Abstract
DDx, PCBs, PBDEs, Hg, and As contamination in sediments and aquatic organisms of different trophic levels (zooplankton, mussel, fish) were analyzed in Lake Maggiore and Lake Lugano, two large deep perialpine lakes. In the period 2001–2015, we analyzed the spatial and temporal trends of the considered pollutants to detect potential contamination sources and to compare concentrations with Sediment Quality Guidelines (SQGs) or existing Quality Standards (QSs). DDx and Hg contamination deriving from past industrial activities in the Pallanza Basin still exceeded SQGs in sediments and QSs in fish, with potential risks for the ecosystem. Banned in Europe in 1985, PCBs showed low residual values, while recent PBDE peaks resulted in the exceedance of the QSs for biota in both lakes, probably due to current industrial activities. Arsenic mainly derives from geochemical origin. The analysis of the biomagnification of toxicants in a pelagic food chain in Lake Maggiore (zooplankton–fish) according to a stable isotope approach is also presented, according to both the Trophic Magnification Factor and the Trophic Level-adjusted BioMagnification Factor: the importance of seasonality and a Hg > DDx ≈ PBDEs biomagnification capacity were observed. Low PCB bioaccumulation was detected in biota, probably because equilibrium was not reached yet in young fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake Maggiore and Lake Lugano are large deep alpine lakes located at the boundary between Italy and Switzerland with an important role for recreational use, irrigation, and fishery. Lake Maggiore has a long history of severe pollution, including both inorganic compounds, in particular Hg, and persistent organic pollutants, such as dichlorodiphenyltrichloroethane and its metabolites. On the contrary, Lake Lugano shows a more pristine condition: in the last century this ecosystem was less impaired by industrial activities, even though a diffuse pollution was recorded in the lake waters, also deriving from atmospheric transport of contaminated particles.
The International Commission for the Protection of the Italian-Swiss Common Waters (CIPAIS) supports monitoring activities in both lakes, with a particular focus on priority substances according to European Directive 2013/39/EU. The study of DDx contamination in Lake Maggiore started in 1998 like a consequence of a known industrial discharge, while in Lake Lugano both sediments and biota were monitored since 2009. CIPAIS activities comprise the monitoring of different persistent organic pollutants (POPs), a heterogeneous group of chemicals, including pesticides, such as DDx, and other organic chemicals of industrial origin such as polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs). All these substances may have harmful effects on human health and on the environment (Eljarrat & Barceló, 2003; Ruiz-Fernandez et al., 2014). Being semi-volatile, toxic, and persistent, these toxicants are mainly bound to particulate matter in the water column and in sediments. Changes in physical and chemical conditions may cause their partial release into the dissolved phase, with increased bioavailability for organisms (Pisanello et al., 2016). Because of their physico-chemical properties, POPs can be detected everywhere, including worldwide remote regions located far from emission sites, such as polar or Alpine ecosystems (Tremolada et al., 2008; Hung et al., 2010; van Drooge et al., 2014; Guzzella et al., 2016).
The POPs on which this study is focused were chosen on the basis of their use in the lake basins: DDx was extensively produced in an industrial site located along the Toce River (a tributary of Lake Maggiore) until 1996; PCBs were commonly used as industrial compounds, until they were banned in the European Union (EU) in 1985; PBDEs are a class of halogenated flame retardants extensively used in consumer, commercial, and industrial products such as polyurethane foam, thermoplastics, textiles, and circuit boards (de Wit, 2002). Pentabrominated diphenyl ether- and Octabromodiphenyl ether-containing mixtures were banned in Europe in 2003 (Directive 2003/11/EC), while the UE’s Restriction of Hazardous Substances Directive (RoHS) banned the Decabromodiphenyl ether (DBDE, containing 97% of deca-BDE) from electronics in 2006.
By contrast, trace elements in aquatic environments may derive both from natural process, such as biogeochemical recycling, and from anthropogenic activities, such as industrial activities comprising metal processing (Tessier & Campbell, 1987; Wu et al., 2014). Among them, Hg is a priority hazardous substance according to European Directive 2013/39/EU because of its persistence, its capacity to bioaccumulate and biomagnify in food webs, as well as to exert toxic effects at environmental concentrations (Ullrich et al., 2001). Besides, As is widely distributed in aquatic environments and its presence in sediments and in organisms can be primarily associated to natural processes, even if an anthropogenic contribution can be due to metallurgical and agricultural applications and to the manufacture of wood preservatives (Wang & Mulligan, 2006). Notwithstanding this, As is well known to be toxic for aquatic organisms and humans (Sharma & Sohn, 2009), and it is considered as additional parameter to be monitored in freshwater ecosystems according to the Italian legislation on priority substances (D. Lgs. 172/2015). In particular, Lake Maggiore ecosystem was severely affected by Hg pollution in the last century due to the presence of a mercury-cell chlor-alkali plant located on the River Toce (Pisanello et al., 2016; Marziali et al., 2017). High concentrations of As were also found in both lakes, primarily due to natural weathering and erosion processes, even if a contribution from anthropogenic activities is also documented for Lake Maggiore (Marziali et al., 2017).
Quality Standards for Biota (QSBs) are defined in European Directive 2013/39/EU as well as in national legislations for the most bioaccumulative and biomagnifying substances, among which are DDx, PBDEs, and Hg. For these toxicants, official limits for human consumption are also established. On the contrary, no regulation regarding freshwater sediment quality standards is nowadays shared by all European Countries, so each EU nation is following a different approach in environmental evaluations. The definition of common sediment quality standards, in fact, would require the evaluation of many physical–chemical characteristics of sediments that may influence the potential risk for aquatic organisms. Among existing Sediment Quality Guidelines (SQGs), consensus-based thresholds are widely used in risk analysis, e.g., Threshold Effect Concentrations (TECs), i.e., the concentrations below which effects on aquatic communities are not expected, and Probable Effect Concentration (PECs), i.e., the values above which effects are probable (MacDonald et al., 2000; Conder et al., 2015).
To analyze bioaccumulation of contaminants in the aquatic food web, organisms belonging to different trophic levels are generally considered. Zooplankton represents the main link between pelagic primary producers to planktivorous fish, favoring trophic transfer and biomagnification. It is also widely considered an early warning bioindicator of contamination (Bettinetti et al., 2012a, b). Zebra mussel is also commonly used as sentinel organism to assess water contamination, especially because of its ability to bioaccumulate chemicals and its resistance to a broad range of pollutants (Kwan et al., 2003; Bervoets et al., 2005). Being a sedentary and benthic organism bound to the detritus cycle, with a relative long life cycle, it often mirrors site-specific pollution. In order to obtain a more complete view of the trophic chain and the bioaccumulation phenomena in our ecosystems, we considered then zooplankton, zebra mussels, and three pelagic fish species: shad, whitefish, and perch. These species are commercially important and ecologically precious for aquatic ecosystems. Different approaches can be used to evaluate biomagnification of contaminants in food webs: among them, the Trophic Magnification Factor (TMF) represents the biomagnification behavior of a chemical substance in food webs (Conder et al., 2012), while the trophic level-adjusted biomagnification factor (BMFTL) provides a closer insight on the individual predator–prey relationships (Cullon et al., 2012). The general scientific consensus is that an increase in chemical concentration with increasing trophic level (i.e., biomagnification) results in a BMFTL and/or TMF above 1, while decreasing concentrations with increasing trophic position (TMF < 1) indicates trophic dilution (Arnot & Gobas, 2006). In this study, both BMFTL and TMF were calculated for DDx, PCBs, and Hg in Lake Maggiore.
The objectives of the present investigation were: (i) to analyze the temporal and spatial trends of DDx, PCBs, PBDEs, Hg, and As in sediments and aquatic organisms belonging to three different trophic levels (zooplankton, zebra mussels, and fish) in Lake Maggiore and Lake Lugano; (ii) to compare the contaminant concentrations with existing QSBs for biota and SQGs for sediments and to compare contamination level between the two lakes; (iii) to focus on bioaccumulation/biomagnification effects of contaminants in the aquatic trophic web (from zooplankton to fish level) according to a Stable Isotope Approach (SIA).
