grow slower in acidified water. Moreover, it was shown that, if occasional exposures to higher acidity are relatively well supported by adult crayfish, a gradual increase could produce sublethal effects such as altered reproductive activity or dramatic changes in early larval development, then consequently affect the survival of populations.
As ecological engineers, crayfish presence, absence or extirpation due to acidification may have significant impacts on their habitat such as in the different ponds of the Pinail. Crayfish accelerate biomass turnover by consuming vegetable and animal detritus and corpses (Creed, 1994; Reynolds et al., 2013). Accordingly, scuba diving performed in 2015 in some of the studied uninhabited ponds showed a relatively important amount of necromass at the bottom by contrast with other inhabited ponds, demonstrating a regulator role of the crayfish in the trophic web. These impacts warrant further investigation.
After modeling changing pH in the ten ponds inhabited by A. pallipes, we found that 20% of these ponds would be too acidic at 610 ppm of CO2 for the survival of these crayfish (with a general decrease of 0.2 of the pH, the two ponds with the lowest pH (6.8 and 7.0) will fall below the critical limit of pH < 6.8 found in this study. This amount of carbon dioxide could be reached in 2060 if we do not change our fossil fuel habits. In this simplistic and conservative model, only the increase of atmospheric CO2 was taken into account and we have considered the other parameters such as temperature as constant. However, future conditions would also increase global temperatures, also resulting in a decrease in pH (Cottin et al., 2012; Ivanina et al., 2013; Kroeker et al., 2013). In addition to the negative effects on crayfish physiology, higher temperatures will decrease oxygen concentration in water. Our estimation must then be considered as optimized and optimistic.
Our findings are of great interest not only as an example of the impacts of freshwater acidification but secondarily for reintroduction projects where pH may be underestimated, including ark site projects and conservation actions plan to transfer endangered populations of crayfish into lentic systems such as ponds, pools or quarry pools. Such waters are isolated from invasive crayfish and associated crayfish plague. Thus, it is of prime importance to highlight the main limiting factors such as pH < 6.8, to select the most hospitable place for rescuing white-clawed crayfish. Our recommendation for suitable water bodies is pH ≫ 7 in anticipation of a future pH decrease. We are now searching for such ponds where conditions are suitable for A. pallipes but where populations have not yet colonized with the help of humans (Beaune et al., 2017).
References
Appelberg, M., 1985. Changes in haemolymph ion concentrations of Astacus astacus L. and Pacifastacus leniusculus (Dana) after exposure to low pH and aluminium. Hydrobiologia 121: 19–25.
Beaune, D., Y. Sellier, E. Lambert & F. Grandjean, 2017. The use of Chara spp. (Charales: Characeae) as a bioindicator of physico-chemical habitat suitability for an endangered crayfish Austropotamobius pallipes in lentic waters. Aquatic Conservation: Marine and Freshwater Ecosystems. https://doi.org/10.1002/aqc.2847.
Bechmann, R. K., I. C. Taban, S. Westerlund, B. F. Godal, M. Arnberg, S. Vingen, A. Ingvarsdottir & T. Baussant, 2011. Effects of ocean acidification on early life stages of shrimp (Pandalus borealis) and mussel (Mytilus edulis). Journal of Toxicology and Environmental Health Part A 74: 424–438.
Borowitzka, M. A., 1984. Calcification in aquatic plants. Plant, Cell & Environment 7: 457–466.
Cairns, A. & N. Yan, 2009. A review of the influence of low ambient calcium concentrations on freshwater daphniids, gammarids, and crayfish. Environmental Reviews 17: 67–79.
Caldeira, K. & M. E. Wickett, 2003. Anthropogenic carbon and ocean pH. Nature 425: 365-365.
Caldwell, G. S., S. Fitzer, C. S. Gillespie, G. Pickavance, E. Turnbull & M. G. Bentley, 2011. Ocean acidification takes sperm back in time. Invertebrate Reproduction & Development 55: 217–221.
Carpenter, S. R., S. G. Fisher, N. B. Grimm & J. F. Kitchell, 1992. Global change and freshwater ecosystems. Annual Review of Ecology and Systematics 23: 119–139.
Chen, S. M. & J. C. Chen, 2003. Effects of pH on survival, growth, molting and feeding of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 218: 613–623.
