Abstract
The acorn barnacle Perforatus perforatus has a defined breeding temperature range and reproductive season that varies geographically. This study aims to investigate the influence of reproductive parameters of P. perforatus in species distribution ranges in the NE Atlantic. The hypothesis tested is that the breeding season of P. perforatus off NW Portugal begins earlier and is longer than at the northern distribution limit of this species, and that fecundity is higher in terms of number of broods per individual per breeding season. The span of the breeding season and fecundity indices were assessed based on the presence and maturation of ovigerous lamellae and correlated with temperature. Results showed that the breeding season in the NW Portuguese coast lasts over 10 months (February–November) and the number of broods was determined to be 9.2 ind/year. Temperature seems to be a primary factor determining the breeding season, but other factors, such as food availably, light and photoperiod, are also of great importance. However, the higher quantity of embryos produced in NW Portugal is not reflected in a higher abundance of settled adults in rocky shores. Contrarily, the species is particularly abundant in artificial substrata offshore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As adults, barnacles (Cirripedia) are exclusively marine sessile crustaceans that function as filter feeders in rocky shore ecosystems and compete for space with molluscs such as limpets and mussels. The occurrence, abundance and latitudinal distribution of barnacle species depend on the climatic regime, and temperature is a crucial factor determining their reproductive success (Norris & Crisp, 1953; Patel & Crisp, 1960a; Barnes, 1989; Southward, 1991; Poloczanska et al., 2008). Namely, the number of broods, timing and extent of the breeding season are species-specific parameters that define barnacles as being either boreo-arctic or temperate-tropical species. Boreo-arctic species (e.g., Semibalanus balanoides and Balanus balanus) produce a single annual brood, gonadal development occurs during summer and early autumn, and embryos incubate during the winter (Barnes, 1963). In contrast, the pattern corresponding to tropical and temperate barnacles (e.g., Perforatus perforatus and Chthamalus montagui and C. stellatus) is characterised by a defined breeding season with multiple broods (Norris & Crisp, 1953; Burrows et al., 1999; Herbert et al., 2003; Macho et al., 2010). However, other cirripedes (e.g., Austrominus modestus and Amphibalanus amphitrite) are strongly eurythermal, having a broad distribution range (comprising various continents) and breeding over a wide range of temperatures. Perforatus perforatus is found on a wide range of hard substrata along wave-exposed shores and in ria-type estuaries in the lower half of the littoral zone, (Evans, 1947; Norris & Crisp, 1953; Herbert et al., 2003; Macho et al., 2010) and also in the sublittoral zone (Herbert et al., 2003; Neal & Yule, 1994). It commonly inhabits semi-exposed and shady rock surfaces, crevices and overhangs that reach the mid tide level zone (Evans, 1947; Herbert et al., 2003). P. perforatus occurs naturally along the NE Atlantic seashore from SW Wales to West Africa, ranging between 31°N and 52°N and 9°W and 41°E (Choi et al., 2013), as well as in the Mediterranean Sea. However, in the English Channel, at the northern limit of its distribution, the species has extended its range eastwards in response to rising sea temperatures following the severe winter of 1962–1963 (Crisp, 1964; Herbert et al., 2003). The intolerance of adults to low air temperatures also defines the geographic limits of the northern edge of the distribution of the species (Herbert et al., 2003). Also, an increase in occurrence has been noticed in artificial substrates, including piers, breakwaters, marinas and sea defences that produce embayed zones and may facilitate colonisation (Herbert et al., 2003). More recently, P. perforatus was found to have been spread globally (e.g., the East China Sea and Sea of Japan) through discharges of ballast waters, fouled ship hulls (Torres et al., 2012; Choi et al., 2013) and plastic flotsam (Rees & Southwards, 2009), and in the NE Atlantic and the North Sea, it represents one of the most important species involved in biofouling (Fragoso & Icely, 2009; Kerckhof et al., 2010).
Previous studies show that P. perforatus only breeds over a limited range of temperature and has a defined breeding season that varies according to geographic location (Patel & Crisp, 1960a; Macho, 2006; Macho et al., 2010). In the present study, the reproductive parameters of P. perforatus on the NW coast of Portugal, the centre of the natural distribution of P. perforatus, were compared to those at the northern limit of the species distribution, as well as to those of other cirripedes species of the NE Atlantic coast. It is predicted that the higher temperatures at the lower latitudes of the NW coast of Portugal determine the reproductive parameters of P. perforatus and the abundance of adults on the rocky shores.