Materials and methods
Study area: Lake Maggiore
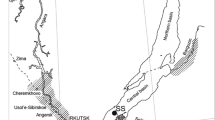
Lake Maggiore is a large (surface area = 213 km2, volume = 38 km3), deep (maximum depth = 370 m), oligo-mesotrophic lake located at the Southern foothills of the Central Alps, shared between Italy (ca. 80%) and Switzerland (ca. 20%; Fig. 1a). Lake Maggiore was formed by glacial erosion in a pre-existing fluvial valley and is considered holo-oligomictic, rarely undergoing complete mixing (Barbanti & Ambrosetti, 1989). It has a relatively large watershed (6,599 km2), mostly formed by mountain areas, with a complex geological structure, as detailed by Baudo (1989). In the watershed, arsenopyrite veins are present, located both in the Toce catchment and close to Lake Lugano, and As was found in the dumps of gold and iron mines dating back to the Roman Age (Pfeifer et al., 2000). Lake Maggiore has 33 tributaries and one outlet, the River Ticino (CIPAIS, 2015a). The mean theoretical water residence time is 4 years.
Sampling sites in Lake Maggiore (a) and Lake Lugano (b). Red dots: sediment cores; yellow square: zooplankton; green triangles: D. polymorpha. B3, 1, 17, 16, 13: cores in the Pallanza Bay; 29: core in the Northern part of the lake; 57: core in the central part of the lake; 28: core in the Southern part of the lake
Like most lakes in Italy and Central Europe, Lake Maggiore underwent anthropogenic eutrophication during the second half of the 20th century (Salmaso & Mosello, 2010). In the following decades, the building of sewage treatment plants and the progressive ban of phosphorus from detergents caused a decrease of P load, until values close to pre-industrial concentrations (Marchetto et al., 2004). Beside eutrophication, in the last two centuries, Lake Maggiore was affected by a number of human pressures. Since 1808, industrial production led to the discharge in the lake and in its tributaries of several pollutants, among which Hg used in a hat-making industry. In 1915, a large chemical factory was built up at Pieve Vergonte, along the River Toce. From the ‘40 s, DDT production and a mercury-cell chlor-alkali plant caused intense DDx and Hg pollution, until DDT production was stopped in 1996. Peak concentrations in sediments in the Pallanza Basin reached values up to 13.000 µg kg−1 OC d.w. for total DDx and up to 26 mg kg−1 d.w. for total Hg (Guzzella et al., 1998; Guilizzoni et al., 2012); commercial fishing was also banned, because DDx concentrations reached values up to 3 mg kg−1 w.w. in the fish muscle, largely exceeding the limit for human consumption (50 ng/g w.w. and 100 ng/g w.w. for fish with a lipid content lower or higher than 5%, respectively; Ordinanza Ministero della Sanità 18-7-1990; D. Lgs. 172/2015). Thanks to the drastic reduction of the industrial activities, the treatment of effluents and the stabilization of contaminated soils, the contaminant inputs into the aquatic ecosystem dropped significantly. The atmospheric pollution in the neighboring Po plain also contributes to the atmospheric deposition into the lake and into its catchment of several compounds, especially volatile organic pollutants (Grimalt et al., 2001): the atmospheric deposition was considered the main source of dioxin-like PCBs (Vives et al., 2007).
Study area: Lake Lugano
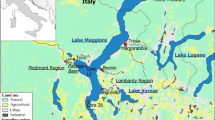
Lake Lugano (surface area = 49 km2, volume = 6.5 km3), whose basin is shared between Italy and Switzerland, lies at the southern foothills of the Central Alps in a valley mainly resulting from fluvial erosion with a strong morphological overprinting during the Pleistocene alpine glaciations (Fig. 1b). The lake is divided into two parts by a morainic front, on which an artificial dam—the ponte-diga in Melide—was built before 1850. Consequently, Lake Lugano is divided into two distinct basins with specific characteristics and different morphological and hydrological features. The Northern basin is mesotrophic, the Southern basin is eutrophic (Barbieri & Polli, 1992). Lake Lugano has 6 major and 2 middle-sized tributaries. The outlet, the River Tresa, flows into Lake Maggiore (IST-SUPSI, 2016). The Northern basin, located between Melide and Porlezza, is deeper than the Southern one (maximum depth = 288 m). Its limited volume (4.69 km3) results in a long mean water residence time (12.3 years). The Southern basin, between Capolago and Agno–Ponte Tresa, presents a smaller water volume (1.14 km3) and the mean water residence time is shorter (1.4 years) (IST-SUPSI, 2016).
In Lake Lugano catchment is mainly characterized by forests and semi-natural areas, while urban residential areas, significantly increased over the last decades, accounting for about 8% of the catchment area. Industrial or commercial activities are mostly located in the Swiss part of the watershed, while agricultural areas are mainly located in Italy (IST-SUPSI, 2016).
The industrial development in the Region started slowly in the 1890 s, while the most relevant expansion occurred mostly after World War II. These activities are limited and did not have a relevant impact on Lake Lugano (CIPAIS, 2010). Nevertheless, in the last 20 years the lake had to face anthropogenic eutrophication phenomena, which reached their highest impact between the 1970s and the 1980s. Afterwards, the extension of sewage and the construction of wastewater treatment plants, combined with the ban of phosphorus from detergents in 1986, led to a decrease of the phosphorus load, as reported above for Lake Maggiore. However, the delicate phase of recovery is still not concluded. Moreover, diffuse source pollution and atmospheric depositions are considered responsible for significant impacts on the lake (FOEN, 2006; Schmid et al., 2010). Point sources of emerging/current-use contaminants are also reported (CIPAIS, 2016).
Sampling sites
In April 2014, five sediment cores were collected in the Pallanza Basin of Lake Maggiore (cores B3, 1, 17, 16, and 13) and three along the main axis of the lake in the Northern (core 29), central (core 57), and Southern (core 28) part of the lake (Fig. 1a). The sites were selected on the base of previous screening studies to be the most representative of the contamination status of the different areas (CIPAIS, 2011). In Lake Lugano (Fig. 1b), two sediment cores were collected in December 2011, near Melide and Figino, respectively. All sediment cores were collected using a gravity corer and then dated using 210Pb and 137Cs analysis, as detailed in Marchetto et al. (2004) for Lake Maggiore and in CIPAIS (2013a) for Lake Lugano. In this investigation, the most recent layers of sediment cores were considered, covering a period between 1999 and 2014 for Lake Maggiore and between 2009 and 2011 for Lake Lugano.
Zooplankton organisms were collected seasonally from 2008 to 2015 in Lake Maggiore at Ghiffa, using a nylon net (58 cm diameter, 450 µm mesh), 0–50 m depth vertical hauls, in order to reach 1 g d.w. for each sample. Samples were filtered on a 1.2 µm pore glass-fiber filter (4.7 cm of diameter) and frozen at − 20°C until analyses.
Samples of zebra mussel (Dreissena polymorpha, Pallas, 1771) were collected in Lake Maggiore by a scuba diver every year between 2008 and 2015 at 3 sites: Brissago, Pallanza, and Baveno. The sampling was carried out during the organisms’ pre-reproductive period (from early May to early June) to reduce differences of chemical concentrations caused by physiological and/or environmental changes. Specimens were washed with lake water, transported to the laboratory in refrigerated bags, and then frozen at − 20°C until analyses. In Lake Lugano, D. polymorpha was sampled with the same procedure in 2010 and 2011 at Figino, Melide, and Gandria basins.
In Lake Maggiore, two fish species were sampled: shad (Alosa fallax lacustris, Scopoli, 1786) and whitefish (Coregonus lavaretus, Linnaeus, 1758). Fish samples were collected with pelagic gill nets in the central part of the lake. Sampling campaigns were carried out seasonally every year between 2001 and 2015. 2- to 3-year-old fish were selected and fillet was frozen at − 20°C until analyses, according to the historical data series (CIPAIS, 1999). In Lake Lugano, five sampling campaigns were performed for shad and European perch (Perca fluviatilis, Linnaeus, 1758) in the period 1993–2015. Fish of similar size and collected in the same basin (North vs. South) were pooled and the caudal fillet was frozen at − 20°C until analyses.
Target analytes
Target analytes were selected on the basis of pre-existing contamination data and of their classification as priority substances according to Directive 2013/39/EU. Selected organic target analytes were: 14 PCBs (PCB-18, -28, -31, -44, -52, -101, -118, -149, -138, -153, -170, -180, -194, and -209), 6 DDx (o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, and p,p′-DDT), and 8 PBDEs (BDE-28, -47, -99, -100, -153, -154, -183, and -209). Mercury (Hg) and arsenic (As) were considered as inorganic target analytes.