Cottin, D., D. Roussel, N. Foucreau, F. Hervant & C. Piscart, 2012. Disentangling the effects of local and regional factors on the thermal tolerance of freshwater crustaceans. Naturwissenschaften 99: 259–264.
Creed, R. P, Jr. 1994. Direct and indirect effects of crayfish grazing in a stream community. Ecology 75: 2091–2103.
DiStefano, R. J., R. J. Neves, L. A. Heldrich & M. C. Lewis, 1991. Response of the crayfish Cambarus bartonii bartonii to acid exposure in southern Appalachian streams. Canadian Journal of Zoology 69: 1585–1591.
Doney, S. C., V. F. Fabry, R. A. Feely & J. A. Kleypas, 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science 1: 169–192.
Fabry, V. J., B. A. Seibel, R. A. Feely & J. Orr, 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. Journal of Marine Science 65: 414–432.
Favaro, L., T. Tirelli & D. Pessani, 2010. The role of water chemistry in the distribution of Austropotamobius pallipes (Crustacea Decapoda Astacidae) in Piedmont (Italy). Comptes Rendus Biologies 333: 68–75.
Foster, J., 1995. Factors influencing the distribution and abundance of the crayfish Austropotamobius pallipes (Lereboullet) in Wales and the Marches, UK. Freshwater Crayfish 8: 78–98.
France, R. L., 1984. Comparative tolerance to low pH of three life stages of the crayfish Orconectes virilis. Revue Canadienne de Zoologie 62: 2360–2363.
France, R. L., 1987. Calcium and trace metal composition of crayfish (Orconectes virilis) in relation to experimental lake acidification. Canadian Journal of Fisheries and Aquatic Science 44: 107–113.
France, R. L., 1993. Influence of lake pH on the distribution, abundance and health of crayfish in Canadian Shield lakes. Hydrobiologia 271: 65–70.
France, R. L. & N. C. Collins, 1993. Extirpation of crayfish in a lake affected by long-range anthropogenic acidification. Conservation Biology 7: 184–188.
Füreder, L., F. Gherardi, D. Holdich, J. Reynolds, P. Sibley & C. Souty-Grosset, 2010. Austropotamobius pallipes. The IUCN Red List of Threatened Species 2015-4. Endangered A2ce ver 3.1. http://www.iucnredlist.org/details/2430/0.
Grandjean, F., B. Cornuault, S. Archambault, M. Bramard & G. Otrebsky, 2000. Life history and population biology of the white-clawed crayfish, Austropotamobius pallipes pallipes, in a brook from the Poitou-Charentes region (France). Bulletin Français de la Pêche et de la Pisciculture 356: 55–70.
Greenaway, P., 1985. Calcium balance and molting in the Crustacea. Biological Reviews 60: 425–454.
Haddaway, N. R., R. J. G. Mortimer, M. Christmas & A. M. Dunn, 2013. Effect of pH on growth and survival in the freshwater crayfish Austropotamobius pallipes. Freshwater Crayfish 19: 53–62.
Haddaway, N. R., R. J. G. Mortimer, M. Christmas & A. M. Dunn, 2015. Water chemistry and endangered white-clawed Crayfish: a literature review and field study of water chemistry association in Austropotamobius pallipes. Knowledge and Management of Aquatic Ecosystems 416: 01.
Hammond, K. S., J. W. Hollows, C. R. Townsend & P. M. Lokman, 2006. Effects of temperature and water calcium concentration on growth, survival and moulting of freshwater crayfish, Paranephrops zealandicus. Aquaculture 251: 271–279.
Hasler, C. T., D. Butman, J. D. Jeffrey & C. D. Suski. 2016. Freshwater biota and rising pCO2? Ecology Letters, 19: 98–108.
Hasler, C. T., J. D. Jeffrey, E. V. Schneider, K. D. Hannan, J. A. Tix & C. D. Suski. 2018. Biological consequences of weak acidification caused by elevated carbon dioxide in freshwater ecosystems. Hydrobiologia, 806: 1–12.
Heino, J., R. Virkkala & H. Toivonen, 2009. Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions. Biological Reviews 84: 39–54.