Considering previous work on the effects of temperature on breeding in marine organisms, this study aims to test the hypothesis that the breeding season of P. perforatus on the NW Atlantic coast of Portugal begins earlier and is longer than that at the northern distribution limit of this species; and that the species has higher fecundity in terms of number of broods per individual per breeding season.
Materials and methods
Adult Perforatus perforatus were collected fortnightly at Praia da Memória beach (41°13′47.17″ N; 8°43′21.33″W), Porto, Portugal during low spring tides over 23 months, from August 2013 to June 2015. In total, 1839 adult barnacles were collected and sample size was 60–70 individuals when possible. The location was selected due to the abundance of P. perforatus, the morphology of the beach, easy access and proximity to a reference area with low levels of contamination (Cunha et al., 2005). At Praia da Memória beach, there is approximately 1 km of rocky shorefront and the intertidal zone is quite wide at low tide. Barnacles were collected by hand using a small hammer and a chisel from the low intertidal zone shady rocks, in crevices and beneath overhangs. Juvenile barnacles were excluded from the study by selecting individuals whose apical diameter along the rostro-carinal axis was >5 mm (Iwaki & Hattori, 1987) and 10 mm for the basal diameter (Herbert et al., 2003). During January and February, samples were only collected once a month due to the severe weather and sea conditions.
Mean monthly seawater temperatures were provided by the Hydrographic Institute of the Portuguese Navy (Portugal; http://www.hidrografico.pt). Sea temperatures were obtained from an ondograph buoy situated approximately 10 km north and 20 km west (41°19′00″N; 8°59′00″N) of the sampling point (Praia da Memória).
The examination and isolation of mature ovigerous lamellae was performed immediately upon arrival at the laboratory. The presence/absence of lamellae in all individuals was recorded to determine the fecundity index (F i; %), i.e., the average percentage of the population with embryos over the year, and the relationship between this parameter and temperature. The number of broods (N B) released per individual per year was determined by applying the formula (Burrows et al., 1992):
L B = number of days that an individual spends carrying embryos, given as L B = Σ P B × ∆t), where P B is the average proportion of the population carrying embryos during the sampling interval (F i/100), and Δt is the time interval between consecutive samplings.
L E = the number of days needed for complete embryo development from oviposition to larval release. LE is 6 days and at 14.5°C and 4 days at 20°C.
The time that individuals spend carrying eggs was corrected for the mean seawater temperature during each sampling interval, assuming a linear relationship between temperature and the time spent carrying broods, based on the values provided by Patel & Crisp (1960a) for this species at 14.5 and 20°C.
The length of the breeding season was defined by counting the number of months in which ovisacs were present in the pallial cavity of adult females, and the optimum breeding temperature corresponded to the highest observed fecundity indices. It was not possible to accurately determine the minimum and maximum critical breeding temperatures since individuals were always observed carrying ovisacs at the minimum and maximum temperatures recorded during the 2 years of sampling. Therefore, we could only determine that the true values of those two temperatures are below and above the minimum and maximum recorded temperatures, respectively.
Results
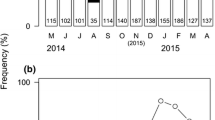
A total of 1839 adult barnacles were collected and examined, and the mean sample size was 57.5 ± 25.6 ind/sampling (mean ± standard deviation of the mean). The target sample size was 60–70 individuals when possible, and at least 19 individuals were sampled when the weather conditions were not favourable (Fig. 1).
Perforatus perforatus fecundity index (below) and monthly mean seawater temperature (above) throughout the year. The fecundity index was calculated as the percentage of individuals presenting ovigerous lamellae on the pallial cavity and determined over 23 months, from August 2013 to June 2015. The small numbers near the fecundity curves indicate sample size
The fecundity indices calculated for adult P. perforatus collected between August 2013 and June 2015 are presented in Fig. 1. The presence of ovigerous lamellae was first observed in February, and the maximum fecundity was observed in late April of 2014, when 52% of the individuals presented mature ovigerous lamellae in the pallial cavity. Lamellae are pale yellow and turn to dark orange in the final developmental stage, as they become larger and stiffer. All specimens presented 2 lamellae bilaterally, and mature individuals with lamellae were found from 5 mm apical diameter. During autumn and winter, lamellae were scarce or absent, corresponding to a fecundity index below 20% from September to February or actually nil in various samples (Fig. 1).