Sediment sample analysis
Sample preparation and analysis of PCBs, DDx, and PBDEs were performed as detailed by Guzzella et al. (2016). Briefly, sediments were lyophilized and a variable amount of sample (0.5–1.0 g d.w.) was spiked with an internal standard solution containing the labeled compounds (o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, p,p′-DDT, PCB-28, -52, -101, -138, -153, -180, PBDE-28, -47, -99, -100, -153, -154, -183, -209; Wellington Laboratories, Inc., Canada). The samples were extracted with a hot Soxhlet apparatus (Büchi, Switzerland) using a n-hexane/acetone (3:1, v/v) mixture. The clean-up procedure was performed on a multi-layer column packed with acidified silica gel (30% w/w sulfuric acid, Sigma-Aldrich, Germany) and Florisil® (100–200 mesh, Sigma-Aldrich, Germany). GC analysis was performed using a Thermo Electron Trace GC 2000 coupled with a PolarisQ Ion Trap (Thermo Electron—Austin, Texas) mass spectrometer, equipped with a PTV injector and an AS-3000 auto sampler. The system was managed by Thermo Finnigan Xcalibur software version 1.4.1., as detailed in Guzzella et al. (2016).
Performance method for DDx and PCBs was evaluated using 1939a SRM river sediment (National Institute of Standard and Technology, Gaithersburg, MD, USA). The performance for PBDE analysis was evaluated using the candidate-certified sediment BROC-2 CRM (Netherlands Institute of Fisheries Research). Using a signal-to-noise ratio of 3:1 calculated by Xcalibur 1.4.1 software during the instrument calibration, the limits of detection (LODs) were estimated as 0.05 ng g−1 d.w. for each compound in sediment samples. A procedural blank was also analyzed every eight samples to check laboratory contaminations. The results of the procedural blanks were always lower than the LOD values.
Total Hg concentrations in sediments were determined by thermal decomposition, amalgamation, and atomic absorption spectrometry according to the US-EPA method 7473 (US-EPA, 1998), using an Automated Mercury Analyzer (AMA254, FKV, Bergamo, Italy). After freeze-drying and homogenizing, each sample (0.1 g) was analyzed in triplicate and results with precision below 5% were accepted. Accuracy was checked using the certified reference material GBW07305 from the National Standard Centre of China for sediments and resulted within ± 10% of certified values.
For As determination, a microwave-assisted digestion (Preekem EU Excel 2000) of dried samples (0.2 g) was performed, with concentrated HNO3 (VWR, Suprapur) and MilliQ water. Concentrations were measured using atomic absorption spectrometry (AAS) with a Perkin Elmer AAnalyst 600 with Zeeman correction, equipped with a graphite atomization system (GF-AAS). Procedural blanks were regularly checked for ambient or reagent contamination. For each sample, analysis was run in duplicate and results with precision below 5% were accepted. The accuracy of recovery was between 75 and 100%, as estimated using the reference material GBW07305.
Organic carbon (OC) content of the sediments was determined by back titration after oxidation with potassium dichromate in the presence of sulfuric acid, as detailed in the method by Walkley and Black (1934).
Zooplankton and zebra mussel analysis
Continuous-flow isotope ratio mass spectrometry (CF-IRMS) Stable Isotope Analysis (SIA) was performed on zooplankton for measuring carbon (δ13C‰) and nitrogen (δ15N‰) (activity undertaken by G.G. Hatch Stable Isotope Laboratory of University of Ottawa, Ontario, Canada). A detailed description of lipid extraction procedure and DDx and PCBs analytical methods for zooplankton is reported in Bettinetti et al. (2012b). The accuracy of recovery was estimated using the reference materials BCR 598 for DDx and BCR 349 for PCBs. The lipid content values of zooplankton are reported in Tables S1 and S2.
About 150 adult specimens of D. polymorpha with a shell length greater than 15 mm (more than 1-year-old individuals) were sampled at each sampling sites. After collection, mussels were defrosted, shell and byssus were removed, and soft tissues were freeze-dried (Freeze-dryer Edwards mod. 24). The lipid content of mussels was determined gravimetrically extracting 1.5 g of sample with solvent (Tables S1, S2). After the lipid extraction, the digestion of the organic phase, and the subsequent clean-up, samples were injected into a gas chromatograph for the quantitative evaluation of DDx, PCBs, and PBDEs, as detailed in Binelli et al. (2008) and Riva et al. (2010).
For Hg analysis in zooplankton and for Hg and As in D. polymorpha, the same procedures used for sediments were adopted, with the only exception of the amount of sample for Hg analysis (0.04 mg d.w.). Accuracy was checked using the certified reference material BCR 278 Mussel tissue from the Bureau Communautaire de Référence and was within ± 10% of certified values for Hg. Recovery for As was between 84 and 100%.
Fish sample analysis
Fish samples were prepared for organic compound analysis as follows: after lyophilization, a variable amount of dried fish tissue (0.5–1.0 g d.w.) was spiked with the same internal standard solution, containing the same labeled compounds used for sediments. The same procedure used for sediments was adopted for the extraction and clean-up of fish samples, with the only exception of an additional clean-up step after the extraction with Gel Permeation Chromatography (LCTech GmbH, Dorfen, Germany).
The validation of the analytical method for all organic compounds in fish samples was performed using the SRM NIST 1947—Lake Michigan Fish Tissue (National Institute of Standard and Technology, USA). Using a signal-to-noise ratio of 3:1 calculated by Xcalibur 1.4.1 software during the instrument calibration, the LODs were estimated as 0.01 ng g−1 d.w. in fish samples for each chemical. Like for sediments, a procedural blank was analyzed every eight samples to check laboratory contaminations. For Hg and As extraction, the same procedure described above was adopted. The lipid content of fish tissues was determined gravimetrically as for mussels (Tables S1, S2).
For Lake Lugano, DDx and PCBs in fish were extracted according to the method published by Steinwandter (1985). Briefly, 50 g of fish samples were homogenized and subsequently purified with Gel Permeation Chromatography (GPC) and silica micro-column fractioning (Specht & Tillkes, 1980, 1985). Finally, DDx and PCBs were quantified as reported in Ceschi et al. (1996). Quality control of the validated method included the regular determination of a cod-liver oil standard reference material (cod-liver oil BCR 598). Recoveries and limit of quantifications were in the range 85–110% and < 1 μg kg−1, respectively. The results of proficiency testing showed suitable standard deviations for repeatability and reproducibility (sr ~ 14% and sR ~ 26%) (Solcà et al., 2006).
Biomagnification factor and Trophic Magnification Factor
In this study, the Trophic Level-adjusted BioMagnification Factor (BMFTL) and the TMF were calculated for DDx, PCBs, and Hg in Lake Maggiore, considering zooplankton as prey and fish (shad and whitefish) as predators. For the BMFTL and TMF calculation, only pelagic fish were considered, being directly related to zooplankton samples. The values of BMFTL were then compared with those of TMF to determine if certain combinations result in higher or lower accumulation factor. The Trophic Level (TL) was estimated for zooplankton and all the considered fish species as reported in Poma et al. (2014), according to the SIA.
BMFTL was calculated using the following equation (Conder et al., 2012):
where Cpredator and Cprey are lipid-normalized values (for organic chemicals) in the predator and in the prey, respectively; TLpredator and TLprey are the trophic levels of the predator and the prey, respectively.
TMF was determined by linear regression between the lipid-normalized concentrations in biota, logarithmically transformed, and the relative trophic position of the same biota as follows (Borgå et al., 2012):
where Cb is the contaminant concentration in the biota. Subsequently, TMF was calculated using the formula:
where m was the slope of the previous linear regression.
Statistical analysis
Time trend tests were performed using the Seasonal Kendall test with R Software, as detailed in Marchetto et al. (2013). All other statistical analyses were performed with Statistica 7 Software (StatSoft). Differences in contamination between organisms (zooplankton, zebra mussels, and fish) sampled in Lake Maggiore vs. Lake Lugano were tested using Student’s t test, after testing data normality with Kolmogorov–Smirnov test. The criterion for significance was set at P < 0.05. In order to evaluate seasonal differences in biota, 2008–2015 data of whitefish and zooplankton contamination were first tested for normality and homoscedasticity, and then tested by one-way ANOVA and Tukey’s post hoc comparison tests, or by non-parametric tests, as appropriate. TMF and BMFTL correlation was evaluated through the results of the regression lines, as reported in Poma et al. (2014).
Results
Micropollutants in sediments and comparison with SQGs
In this investigation, we considered only recent sediment layers (corresponding to the last 10–15 years), with the aim of comparing contamination in sediment and biota samples.