Hessen, D., G. Kristiansen & I. Lid, 1991. Calcium uptake from food and water in the crayfish Astacus astacus (L., 1758), measured by radioactive 45Ca (Decapoda, Astacidea). Crustaceana 60: 76–83.
Ivanina A. V., G. H. Dickinson, O. B. Matoo, R. Bagwe, A. Dickinson, E. Beniash & I. M. Sokolova. 2013. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comparative Biochemistry and Physiology Part A: Molecular Integrative Physiology, 166: 101–111.
Jay, D. & D. M. Holdich, 1977. The pH tolerance of the crayfish Austropotamobius pallipes (Lereboullet). Freshwater Crayfish 3: 363–370.
Jay, D. & D. M. Holdich, 1981. The distribution of the crayfish, Austropotamobius pallipes, in British waters. Freshwater Biology 11: 121–129.
Kozák, P., L. Füreder, A. Kouba, J. Reynolds & C. Souty-Grosset, 2011. Current conservation strategies for European crayfish. Knowledge and Management of Aquatic Ecosystems 401: 01.
Kroeker, K. J., R. L. Kordas, R. N. Crim & G. G. Singh, 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters 13: 1419–1434.
Kroeker, K. J., R. L. Kordas, R. N. Crim, I. E. Hendriks, L. Ramajo, G. S. Singh, C. M. Duarte & J. P. Gattuso, 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biology 19: 1884–1896.
Krumbein, W. E., 1979. Calcification by bacteria and algae. In Trudinger, P. A. & D. J. Swaine (eds), Biogeochemical cycling of Mineral-Forming Elements. Elsevier, Amsterdam: 47–68.
Kunkel, J. G., 2013. Modeling the calcium and phosphate mineralization of American lobster cuticle 1. Canadian Journal of Fisheries and Aquatic Sciences 70: 1601–1611.
Long, W. C., K. M. Swiney, C. Harris, H. N. Page & R. J. Foy, 2013. Effects of ocean acidification on juvenile red king crab (Paralithodes camtschaticus) and tanner crab (Chionoecetes bairdi) growth, condition, calcification, and survival. PloS ONE 8: e60959.
Lowenstam, H. A. & S. Weiner, 1989. On Biomineralization. Oxford University Press, New York.
Lowery, R. S., 1988. Growth, moulting and reproduction. In Holdich, D. M. & R. S. Lowery (eds), Freshwater Crayfish: Biology, Management and Exploitation. Croom Helm (Chapman & Hall), London: 83–113.
Luquet, G., 2012. Biomineralizations: insights and prospects from crustaceans. Zookeys 176: 103–121.
Malley, D. F., 1980. Decreased survival and calcium uptake by the crayfish Orconectes virilis in low pH. Canadian Journal of Fisheries and Aquatic Sciences 37: 364–372.
Mauro, N. A. & G. W. Moore, 1987. Effects of environmental pH on ammonia excretion, blood pH, and oxygen uptake in fresh water crustaceans. Comparative Biochemistry and Physiology 87C: 1–3.
Morgan, D. O. & B. R. McMahon, 1982. Acid tolerance and effects of sublethal acid exposure on iono-regulation and acid-base status in two crayfish Procambarus clarki and Orconectes rusticus. Journal of Experimental Biology 97: 241–252.
Økland, K. A. & J. Økland, 1985. Factor interaction influencing the distribution of the freshwater “shrimp” Gammarus. Oecologia 66: 364–367.
Orr, J. C., V. F. Fabry, O. Aumont, L. Bopp, S. C. Doney, R. A. Feely, A. Gnanadesikan, N. Gruber, A. Ishida & F. Joos, 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681–686.
Phillips, J. C., G. A. McKinley, V. Bennington, H. A. Bootsma, D. J. Pilcher, R. W. Sterner & N. R. Urban. 2015. The potential for CO2-induced acidification in freshwater: A Great Lakes case study. Oceanography, 28: 136–145.
Préau, C., P. Dubech, Y. Sellier, M. Cheylan, F. Castelnau & D. Beaune, 2017. Amphibian Response to the Non-Native Fish, Lepomis gibbosus: The Case of the Pinail Nature Reserve, France. Herpetological Conservation and Biology 12: 616–623.
R Development Core Team, 2011. R: A Language and Environment for Statistical Computing. The R Foundation for Statistical Computing, Vienna.