The fecundity indices were correlated with the seawater temperature during the month of sampling, as well as 1, 2 and 3 months earlier. A second-order polynomial function was fitted to data, and the best coefficient of determination (R 2 = 0.39) was obtained when the fecundity data were modelled as a function of seawater temperature 2 months before sampling date (Fig. 2; Table 1). Accordingly, the highest fecundity index was observed when the mean seawater temperature was 14.9°C. The minimum critical breeding seawater temperature was below 12.6°C, and the maximum was above 19.7°C, the lowest and highest temperatures registered during the sampling period, respectively (Fig. 1).
The number of broods per individual per year (N B) for P. perforatus was calculated to be 9.18 based on the P B determined for each sampling interval (the values in Fig. 1 transformed into proportions) and the duration of the interval between samplings (days).
Discussion
The breeding season of P. perforatus on the NW Portuguese coast starts in February and ends in November. Previous studies have determined that this species breeds during the summer months in England, from the middle of June to August (Norris & Crisp, 1953; Patel & Crisp, 1960a, b) and, more recently, from May to September (Herbert et al., 2003). In NW Spain breeding occurs from March to November (Macho, 2006; Macho et al., 2010) and in April in the Mediterranean, becoming less frequent later in the season (Lochhead, 1936) (Table 2). In summary, the breeding season begins earlier in Portugal (in late winter) followed by Spain and Italy (in spring) and then only in late spring and summer further north, i.e., South Wales and the English Channel, which indicates a strong relationship between temperature and the beginning of the breeding period. This corresponds to the prediction of Orton (Orton, 1920) that in the northern hemisphere, reproduction occurs progressively later toward the species northern edge of its geographic range.
In addition to the higher air and water temperatures observed further south, food availability is a factor that may also explain the earlier occurrence of the breeding season in NW Portugal by nearly 4 months relatively to South Wales and the English Channel (Table 2). Along the W coast of Portugal, the Canary/Iberian upwelling occurs from March to October (Wooster et al., 1976; Fiúza, 1983) and is responsible for the high productivity in the area at that time. Towards the end of the 20th century, the onset of the upwelling season has occurred progressively earlier in Portugal (Lemos & Pires, 2004), nearly corresponding to the beginning of the P. perforatus breeding season in February. In the English Channel, two major blooms are identified. The first occur from mid-April to mid-May, and the second, of slightly lower maximum chlorophyll concentration but longer duration, from early July to late September (Southward et al., 2005; Ward et al., 2011). The major role played by effective feeding on breeding success was confirmed in starved P. perforatus and C. stellatus (Patel and Crisp, 1960a). Also, in another barnacle species, Amphibalanus (as Balanus) amphitrite, the breeding potential at any given temperature is known to be regulated by food concentration (Desai et al., 2006).
On the NW Portuguese coast, the fecundity index peaks appear in April and May (corresponding to a seawater temperature of 14.9°C), 2 months after the lowest temperatures of the year (12.5°C in February). The time required from oocyte proliferation to the first brood has not been determined experimentally in P. perforatus. However, according to our data, it seems to be approximately 2 months of development, since the fecundity index is better correlated with seawater temperature 2 months before each sample was collected than with the temperature at the sampling time (Fig. 2). In contrast, Patel & Crisp (1960a) determined that, in most cases, P. perforatus is able to breed 2–3 weeks after being kept at temperatures above the minimum critical temperature for breeding, which is less time than inferred from our data. The minimum critical breeding temperatures of 15–16°C considered by Patel & Crisp (1960a), at which they performed their laboratory experiments, may explain the shorter duration of ovary development than observed in the field in NW Portugal, where the minimum critical breeding temperature appeared to be less than 12.6°C (Fig. 1).