In Lake Maggiore the considered sediment layers covered the period between 1999 and 2014, showing an increasing trend of DDx, PBDEs, and Hg concentrations from the upper part of the lake (core 29) to the South (cores 57 and 28), following the hydraulic paths of the lake and according to the localization of the contaminant sources (Figs. 2a and S1). In particular, DDx and Hg were mainly transported from the Pallanza Basin, as shown by the higher concentrations in sediment cores B3, 1, 17, 16, and 13, deriving from the activities of the industrial plant located on the Toce River. PBDEs were mainly derived from industrial activities located in Lombardy region, with the highest values in core 28. Values increased up to 134 times for DDx, up to 43 times for Hg and up to 3 times for PBDEs along the main lake axis. On the contrary, PCBs and As showed slightly higher values in upper part of the lake (core 29). In particular, PCBs derive from industrial oil machines, while As is driven by the Tresa River due to natural leaching of As-rich rocks (Pfeifer et al., 2004).
Concentrations of DDx (ng g−1 normalized on 1% OC) and Hg (mg kg−1 d.w.) in the upper layers of the sediment cores collected in Lake Maggiore for the period 1999–2014 (a) and in Lake Lugano for the period 2011–2014 (b). Sediment thresholds according to MacDonald et al. (2000) are also reported where indicated. B3, 1, 17, 16, 13: cores in the Pallanza Bay; 29: core in the Northern part of the lake; 57: core in the central part of the lake; 28: core in the Southern part of the lake
Figure 2a also shows that contaminants deriving from past industrial activities, such as DDx and Hg in the Pallanza Basin, show a decreasing temporal trend in the most recent sediment layers (DDT production was banned in 1996 and industrial activities were drastically reduced). The highest values were measured in the oldest sediment layers (1999–2008) in the Pallanza Basin (cores 17 and 16) both for DDx and Hg, where contaminants were driven from the Toce River, and in the Southern part of the Lake (core 28). A similar trend can be observed for PCBs (Fig. S1), whose production and use were banned in the European Union. On the contrary, PBDE values showed an increasing trend following more intense industrial use in the most recent period. The highest PBDE concentrations were measured in cores 57 and 28, reaching 50 ng g−1 d.w. in recent years (2011–2014) and sporadically in some samples (core 17) of the Pallanza Basin. Lastly, As concentrations (Fig. S1) showed no clear temporal trends, showing that this contaminant is mainly derived from natural geochemical weathering.
Contaminant concentrations in sediments were compared with TECs and PECs values by MacDonald et al. (2000). Only in core 29 (Fig. 2a), located in the Northern part of the Lake, DDx concentrations were lower than the TEC value (5.28 ng g−1 d.w. as sum of all six compounds and referred to 1% TOC sediments). For PCBs (Fig. S1), all values were lower than the TEC (59.8 ng g−1 d.w.), while no threshold was defined by MacDonald et al. (2000) for PBDEs. Hg values (Fig. 2a) were below the TEC (0.18 mg kg−1 d.w.) only in the Northern part of the lake (core 29), while they exceeded the PEC (1.06 mg kg−1 d.w.) in the Southern area (cores 57 and 28). In consideration of the local geochemistry, a background value for the Pallanza Basin was calculated as 0.044 mg Hg kg−1 d.w. (Vignati & Guilizzoni, 2011): present concentrations are markedly higher in the Pallanza Basin (up to 0.604 mg kg−1 d.w.) and in the Southern part of the lake (up to 3.07 mg kg−1 d.w.), showing a strong anthropogenic enrichment. Concerning As, the background value estimated for the Pallanza Basin (34 mg kg−1 d.w.) is higher than the PEC value (33 mg kg−1 d.w.). The peaks found in core B3 (Fig. S1) are possibly due to the local accumulation of sediment fine fractions or to phenomena of remobilization of residual industrial contamination driven from the Toce River during intense hydrological events.
For Lake Lugano, the sediment layers here considered covered the period between 2009 and 2011. DDx and PCB values were higher in Melide Basin than in Figino Basin (Figs. 2b and S2) and concentrations were very low (< 0.2 ng g−1 d.w. and < 0.9 ng g−1 d.w., respectively for DDx and PCBs, both below the TEC value), and may be considered as diffuse pollution, possibly deriving from atmospheric deposition of contaminated particles. Hg and As values were higher in Figino Basin than in Melide Basin (Figs. 2b and S2). For Lake Lugano, background values were not determined, but both elements are supposed to derive mainly from geochemical origin, since anthropic point sources are not present on the lake. In particular, the high As values in Figino Basin (up to 166 mg kg−1 d.w., well above the PEC value, Fig. S2) cannot be ascribed to local anthropogenic sources, and therefore should be considered natural (Pfeifer et al., 2004).
Micropollutants in lake biota and comparison with QSBs
Dreissena polymorpha
Samples of D. polymorpha in Lake Maggiore were collected between 2008 and 2015 in 3 stations: Brissago, located in the upper part of the lake; Baveno, located in the Pallanza Basin; and Pallanza, located just outside this basin. These stations allowed the determination of spatial contamination trends in biota (CIPAIS, 2015b, 2016). In particular, the same gradients described for sediments were found for DDx and Hg (Figs. 3a and S3): significantly higher values were found in the Pallanza Basin (up to 53 ng g−1 w.w. for DDx and 18 µg kg−1 w.w. for Hg) than in the upper part of the lake (up to 29 ng g−1 w.w. for DDx and 7 µg kg−1 w.w. for Hg), in agreement with the localization of the contamination sources. Consistently, PCBs, PBDEs, and As showed similar values in the different lake areas, suggesting a diffuse contamination origin or a lower bioaccumulation capacity than DDx and Hg.
a Concentrations of DDx and PBDEs (ng g−1 w.w.) in D. polymorpha and two species of fish (shad and whitefish) sampled in Lake Maggiore. Existing QSBs are also reported. Red full points: samples exceeding the existing BSQ for DDx; b concentrations of DDx, PCBs (ng g−1 w.w.), and Hg (µg kg−1 w.w.) in two species of fish (shad and perch) sampled in Lake Lugano. **Indicates significant differences between fish species (P < 0.01)
The concentration found in D. polymorpha were compared to QSBs. Existing thresholds are referred to fish, so the comparison should be considered as an attempt approach only. The exceedance of QSBs at lower levels of the trophic chain, however, may be worthy of attention, since it may potentially result in high tissue concentrations in predator organisms (e.g., fish), especially for magnifying contaminants such as DDx and Hg.
Figures 3a and S3 show that all DDx, PCB, and Hg values were below SQBs (50–100 ng DDx g−1 w.w. for organisms with less and more than 5% of lipid content, respectively, according to Directive 2013/39/EU; 125 ng PCBs g−1 w.w. according to EU Regulation 1259/2011 for freshwater organisms; 20 µg Hg kg−1 w.w. according to Directive 2013/39/EU). On the contrary, all PBDE concentrations were above the QSB fixed at 0.0085 ng g−1 w.w. by Directive 2013/39/EU.
In Lake Lugano, D. polymorpha samples were collected in 3 sites, corresponding to different lake zones (Northern part, Figino Basin, and Melide Basin). Values of DDx, PCBs, and Hg were similar in the 3 sites, suggesting diffuse pollution for these contaminants (Fig. S4), and were all below the QSBs. PBDEs and As were not investigated in D. polymorpha of Lake Lugano.
Fish
Whitefish and shad were sampled in Lake Maggiore from 2008 to 2016. In most cases, the concentrations of the studied contaminants (DDx, PCBs, PBDEs, and Hg) were higher in shad than in whitefish (Fig. 3a). Indeed, in shad DDx concentrations frequently exceeded the limit set for human consumption, mainly in the period 2009–2011 (D. Lgs. 172/2015). Because of the exceedance of this threshold, professional fishing of the shad in the Italian part of Lake Maggiore was forbidden in 1996. In this study, we found that, although rarely, also whitefish samples exceeded the QSB for DDx (Fig. 3a). On the contrary, in both fish species, PCB concentrations in the 2008–2016 period were lower than the limit set for human consumption (125 ng g−1 w.w.; EU Regulation 1259/2011, Fig. S3). A highly significant correlation between δ15N signatures and PCB and DDx concentrations in fish (expressed as natural logarithm) was found for the samples in the period 2010–2015 (R2 = 0.218; n = 24; F = 6.126; P = 0.022 for DDx; R2 = 0.330; n = 28; F = 12.797; P = 0.001 for PCBs).