Renberg, I., T. Korsman & N. J. Anderson, 1993. A temporal perspective of lake acidification in Sweden. Ambio 22: 264–271.
Reynolds, J. D., 2002. Growth and reproduction. In Holdich, D. M. (ed.), Biology of freshwater crayfish. Blackwell Science, New York: 152–191.
Reynolds, J., C. Souty-Grosset & A. Richardson, 2013. Ecological roles of crayfish in freshwater and terrestrial habitats. Freshwater Crayfish 19: 197–218.
Riebesell, U., I. Zondervan, B. Rost, P. D. Tortell, R. E. Zeebe & F. M. Morel, 2000. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407: 364–367.
Roer, R. & R. Dillaman, 1984. The structure and calcification of the crustacean cuticle. American Zoologist 24: 893–909.
Rukke, N. A., 2002. Effects of low calcium concentrations on two common freshwater crustaceans, Gammarus lacustris and Astacus astacus. Functional Ecology 16: 357–366.
Scalici, M. & G. Gibertini, 2009. Molt and gastroliths in Austropotamobius pallipes (Lereboullet, 1858). Knowledge and Management of Aquatic Ecosystems 14: 394–395.
Schindler, D. W., 1988. Effects of acid rain on freshwater ecosystems. Science 239: 149–157.
Stumm, W. & J. J. Morgan, 2012. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. Wiley, New York.
Taugbøl, T., S. B. Wærvågen, A. N. Linløkken & J. Skurdal, 1997. Post-molt exoskeleton mineralization in adult noble crayfish, Astacus astacus, in three lakes with different calcium levels. Freshwater Crayfish 11: 219–226.
Taylor, E. W. & N. M. Whiteley, 1989. Oxygen transport and acid-base balance in the haemolymph of the lobster, Homarus gammarus, during aerial exposure and resubmersion. Journal of Experimental Biology 144: 417–436.
Travis, D. F., 1960. The deposition of the skeletal structures in the Crustacea. I. The histology of the gastrolith skeletal tissue complex and the gastrolith in the crayfish, Orconectes (Cambarus) virilis Hagen – Decapoda. Biological Bulletin 118: 137–149.
Trouilhé, M. C., C. Souty-Grosset, F. Grandjean & B. Parinet, 2007. Physical and chemical water requirements of the white-clawed crayfish (Austropotamobius pallipes) in western France. Aquatic Conservation: Marine and Freshwater Ecosystems 17: 520–538.
Turley, C., J. Blackford, S. Widdicombe, D. Lowe, P. D. Nightingale & A. P. Rees, 2006. Reviewing the impact of increased atmospheric CO2 on oceanic pH and the marine ecosystem. Avoiding Dangerous Climate Change 8: 65–70.
U.I.C.N. MNHN, 2014. La Liste rouge des espèces menacées en France – Chapitre: Crustacés d’eau douce de France métropolitaine. Paris, France.
Whiteley, N. M., 2011. Physiological and ecological responses of crustaceans to ocean acidification. Marine Ecology Progress Series 430: 257–271.
Whiteley, N. M. & E. W. Taylor, 1992. Oxygen and acid-base disturbances in the hemolymph of the lobster Homarus gammarus during commercial transport and storage. Journal of Crustacean Biology 12: 19–30.
Acknowledgements
We are grateful to the DREAL (Directions Régionales de l’Environnement, de l’Aménagement et du Logement) Nouvelle-Aquitaine, the Communauté d’Agglomération de Grand Châtellerault, the Syndicat de rivière Vienne et Affluents (SyRVA) and the Agence de l’Eau Loire-Bretagne for financial contributions. We thank A. Zylinsky, D. Jeune, P. Dubech, B. Menard, B. Parinet for field contributions and anonymous reviewers for valuable suggestions. We are also indebted to Pr. Julian Reynolds, from Trinity College of Dublin (Ireland), for improving the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Rights and permissions
About this article
Cite this article
Beaune, D., Sellier, Y., Luquet, G. et al. Freshwater acidification: an example of an endangered crayfish species sensitive to pH. Hydrobiologia 813, 41–50 (2018). https://doi.org/10.1007/s10750-018-3504-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3504-4