Additionally, the minimum critical breeding temperature (15–16°C) determined by Patel & Crisp (1960a) in the laboratory corresponds to the optimum breeding temperature determined through field sampling in NW Portugal in this study (14.9°C). These differences might be due to the experimental context (laboratory vs field) and other factors associated with the life cycle of the species besides temperature. Although temperature has been invoked as the most important factor determining the onset of reproduction, breeding period length and breeding success (Orton, 1920), and other environmental factors, such as food availability (Olive, 1981), photoperiod (Garwood & Olive, 1982; Clark, 1988), hydrodynamics or gamete growth rates (Clark, 1988) have been recognised as equally important and can also modulate the reproductive response to temperature in invertebrates. Temperature might regulate the embryonic development directly, but the time of fecundity is more likely connected to food availability rather than temperature per se, influencing the start of the breading season and gamete production (Patel & Crisp, 1960a). Food availability may justify the lag of time of about 2 months observed between the rise of temperature and fecundity, as discussed above.
The minimum and maximum critical temperatures for breeding in this species, as well as the duration of ovary development from ovule proliferation to first brood, need to be further investigated and specifically targeted in future studies of P. perforatus, since there are significant differences between the values previously determined in the laboratory (Patel & Crisp, 1960a) and the values determined from the field in the present study.
The duration of embryonic development in P. perforatus was 6 days at 14.5°C and 4 days at 20°C in the English Channel (Herbert et al., 2003). These short periods of time allow for multiple broods during the breeding season. The number of broods estimated from two different locations in the southern UK at those two temperatures was 2.3 and 3.5 broods per individual per year, respectively (Herbert et al., 2003). In the present study in Portugal, the estimated number of broods was 9.2 per year, a consequence of the mean temperatures being above the minimum critical breeding temperature for a longer time, being 2.5–4 times higher than in the southern UK.
The higher number of broods and fecundity indices observed in NW Portugal should be positively related with the abundance of the species on the shores, if the only parameter determining recruitment was egg abundance. However, the analysis of P. perforatus abundance data from surveys on the W coast of Portugal (Santos, 1994, 2000) and the English Channel (Crisp & Southward, 1958) did not indicate a higher abundance of this barnacle in Portugal as would be expected from the fecundity parameters. On the contrary, none of the 35 sampling sites in Portugal, analysed from 1992 to 1999, had an abundance of P. perforatus greater than 0.01 ind/cm2, while many of the sites in southern England reached values well above that. Moreover, despite these low abundance values in natural substrates on rocky shores along the west coast of Portugal, this barnacle is very abundant on artificial substrates and mollusc shells in offshore aquaculture farms in southern Portugal (Fragoso & Icely, 2009). This indicates that other factors contribute to the lower abundance of natural populations of P. perforatus along the Portuguese coast and higher abundances on artificial substrates. Many factors other than egg abundance are involved in larval and post-settlement survival and recruitment, including food availability, larval transport, predation, competition, the availability of suitable settlement sites and settler dislodgment among others, and may act in combination. Along the coast of western Portugal the Canary/Iberian upwelling system displaces coastal surface waters offshore from March to October (Wooster et al., 1976; Fiúza, 1983), which in some cases contributes to offshore dispersal pathways and in others to near-shore retention depending on the diel vertical migration of plankton (Marta–Almeida et al., 2006; Peliz et al., 3). Morphologically the north-central Portuguese coast is primarily a low-lying coastal plain with sand dunes and beaches, from which rock outcrops occasionally emerge. Therefore the standing spawning biomass may be decreased, due to the reduced area of rocky shore that serves as habitat for adults, in contrast to southern England. The transport of larvae offshore by the upwelling currents may also explain the higher abundance of adult P. perforatus on offshore aquaculture structures (Fragoso & Icely, 2009). Variation in larval supply may contribute to explain the differences in the abundance of P. perforatus on the Portuguese coast compared to southern England (Underwood & Fairweather, 1989; Miron et al., 1995). Moreover, this area is one of the most energetic in the world with very high hydrodynamic forces (Barbosa et al., 2005) that may contribute to the low settlement and high mortality rates of recruits due to strong wave exposure. Additional factors that may influence the abundance of adult P. perforatus on the W Atlantic border, to be considered in future research, are the causes of mortality in larval, post-settled and juvenile individuals due to differences in shore conditions, predation, competition and fitness, among others. Another fact that needs to be considered is the subtidal abundance of the species. The habitat of this species may extend into the sublittoral zone and recruitment in Wales and South England may be also dependent on individuals from shallower or deeper waters. However, sublittoral surveys performed in the final of 20th century have failed to locate B. perforatus along the southeast coast of England (Herbert et al., 2003). Regarding the abundance of P. perforatus in subtidal zone of the Portuguese coast, at the best of our knowledge no data could be found.