A great environmental concern comes from PBDEs and Hg contamination: in all examined fish samples, concentrations exceeded the QSBs of 0.0085 ng g−1 w.w. for PBDE and of 20 µg kg−1 w.w. for Hg according to Directive 2013/39/EU. These very low limits account for the high bioaccumulation capacity of these toxicants. In particular, the threshold for Hg is considered protective for top predators of food webs from the risk of secondary poisoning deriving from the biomagnification potential of this metal. QSB value for PBDE was calculated in Directive 2013/39/EU starting from water concentration protective for aquatic organisms and considering the ecotoxicological data of the most hazard DBE: BDE-44.
In Lake Lugano, DDx, PCB, and Hg concentrations were analyzed in shad and perch, as reported in Fig. 3b. Organic chemical values were significantly higher in shad than in perch (P < 0.01). DDx and PCB concentrations in fish samples were in any case always below the QSBs, while all fish samples exceeded the QSB for Hg. This result is not uncommon both in contaminated and pristine water bodies due to diffuse Hg contamination and/or natural background (e.g., Lepom et al., 2012; Hope & Louch, 2013).
Comparison among Lake Maggiore, Lake Lugano, and other Alpine lakes for sediments and biota
A comparison between Lake Maggiore and Lake Lugano on the basis of contaminant concentrations in sediments and biota samples collected in the same temporal period is reported in Table 1. Sediments collected in 2008–2011 in Lake Maggiore showed a consistently higher contamination level than samples from Lake Lugano. For example, DDx values in the Pallanza Basin and in the middle-southern part of Lake Maggiore were 2 orders of magnitude higher than the concentrations found in Lake Lugano. Similarly, Hg concentrations in the middle-southern part of Lake Maggiore were more than 10 times higher. Nevertheless, when considering the whole dataset, no significant difference was found between the two lakes, because of the wide range of values registered and the limited number of samples available for both lakes.
Similarly, DDx and PCB concentrations in D. polymorpha samples collected in 2010 and 2011 were higher in Lake Maggiore (although the difference was not always significant), while Hg contamination was similar in the samples collected in the two lakes. These results confirm a local hot-spot area for DDx contamination, corresponding to the presence of an industrial site located in the Toce basin for the production of DDT technical mixture. Lake Maggiore contamination by PCBs is likely due to the pollution load of some tributaries of the lake (such as Rivers Boesio and Bardello; CIPAIS, 2011).
The comparison between fish samples collected in 2009 (Table 1) showed that DDx and Hg values in Lake Lugano were significantly lower than in Lake Maggiore, while PCB concentrations were similar in the two lakes. These results differ from those obtained for zebra mussels, likely because of a different bioaccumulation capacity of the two organisms or of their ecological niches. Indeed, while mussels are littoral benthic organisms, shad and whitefish are mainly pelagic and therefore have different trophic role and diet (Poma et al., 2014).
A significant difference (P < 0.05) could be observed when comparing PBDE contamination levels in fish sampled in Lake Maggiore and Lake Lugano in 2015 (4 seasonal samples in 1 year, Fig. S5): this preliminary result may represent a warning for the general health status of Lake Lugano ecosystem. This situation might be caused by local industrial or civil sources, although deeper investigations and a specific monitoring program is recommended in order to exactly identify the pollution sources as well as the real extent of the contamination.
Discussion
Long-term biota trend
To analyze the temporal contamination trends, the concentration of organic chemicals in the biota was normalized on the lipid content (Tables S1 and S2) to reduce differences in the bioaccumulation capacity of the organisms according to their physiological and specific characteristics (Fig. 4).
Temporal trends of contaminants in biota sampled in Lake Maggiore. DDx and PCB concentrations (ng g−1 l.w.) in a two species of fish (shad and whitefish) in the period 2001–2015 and b in zooplankton in the period 2008–2015; c comparison between DDx concentrations in shad, whitefish, and zooplankton (ng g−1 l.w.). Arrows highlight a 3–6 months delay for fish in comparison to zooplankton
In Lake Maggiore, temporal analysis of DDx and PCBs in zooplankton showed a significant decreasing trend in the considered 2008–2016 period (Fig. 4b), after the closure of the industrial activities of Pieve Vergonte (data in previous years are not available). In particular, a strong decrease was observed from July 2009, when concentrations diminished about 6 times for DDx and from 3 to 6 times for PCBs when compared to 2008/2009 values. Similarly, a significant decreasing trend was observed in fish between 2001 and 2015 for both DDx and PCBs (Fig. 4a). On the contrary, in the same period, Hg showed no decreasing trend in fish, with peaks corresponding to intense hydrological events (i.e., 2010 and 2014; CIPAIS, 2015a). Similarly, no decreasing trend was also observed in fish for PBDEs, with a peak in the 2011–2014 period, that might be due to the input of local industrial activities (CIPAIS, 2015b).
The literature reports other studies were general recent trends in fish contamination were observed: as an example, decreasing PCBs trends were reported in two salmon species in Lake Michigan in the 1980–2010 period (Rasmussen et al., 2014); on the contrary, a general increasing trend was shown in PBDE contamination in American Great Lakes trout in the 2004–2009 period (Crimmins et al., 2012).
Comparing DDx values, similar trends were observed in whitefish and zooplankton between 2008 and 2015 (Fig. 4c). In particular, in 4 cases peaks for whitefish showed a 3–6 months delay in comparison to peaks for zooplankton, underlining a possible role of zooplankton as an early warning bioindicator of hot-spot contamination (Bettinetti et al., 2012a, b). Besides, as whitefish is mainly zooplanktivorous (Visconti et al., 2013), this time shift could be ascribed to the time needed for the transfer of this pollutant from zooplankton to fish. The misalignment between the zooplankton and shad peaks was less evident, possibly because shad generally changes its diet seasonally: in winter this fish is generally more pelagic and zooplanktivorous, while in summer is more littoral and benthivorous (Bettinetti et al., 2012a; Poma et al., 2014). Concerning D. polymorpha, no significant decreasing trend was observed for DDx and PCBs in the 1996–2015 period, nor was for Hg and As in the 2008–2013 period (CIPAIS, 2013b). For Lake Lugano, the temporal trend was analyzed for shad and perch considering data collected in 1993, 2000, 2007, and 2009 (Fig. S6 reported the average of the four seasons analyzed): DDx and PCB concentrations decreased in time, especially in the case of shad, but more data would be necessary to test the significance of these trends.
Comparing D. polymorpha results in Lake Maggiore (Pallanza Bay) and Lake Lugano (2010–2011) with two other Alpine deep large lakes located in Northern Italy (Lake Como and Lake Iseo in 2006–2007) (Bettinetti et al., 2008), DDx values of the latter lakes showed an intermediate contamination between Lake Maggiore and Lake Lugano. The Authors explained the phenomenon considering a possible input of DDx through the contribution of glacier melting waters (Bettinetti et al., 2008). PCBs contamination in the four lakes seemed to be more homogeneous. For Hg, D. polymorpha in Lake Maggiore (2008) showed in the Pallanza Basin values significantly higher than specimens collected in lakes Como and Iseo, while values for mussels collected along the main axis of Lake Maggiore were similar to the concentrations found in the other lakes (data not shown). These results again emphasize the local origin of Hg contamination in the Pallanza Basin of Lake Maggiore, while diffuse/natural contamination may be responsible of Hg in the other areas and lakes. Shad samples collected in 2008–2009 in Lake Como (Bettinetti et al., 2016) showed DDx and Hg values significantly lower than those in Lake Maggiore while no difference was observed for PCBs. These results confirm that residual DDx and Hg contamination is present in the Pallanza Basin of Lake Maggiore deriving from the industrial plant located on the River Toce.
Seasonal variability in zooplankton and fish in Lake Maggiore
Zooplankton plays a key role in understanding the patterns and dynamics of the transfer of pollutants through the food chain. Zooplankton includes primary and secondary consumers, whose contribution largely varies with time, driving seasonality in carbon and nitrogen isotopic signatures. In fact, the predators are relatively enriched in the heaviest nitrogen isotope, which is less excreted than the lightest one (Cabana & Rasmussen, 1996; Post, 2002; Layman et al., 2012). In the present study, samples displayed an increase in 15N-enriched traces, as reported in more detail in Poma et al. (2014), suggesting a major contribution of predators over primary consumers. Like in all deep subalpine lakes, zooplankton of Lake Maggiore was characterized by higher 15N-enriched signatures in Winter and lower in Spring and Summer (Visconti & Manca, 2011; Visconti et al., 2013; Fadda et al., 2014). Changes in the SIA signatures were also correlated with the seasonality of DDx and PCB concentrations, consistent with the highly significant correlation that we found between δ15N signatures and PCB and DDx concentrations in fish.
Such pattern was more evident for PCBs, probably because this contamination was derived by diffuse sources (Fig. 5a), while DDx concentrations were in a non-equilibrated status, being more dependent on the DDx loads driven by the flood events of the Toce River. No significant seasonal variability was found for D. polymorpha. Concerning fish samples, no significant seasonal variability was found for shad, while in whitefish a significant seasonal variability could be calculated (Fig. 5b), with lower PCB and DDx concentrations in summer samples. Low summer concentrations in whitefish might be related to the maximum fish growth in this season, with a consequent dilution of the contaminants present into their body (Volta et al., 2009).
Biomagnification of toxicants in a pelagic food chain in Lake Maggiore
Previous studies demonstrated that different BDE congeners can biomagnify in aquatic organisms through the food web (Hu et al., 2010). Moreover, Poma et al. (2014) evaluated the potential PBDE bioaccumulation in Lake Maggiore using zooplankton and fish data, by calculating both the BMFTL and the TMF, in order to examine the direct predator/prey relationship. They found BMFTL values higher than 1 for BDE-47, BDE-99, BDE-100, suggesting that these chemicals biomagnified in fish in Lake Maggiore. In the present study, the same calculation was carried out for PCBs, DDx, and Hg in zooplankton and fish, as detailed in Table S3. A different behavior was observed for DDx in the two considered fish species: a BMFTL higher than 1 was obtained for most DDx metabolites in shad but not in whitefish, highlighting a higher biomagnification capacity in shad. Similarly, shad BMFTL values for PCBs were higher in shad, although never exceeding the value of 1. Notably, PCBs with the highest biomagnification values were: PCB-52, PCB-149, PCB-153, and PCB-138. The low calculated values for the biomagnification of PCBs are probably due to the young age of the fish considered in this study (2-year-old species): in fact, Volta et al. (2009) demonstrated that PCBs bioaccumulation in whitefish is strictly age-dependent and that a steady state is reached only at the age of 5. For PCBs, the same age-dependent behavior was also published by El-Shaarawi et al. (2011). It is thus likely that in the present investigation the fish species analyzed were too young and that the partitioning equilibrium between zooplankton and fish was not reached yet. Significantly higher BMFTL values were calculated for Hg, being > 6 for shad, and ≥ 3 for whitefish. This is in agreement with previous studies, showing that the slope of the relationship between δ15N and contaminant concentration in fish is steeper for Hg than for several other pollutants (Matthews & Mazumder, 2005).
We compared the obtained BMFTL with the TMF values in order to determine whether the information indicated by the calculated BMFTL for specific predator/prey combinations results in higher or lower accumulation than calculated according to TMFs. A strongly significant correlation could be observed both for shad and whitefish, likely because the whole food web depends mostly on the fish-zooplankton relationships. However, the different slopes of the regression line calculated for whitefish (0.962) and shad (0.516) might derive from trophic contribution of other aquatic organisms to shad diet, besides zooplankton, as previously reported. Based on these results, we suggest a possible use of BMFTL values, as alternative to TMF to investigate the biomagnification potential of organic and inorganic chemicals in the evaluation of simple trophic food webs.
When comparing our results with previously published studies, the TMFs reported here for Lake Maggiore are very similar to those calculated in several other investigations (Hu et al., 2010; Ren et al., 2010; Yu et al., 2012), thus confirming that DDx, many PCBs, and PBDEs are able to biomagnify within food webs. A similar situation can be reported also for Hg, with an average calculated TMF of 4.3 for freshwater ecosystems around the world (Lavoie et al., 2013).
Conclusion
In this paper, a critical analysis of the data deriving from CIPAIS monitoring programs in two large deep lakes in the pre-alpine Italian-Swiss area is reported. In particular, the main spatial and temporal trends were derived for sediments and biota and comparisons between the two lakes were also carried out, though partially fragmentary sequences in the dataset. Point and diffuse sources of target toxicants were identified and the time trends allowed to emphasize the contribution of legacy and current contamination. Besides, in order to assess the biomagnification in food webs in Lake Maggiore, two different models were compared.
We conclude that, in Lake Maggiore, DDx and Hg contamination derive from past industrial activities along the Toce River and we also highlight potential risks for the ecosystem: in sediments, DDx and Hg values exceeded PEC thresholds according to MacDonald et al. (2000) and the European QSBs were exceeded in fish. Even if the concentrations of pollutants are decreasing in time in sediments samples, in biota the same trends were not observed for all contaminants. For example, Hg showed no decreasing trend in fish, pointing out that this toxicant is still bioavailable to aquatic organisms. Besides, in both lakes the current industrial use for PBDEs, the past use for PCBs and the prevailing natural origin for As were highlighted.
Through the analysis of the biomagnification in a pelagic food chain in Lake Maggiore, we showed the crucial role of timing for the contamination transfer to the higher trophic levels; as well, the SIA analysis of seasonality showed that food chains may change with the season, according to the ecological/behavioral traits of the considered species. In addition, we hypothesize that the observed difference in the slope of the regression line between TMF and BMFTM for whitefish and shad mirrors a trophic contribution of other food sources, in addition to zooplankton, to shad diet. Lastly, for the considered pelagic chain (zooplankton–fish), TMF and BMFTL values showed a Hg > DDx ≈ PBDEs biomagnification capacity, while limited values were calculated for PCBs, probably because of an age-dependent relationship in the bioaccumulation process.
Lake Lugano showed lower contamination than Lake Maggiore as expected, with a diffuse origin of the analyzed toxicants, with the exception of PBDEs in fish, for which concentrations were higher than in Lake Maggiore: their origin and their bioaccumulation in biota deserves a deeper analysis to identify potential point sources in Lake Lugano.
References
Arnot, J. A. & F. A. P. C. Gobas, 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environmental Reviews 14(4): 257–297.
Barbanti, L. & W. Ambrosetti, 1989. The physical limnology of Lago Maggiore: a review. Memorie dell’Istituto Italiano di Idrobiologia 46: 41–68.
Barbieri, A. & B. Polli, 1992. Description of lake Lugano. Aquatic Sciences 54: 181–183.
Baudo, R., 1989. Metals in Lake Maggiore. Memorie dell’Istituto Italiano di Idrobiologia 46: 261–286.
Bervoets, L., J. Voets, A. Covaci, S. Chu, D. Qadah, R. Smolders, P. Schepens & R. Blust, 2005. Use of transplanted zebra mussels (Dreissena polymorpha) to assess the bioavailability of microcontaminants in Flemish surface waters. Environmental Science & Technology 39: 1492–1505.
Bettinetti, R., S. Quadroni, S. Galassi, R. Bacchetta, L. Bonardi & G. Vailati, 2008. Is meltwater from Alpine glaciers a secondary DDT source for lakes? Chemosphere 73: 1027–1031.
Bettinetti, R., S. Galassi, L. Garibaldi, B. Leoni & S. Quadroni, 2012a. Zooplankton as an early warning signal of POPs contamination in deep lakes. Journal of Limnology 71(2): 335–338.
Bettinetti, R., S. Quadroni, M. Manca, R. Piscia, P. Volta, L. Guzzella, C. Roscioli & S. Galassi, 2012b. Seasonal fluctuations of DDTs and PCBs in zooplankton and fish of Lake Maggiore (Northern Italy). Chemosphere 88: 344–351.
Bettinetti, R., S. Quadroni, E. Boggio & S. Galassi, 2016. Recent DDT and PCB contamination in the sediment and biota of the Como Bay (Lake Como, Italy). Science of the Total Environment 542: 404–410.
Binelli, A., L. Guzzella & C. Roscioli, 2008. Levels and congener profiles of polybrominated diphenyl ethers (PBDEs) in Zebra mussels (D. polymorpha) from Lake Maggiore (Italy). Environmental Pollution 153: 610–617.
Borgå, K., K. A. Kidd, D. C. G. Muir, O. Berglund, J. M. Conder, F. A. P. C. Gobas, J. Kucklick, O. Malm & D. E. Powell, 2012. Trophic Magnification Factors: considerations of ecology, ecosystems, and study design. Integrated Environmental Assessment and Management 8: 64–84.