Conclusions
This study indicates that the breeding season of P. perforatus on the NW Atlantic coast of Portugal begins earlier than at the northern distribution limit of this species, which is in accordance with Orton’s (1920) rules. The time span of the breeding season is longer along the NW coast of Portugal compared to those near the distribution limit of the species. The optimal breeding temperature determined is 14.9°C, and the minimum and maximum critical breeding temperatures were found to be below 12.6°C and above 19.7°C, respectively. The longer breeding season along the NW coast of Portugal gives rise to 2.5–4 times more broods per individual per year than in South Wales and the English Channel. Therefore, the hypothesis proposed was confirmed by our observations. However, the larval cycle, settling and post-settlement of this species deserves further investigation as the higher quantity of embryos produced in NW Portugal seems to be not reflected in a higher abundance of settled adults in rocky shores.
References
Anil, A. C., 1991. Studies on macrofouling ecology of cirripedes in Hamana Bay (Japan). Ph.D. thesis, Faculty of Agriculture, University of Tokyo.
Barbosa, J. P., F. V. & Gomes F. T. Pinto, 2005. Analysis of the Portuguese west coast morphology and morphodynamics, based on aerial images and GIS tools. Proceedings 2nd EARSel Workshop -Remote Sensing of the Coastal Zone: 809–818 pp.
Barnes, H., 1963. Light, temperature and breeding of Balauns balanoides. Journal of the Marine Biological Association of the United Kingdom 43: 717–727.
Barnes, H., 1989. Egg production in cirripedes. Oceanography and Marine Biology Annual Review 27: 91–166.
Burrows, M. T., S. J. Hawkins & A. J. Southward, 1992. A comparison of reproduction in co-occurring chthamalid barnacles Chthamalus stellatus (Poli) and Chthamalus montagui Southward. Journal of Experimental Marine Biology and Ecology 160: 229–249.
Burrows, M. T., S. J. Hawkins & A. J. Southward, 1999. Larval development of the intertidal barnacles Chthamalus stellatus and Chthamalus montagui. Journal of the Marine Biological Association of the United Kingdom 79: 93–101.
Clark, S., 1988. A two phase photoperiodic response controlling the annual gametogenic cycle in Harmothoe imbricata (L.) (Polychaeta: Polynoidae). International Journal of Invertebrate Reproduction and Development 14: 245–265.
Choi, K.-H., H.-W. Choi, I.-H. Kim & J.-S. Hong, 2013. Predicting the invasion pathway of Balanus perforatus in Korean Seawaters. Ocean Polar Research 35: 63–68.
Crisp, D. J., 1950. Breeding and distribution of Chthamalus stellatus. Nature (London) 166: 311–312.
Crisp, D. J., 1964. The effects of the severe winter of 1962–63 on marine life in Britain. Journal of Animal Ecology 33: 165–210.
Crisp, D. J. & P. A. Davies, 1955. Observations in vivo on the breeding of Elminius modestus grown on glass slides. Journal of the Marine Biological Association of the United Kingdom 34: 357–380.
Crisp, D. J. & A. J. Southward, 1958. The distribution of intertidal organisms along the coasts of English Channel. Journal of the Marine Biological Association of the United Kingdom 37: 1031–1048.
Crisp, D. J., A. J. Southward & E. C. Southward, 1981. On the distribution of the intertidal barnacles Chthamahts stellatus, Chthamahis montagui and Eumphia depressa. Journal of the Marine Biological Association of the United Kingdom 61: 359–380.
Cruz, T., 2000. Biologia e ecologia do percebe Pollicipes pollicipes (Gmelim, 1790) no litoral sudoeste português. PhD thesis. University of Evora, Portugal: 306 pp.
Cruz, T. & J. Araújo, 1999. Reproductive patterns of Pollicipes pollicipes (Cirripedia: Pedunculata) in the SW coast of Portugal. Journal of Crustacean Biology 19(2): 260–267.
Cunha, I., L. M. Garcia & L. Guilhermino, 2005. Sea-urchin (Paracentrotus lividus) glutathione S-transferases and cholinesterase activities as biomarkers of environmental contamination. Journal of Environmental Monitoring 7(4): 288–294.
Desai, D. V., A. C. Anil & K. Venkat, 2006. Reproduction of Balanus amphitrite Darwin (Cirripedia: Thoracica): influence of temperature and food concentration. Marine Biology 149: 1431–1441.