Cabana, G. & J. B. Rasmussen, 1996. Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Sciences 93(20): 10844–10847.
Ceschi, M., M. De Rossa & M. Jäggli, 1996. Contaminanti organici, inorganici e radionuclidi nell’ittiofauna dei laghi Ceresio e Verbano (bacini svizzeri). Travaux de chimie alimentaire et d’hygiène 87: 189–211.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 1999. Ricerche sulla distribuzione e gli effetti del DDT nell’ecosistema Lago Maggiore. Rapporto finale sui risultati delle indagini. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2010. Lago Ceresio: indagini su DDT e sostanze pericolose. Rapporto annuale 2009. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2011. Indagini su DDT e sostanze pericolose nell’ecosistema del Lago Maggiore. Rapporto annuale 2011. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2013a. Lago Ceresio: indagini su DDT e sostanze pericolose. Rapporto annuale 2012. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2013b. Indagini su DDT e sostanze pericolose nell’ecosistema del Lago Maggiore. Rapporto annuale 2012. Rapporto finale 2008-2012. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2015a. Studi sull’evoluzione del Lago Maggiore: aspetti limnologici. Programma triennale 2013-2015. Campagna 2014. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2015b. Indagini su DDT e sostanze pericolose nell’ecosistema del Lago Maggiore. Rapporto annuale 2015 e finale 2013-2015. Pallanza, Verbania, Italy.
CIPAIS (Commissione Internazionale per la Protezione delle Acque Italo-Svizzere), 2016. Lago di Lugano: indagine sulle sostanze pericolose. Contaminanti organici persistenti nella fauna ittica. Programma triennale 2013-2015. Campagna 2015. Pallanza, Verbania, Italy.
Conder, J. M., F. A. P. C. Gobas, K. Borgå, D. C. G. Muir & D. E. Powell, 2012. Use of Trophic Magnification Factors and related measures to characterize bioaccumulation potential of chemicals. Integrated Environmental Assessment and Management 8: 85–97.
Conder, J. M., P. C. Fuchsman, M. M. Grover, V. S. Magar & M. H. Henning, 2015. Critical review of mercury sediment quality values for the protection of benthic invertebrates. Environmental Toxicology and Chemistry 34: 6–21.
Crimmins, B. S., J. J. Pagano, X. Xia, P. K. Hopke, M. S. Milligan & T. M. Holsen, 2012. Polybrominated Diphenyl Ethers (PBDEs): turning the corner in Great Lakes Trout 1980–2009. Environmental Science and Technology 46: 9890–9897.
Cullon, D. L., M. B. Yunker, J. R. Christensen, R. W. Macdonald, M. J. Whiticar, N. J. Dangerfield & P. S. Ross, 2012. Biomagnification of polychlorinated biphenyls in a harbor seal (Phoca vitulina) food web from the strait of Georgia, British Columbia, Canada. Environmental Toxicology and Chemistry 31(11): 2445–2455.
de Wit, C. A., 2002. An overview of brominated flame retardants in the environment. Chemosphere 46: 583–624.
Eljarrat, E. & D. Barceló, 2003. Priority lists for persistent organic pollutants and emerging contaminants based on their relative toxic potency in environmental samples. Trends in Analytical Chemistry 22: 655–665.
El-Shaarawi, A. H., S. Backus, R. Zhu & Y. Chen, 2011. Modelling temporal and spatial changes of PCBs in fish tissue from Lake Huron. Environmental Monitoring and Assesment 173: 611–623.
Fadda, A., R. Rawcliffe, B. M. Padedda, A. Luglie, N. Sechi, F. Camin, L. Ziller & M. Manca, 2014. Spatiotemporal dynamics of C and N isotopic signature of zooplankton: a seasonal study on a man-made lake in the Mediterranean region. Annales de Limnologie – International Journal of Limnology 50(4): 279–287.
FOEN (Federal Office for the Environment), 2006. Stockholm Convention on Persistent Organic Pollutants (POPs) – Swiss National Implementation. Berne, 4.2006.
Grimalt, J. O., P. Fernandez, L. Berdie, R. M. Vilanova, J. Catalan, R. Psenner, R. Hofer, P. G. Appleby, B. O. Rosseland, L. Lien, J. C. Massabuau & R. W. Battarbee, 2001. Selective trapping of organochlorine compounds in mountain lakes of temperate areas. Environmental Science and Technology 35: 2690–2697.
Guilizzoni, P., S. N. Levine, M. Manca, A. Marchetto, A. Lami, W. Ambrosetti, A. Brauer, S. Gerli, E. A. Carrara, A. Rolla, L. Guzzella & D. A. L. Vignati, 2012. Ecological effects of multiple stressors on a deep lake (Lago Maggiore, Italy) integrating neo and palaeolimnological approaches. Journal of Limnology 71: 1–22.
Guzzella, L., L. Patrolecco, R. Pagnotta, L. Langone & P. Guilizzoni, 1998. DDT and other organochlorine compounds in the Lake Maggiore sediments: a recent point source of contamination. Fresenius Environmental Bulletin 7: 79–89.
Guzzella, L., F. Salerno, M. Freppaz, C. Roscioli, F. Pisanello & G. Poma, 2016. POP and PAH contamination in the southern slopes of Mt. Everest (Himalaya, Nepal): long-range atmospheric transport, glacier shrinkage, or local impact of tourism? The Science of the Total Environment 544: 382–390.
Hope, B. K. & J. Louch, 2013. Pre-anthropocene mercury residues in North American freshwater fish. Integrated Environmental Assessment and Management 10(2): 299–308.
Hu, G. C., J. Y. Dai, Z. C. Xu, X. J. Luo, H. Cao, J. S. Wang, B. X. Mai & M. Q. Xu, 2010. Bioaccumulation behavior of polybrominated diphenyl ethers (PBDEs) in the freshwater food chain of Baiyangdian Lake, North China. Environmental International 36(4): 309–315.
Hung, H., R. Kallenborn, K. Breivik, Y. Su, E. Brorström-Lundén, K. Olafsdottir, J. M. Thorlacius, S. Leppänen, R. Bossi & H. Skov, 2010. Atmospheric monitoring of organic pollutants in the Arctic under the Arctic Monitoring and Assessment Programme (AMAP): 1993–2006. The Science of the Total Environment 408: 2854–2873.
IST-SUPSI (Istituto Scienze della Terra), 2016. Ricerche sull’evoluzione del Lago di Lugano. Aspetti limnologici. Programma quinquennale 2013-2015. Campagna 2015 e sintesi pluriennale. Commissione Internazionale per la Protezione delle Acque Italo-Svizzere (CIPAIS). Pallanza, Verbania, Italy.
Kwan, K. H. M., H. M. Chan & Y. de Lafontaine, 2003. Metal contamination in zebra mussels (Dreissena polymorpha) along the St. Lawrence River. Environmental Monitoring and Assesment 88: 193–219.
Lavoie, R. A., T. D. Jardine, M. M. Chumchal, K. A. Kidd & L. M. Campbell, 2013. Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environmental Science and Technology 47: 13385–13394.
Layman, C. A., M. S. Araujo, R. Boucek, E. Harrison, Z. R. Jud, P. Matich, C. M. Hammerschlag-Peyer, A. E. Rosenblatt, J. J. Vaudo, L. A. Yeager, D. M. Post & S. Bearhop, 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological Reviews 87(3): 545–562.
Lepom, P., U. Irmer & J. Wellmitz, 2012. Mercury levels and trends (1993–2009) in bream (Abramis brama L.) and zebra mussels (Dreissena polymorpha) from German surface waters. Chemosphere 86: 202–211.
MacDonald, D. D., C. G. Ingersoll & T. A. Berger, 2000. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology 39: 20–31.
Marchetto, A., A. Lami, S. Musazzi, J. Masaferro, L. Langone & P. Guilizzoni, 2004. Lake Maggiore (N. Italy) trophic history: fossil diatoms, plant pigments, chironomids and comparison with long-term limnological data. Quaternary International 113: 97–110.
Marchetto, A., M. Rogora & S. Arisci, 2013. Trend analysis of atmospheric deposition data: a comparison of statistical approaches. Atmospheric Environment 64: 95–102.
Marziali, L., F. Rosignoli, A. Drago, S. Pascariello, L. Valsecchi, R. Bruno & L. Guzzella, 2017. Toxicity risk assessment of mercury, DDT and arsenic legacy pollution in sediments: a triad approach under low concentration conditions. Science of the Total Environment 593–594: 809–821.
Matthews, B. & A. Mazumder, 2005. Consequences of large temporal variability of zooplankton δ15N for modeling fish trophic position and variation. Limnology and Oceanography 50(5): 1404–1414.
Pfeifer, H.R., M.H. Dorron, D. Rey, C. Schlegel, O. Ateeia, R. Dalla Piazza, J.P. Dubois & Y. Mandia, 2000. Natural trace element input to the soil-sediment-water-plant system: examples of background and contaminated situations in Switzerlans, Eastren France and Northern Italy. In: B. Markert & K. Firese (eds), Trace elements – Their distribution and effects in the environment, Elsevier Science B.V.
Pfeifer, H. R., A. Gueye-Girardet, D. Reymond, C. Schlegel, E. Temgoua, D. L. Hesterberg & J. W. Chou, 2004. Dispersion of natural arsenic in the Malcantone watershed, Southern Switzerland: field evidence for repeated sorption–desorption and oxidation–reduction processes. Geoderma 122: 205–234.
Pisanello, F., L. Marziali, F. Rosignoli, G. Poma, C. Roscioli, F. Pozzoni & L. Guzzella, 2016. In situ bioavailability of DDT and Hg in sediments of the Toce River (Lake Maggiore basin, Northern Italy): accumulation in benthic invertebrates and passive samplers. Environmental Science and Pollution Research International 23: 10542–10555.
Poma, G., P. Volta, C. Roscioli, R. Bettinetti & L. Guzzella, 2014. Concentrations and trophic interactions of novel brominated flame retardants, HBCD, and PBDEs in zooplankton and fish from Lake Maggiore (Northern Italy). The Science of the Total Environment 481: 401–408.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83(3): 703–718.
Rasmussen, P. W., C. Schrank & M. C. W. Williams, 2014. Trends of PCB concentrations in Lake Michigan coho and chinook salmon, 1975–2010. Journal of Great Lakes Research 40: 748–754.
Riva, C., M. Parolini, A. Binelli & A. Provini, 2010. The case of pollution of Lake Maggiore: a twelve-year study with the bioindicator mussel Dreissena polymorpha. Water Air and Soil Pollution 210: 75–86.
Ruiz-Fernandez, C. A., F. J. Ontiveros-Cuadras, J. L. Sericano, J. A. Sanchez-Cabeza, L. L. W. Kwong, R. B. Dunbar, D. A. Mucciarone, L. H. Perez-Bernal & F. Páez-Osuna, 2014. Long-range atmospheric transport of persistent organic pollutants to remote lacustrine environments. The Science of the Total Environment 493: 505–520.
Salmaso, N. & R. Mosello, 2010. Limnological research in the deep southern subalpine lakes: synthesis, directions and perspectives. Advances in Limnology and Oceanography 1: 29–66.
Schmid, P., M. Zennegg, P. Holm, C. Pietsch, B. Brüschweiler, A. Kuchen, E. Staub & J. Tremp, 2010. Polychlorierte Biphenyle (PCB) in Gewässer der Schweiz. Daten zur Belastung von Fischen und Gewässern mit PCB und Dioxinen, Situationsbeurteilung. Umwelt-Wissen 1002, Bundesamt für Umwelt: 1–101.
Sharma, V. K. & M. Sohn, 2009. Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environment International 35: 743–759.
Solcà, N., M. De Rossa & M. Jermini, 2006. Monitoring of DDTs in fishes of the Lake Maggiore. Travaux de chimie alimentaire et d’hygiène 97: 348–357.
Specht, W. & M. Tillkes, 1980. Gas-chromatographische Bestimmung von Rückständen an Pflanzenbehandlungs- mitteln nach Clean-up über Gel-Chromatographie und Mini-Kieselgel-Säulen-Chromatographie. 3 Mitteilung. Fresenius’ Journal of Analytical Chemistry 301: 300–307.
Specht, W. & M. Tillkes, 1985. Gas-chromatographische Bestimmung von Rückständen an Pflanzenbehandlungs mitteln nach Clean-up über Gel-Chromatographie und Mini-Kieselgel-Säulen-Chromatographie. 5 Mitteilung. Fresenius’ Journal of Analytical Chemistry 322: 443–455.
Steinwandter, H., 1985. Universal 5 min on-line method for extracting and isolating pesticide residues and industrial chemicals. Fresenius’ Journal of Analytical Chemistry 322: 752–754.
Tessier, A. & P. G. C. Campbell, 1987. Partitioning of trace metals in sediments: relationships with bioavailability. Hydrobiologia 149: 43–52.
Tremolada, P., S. Villa, P. Bazzarin, E. Bizzotto, R. Comolli & M. Vighi, 2008. POPs in mountain soils from the Alps and Andes: suggestions for a ‘precipitation effect’ on altitudinal gradients. Water, Air, & Soil Pollution 188: 93–109.
Ullrich, S. M., T. W. Tanton & S. Abdrashitova, 2001. Mercury in the aquatic environment: a review of factors affecting methylation. Critical Reviews in Environmental Science and Technology 31: 37–41.
US EPA, US Environmental Protection Agency, USACE, US Army Corps of Engineers, 1998. Evaluation of dredged material proposed for discharge in waters of the United States – Testing Manual. U.S. Environmental Protection Agency, U.S. Army Corps of Engineers, Washington DC.
van Drooge, B. L., G. Garriga, K. A. Koinig, R. Psenner, P. Pechan & J. O. Grimalt, 2014. Sensitivity of a remote alpine system to the Stockholm and LRTAP regulations in POP emissions. Atmosphere 5: 198–210.
Vignati, D. A. L. & P. Guilizzoni, 2011. Metalli nel Lago Maggiore: livelli naturali e antropici. Acqua & Aria 1: 22–27.
Visconti, A. & M. Manca, 2011. Seasonal changes in the δ13C and δ15N signatures of the Lago Maggiore pelagic food web. Journal of Limnology 70(2): 263–271.
Visconti, A., P. Volta, A. Fadda, A. Di Guardo & M. Manca, 2013. Seasonality, littoral versus pelagic carbon sources, and stepwise 15N-enrichment of pelagic food web in a deep subalpine lake: the role of planktivorous fish. Canadian Journal of Fisheries and Aquatic Sciences 71(3): 436–446.
Vives, I., E. Canuti, J. Castro-Jimenez, E. H. Cristoph, S. J. Eisenreich, G. Hanke, T. Huber, G. Mariani, A. Mueller, H. Skejo, G. Umlaufa & J. Wollgasta, 2007. Occurrence of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in Lake Maggiore (Italy and Switzerland). Journal of Environmental Monitoring 9: 589–598.
Volta, P., P. Tremolada, M. C. Neri, G. Giussani & S. Galassi, 2009. Age-dependent bioaccumulation of organochlorine compounds in fish and their selective biotransformation in top predators from Lake Maggiore (Italy). Water, Air, & Soil Pollution 197: 193–209.
Walkley, A. & I. A. Black, 1934. An examination of the Deghtareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science 27: 29–38.
Wang, S. & C. N. Mulligan, 2006. Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Science of the Total Environment 366: 701–721.
Wu, B., G. Wang, J. Wu, Q. Fu & C. Liu, 2014. Sources of heavy metals in surface sediments and an ecological risk assessment from two adjacent plateau reservoirs. PLoS ONE 9(7): e102101.
Yu, Y. X., S. H. Zhang, N. B. Huang, J. L. Li, Y. P. Pang, X. Y. Zhang, Z. Q. Yu & Z. G. Xu, 2012. Polybrominated diphenyl ethers and polychlorinated biphenyls in freshwater fish from Taihu Lake, China: their levels and the factors that influence biomagnification. Environmental Toxicology and Chemistry 31(3): 542–549.
Acknowledgements
This work was supported by the International Commission for the Protection of the Italian-Swiss Common Waters (CIPAIS) within the research activity programs 2008–2012 and 2013–2015 “Investigations on DDT and hazardous substances—Lake Maggiore and Lake Ceresio.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Nico Salmaso, Orlane Anneville, Dietmar Straile & Pierluigi Viaroli / Large and deep perialpine lakes: ecological functions and resource management
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guzzella, L.M., Novati, S., Casatta, N. et al. Spatial and temporal trends of target organic and inorganic micropollutants in Lake Maggiore and Lake Lugano (Italian-Swiss water bodies): contamination in sediments and biota. Hydrobiologia 824, 271–290 (2018). https://doi.org/10.1007/s10750-017-3494-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3494-7