Evans, R. G., 1947. The intertidal ecology of selected localities in the Plymouth neighbourhood. Journal of the Marine Biological Association of the United Kingdom 27: 173–218.
Fiúza, A.F.G., 1983. Upwelling patterns of Portugal. in: Coastal upwelling: Its sediment record. In Suess, E., & J. Thiede (eds), Plenum NY, Part A: 85–98.
Fragoso, B. & J. D. Icely, 2009. Upwelling events and recruitment patterns of the major fouling speies on the costal aquaculture (Sagres), Portugal. Journal of Coastal Research 56: 419–423.
Garwood, P. R. & P. J. W. Olive, 1982. The influence of photoperiod on oocyte growth and its role in the control of the reproductive cycle of the polychaete Harmothoe imbricata (L.). Invertebrate. International Journal of Reproduction 5(3): 161–165.
Herbert, R. J. H., S. J. Hawkins, M. Sheader & A. J. Southward, 2003. Range extension and reproduction of the barnacle Balanus perforatus in the eastern English Channel. Journal of the Marine Biological Association of the United Kingdom 83: 73–82.
Iwaki, T. & H. Hattori, 1987. First maturity and initial growth of some common species of barnacles in Japan. Bulletin of the Faculty of Fisheries-Mie University 14: 11–19.
Kerckhof, F., B. Rumes, A. Norro, T. G. Jacques & S. Degraer, 2010. Seasonal variation and vertical zonation of the marine biofouling on a concrete offshore windmill foundation on the Thornton Bank (southern North Sea). In Degraer, S., R. Brabant & B. Rumes (eds), Offshore Wind Farms in the Belgian part of the North Sea: Early Environmental Impact Assessment and Spatio-Temporal Variability. Royal Belgian Institute of Natural Sciences, Management Unit of the North Sea Mathematical Models, Marine Ecosystem Management Unit, Brussels: 53–68.
Lemos, R. T. & H. O. Pires, 2004. The upwelling regine off the west Portuguese coast, 1941–2000. International Journal of Climatology 24: 511–524.
Lochhead, J., 1936. On the feeding mechanism of the nauplius of Balanus perforatus Bruguière. Zoological Journal of the Linnean Society 39: 429–442.
Macho, G., 2006. Ecología reproductiva y larvaria del percebe y otros cirripídeos en Galicia. PhD Thesis, University of Vigo, Spain.
Macho, G., E. Vasquez, R. Giráldez & J. Molares, 2010. Spatial and temporal distribution of barnacle larvae in the partially mix estuary of the Ria de Arousa (Spain). Journal of Experimental Marine Biology and Ecology 392: 129–139.
Marta-Almeida, M., J. Dubert, A. Peliz, & H. Queiroga, 2006. Influence of vertical migration pattern on retention of crab larvae in a seasonal upwelling system. Marine Ecology Progress Series 307: 1–19.
Miron, G. Bernard, B. Boudreau & E. Bourget, 1995. Use of larval supply in benthic ecology: testing correlations between larval supply and larval settlement. Marine Ecology Progress Series 124: 301–305.
Neal, A. L. & A. B. Yule, 1994. The tenacity of Elminius modestus and Balanus perforatus cyprids to bacterial films grown under different shear regimes. Journal of the Marine Biological Association of the United Kingdom 74(1): 251–257.
Norris, E. & D. J. Crisp, 1953. The distribution and planktonic stages of the cirripede Balanus perforatus Bruguière. Proceedings of the Zoological Society of London 123: 393–409.
O’Riordan, R. M., F. Arenas, J. Arrontes, J. J. Castro, T. Cruz, J. Delany, B. Martínez, C. Fernandez, S. J. Hawkins, D. McGrath, A. A. Myers, J. Oliveros, F. G. Pannacciulli, A. M. Power, G. Relini, J. M. Rico & T. Silva, 2004. Spatial variation in the recruitment of the intertidal barnacles Chthamalus montagui Southward and Chthamalus stellatus (Poli) (Crustacea: Cirripedia) over an European scale. Journal of Experimental Marine Biology and Ecology 304: 243–264.
Olive, P. J. W., 1981. Control of the reproductive cycle in female Eulalia Viridis (Polychaeta: Phyllodocidae). Journal of the Marine Biological Association of the United Kingdom 61: 941–958.
Orton, J. H., 1920. Sea temperature breeding and distribution in marine animals. Journal of Marine Biological Association of the United Kingdom 12: 371–378.
Patel, B. & D. J. Crisp, 1960a. The influence of temperature on the breeding and the moulting activities of some warm-water species of operculate barnacles. Journal of the Marine Biological Association of the United Kingdom 39: 667–680.
Patel, B. & D. J. Crisp, 1960b. Rates of development of embryos of several species of barnacles. Physiological Zoology 33: 104–119.
Peliz, A., P. Marchesiello, J. Dubert, M. Marta-Almeida, C. Roy & H. Queiroga, 2007. A study of crab larvae dispersal on the Western Iberian Shelf: physical processes. Journal of Marine Systems 68: 215–236.
Poloczanska, E. S., S. J. Hawkins, A. J. Southward & M. T. Burrows, 2008. Modeling the response of populations of competing species to climate change. Ecology 89: 3138–3149.
Rees, E. I. S. & A. J. Southward, 2009. Plastic flotsam as an agent for dispersal of Perforatus perforatus (Cirripedia: Balanidae). Marine Biodiversity Records 2: 1–3.
Santos, A., 1994. Estudo e caracterização dos povoamentos bentónicos intertidais (substrato rochoso) do Norte de Portugal. Master thesis, University of Porto, Portugal.
Santos, A., 2000. Intertidal Ecology of Northern Portuguese Rocky Shores. University of Southampton, UK: 166.
Southward, A. J., 1991. Forty years of changes in species composition and population density of barnacles on a rocky shore near Plymouth. Journal of the Marine Biological Association of the United Kingdom 71: 495–513.
Southward, A. J., O. Langmead, N. J. Hardman-Mountford, J. Aiken, G. T. Boalch, P. R. Dando, J. Genner, I. Joint, M. A. Kendall, N. C. Halliday, R. P. Harris, R. Leaper, N. Mieszkowska, R. D. Pingree, A. J. Richardson, D. W. Sims, T. Smith, A. W. Walne & S. J. Hawkins, 2005. A review of long-term research in the western English Channel. In Southward, A. J., P. A. Tyler, C. M. Young & L. A. Fuiman (eds), Advances in Marine Biology, Vol. 47. Elsevier Academic Press, San Diego: 3–84.
Torres, P., A. C. Costa & M. A. Dionísio, 2012. New alien barnacles in the Azores and some remarks on the invasive potential of Balanidae. Helgoland Marine Research 66: 513–522.
Underwood, A. J. & P. G. Fairweather, 1989. Supply-side ecology and benthic marine assemblages. Trends in Ecology & Evolution 4(1): 16–20.
Ward, B. B., A. P. Rees, P. J. Somerfield & I. Joint, 2011. Linking phytoplankton community composition to seasonal changes in f-ratio. Multidisciplinary Journal of Microbial Ecology 5(11): 1759–1770.
Wooster, W. S., A. Bakun, & D. R. McLain, 1976. The seasonal upwelling cycle along the eastern boundary of the North Atlantic. Journal of Marine Research 34: 131–141.
Acknowledgements
This research was partially supported by the Structured Program of R&D&I INNOVMAR – Innovation and Sustainability in the Management and Exploitation of Marine Resources (reference NORTE-01-0145-FEDER-000035, Research Line NOVELMAR), which is funded by the Northern Regional Operational Programme (NORTE2020) through the European Regional Development Fund (ERDF) as well as by the Strategic Funding UID/Multi/04423/2013 through national funds provided by FCT – Foundation for Science and Technology and European Regional Development Fund (ERDF), in the framework of the programme PT2020, and also by the Portuguese Foundation for Science and Technology (FCT) through two postdoctoral scholarships to IC (SFRH/BPD/110020/2015) and JRA (SFRH/BPD/87416/2012). We acknowledge the Instituto Hidrográfico of the Portuguese Navy for providing the seawater temperature data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jörg Dutz
Rights and permissions
About this article
Cite this article
Cunha, I., Azevedo, T., Vasconcelos, V. et al. Distribution ranges of the acorn barnacle Perforatus (=Balanus) perforatus (Bruguière, 1789) in the NE Atlantic are influenced by reproductive parameters. Hydrobiologia 806, 227–235 (2018). https://doi.org/10.1007/s10750-017-3362-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3362-5