Abstract
The processes that determine the coexistence of phylogenetically close species are very complex, particularly when species introduction leads to sympatry among species that did not co-evolved. We evaluated possible differences in δ13C and δ15N signatures between two piranha species (Serrasalmus marginatus and S. maculatus), in a system where S. marginatus invaded 30 years ago (floodplain ponds in the Upper Paraná River). We predicted that carbon and nitrogen stable isotope values would not differ between piranha species. Additionally, we evaluated the abundance (CPUE) of both piranha populations along the years (1986–2015). Native and non-native Serrasalmus species have different δ13C signatures, likely exploiting different energy pathways on the food web. Overall, native and non-native piranhas have similar δ15N values and occupy the third trophic level in the food web. Regarding the two piranha population fluctuations, there was an inversion of dominance after the non-native species establishment, where S. marginatus became dominant over S. maculatus (after 1988). Our results showed that trophic niche dimension (revealed by trophic segregation) is not the reason of the observed inversion in the dominant species, and this could be a primary factor driving the persistence of the native species in the ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In ecology, there is a long debate regarding the processes that determine the coexistence of species (Juncos et al., 2015), especially in cases where these species are phylogenetically close (Ricciardi & Mottiar, 2006; Li et al., 2015), and have similar feeding habits (Keppeler et al., 2014). Cases of coexistence need to be extensively studied, particularly when species introduction leads to sympatry among species that did not co-evolve, resulting in coexistence or exclusion (Gause, 1934; Bøhn et al., 2008). In cases in which coexistence is achieved without species exclusion, the introduced species almost always become dominant, changing the community structure and leading to trophic network instability occurred due to competition with native species, and overexploitation of native trophic resources (Latini & Petrere, 2004; Li et al., 2015; Sagouis et al., 2015).
Introduced species are a major threat to the abundance of native species and have been the focus of discussions about biodiversity and functional loss (e.g., Dudgeon et al., 2006; Pelicice et al., 2014; Daga et al., 2015). Human activities have contributed to the introduction of non-native species in freshwater ecosystems, such as the construction of hydroelectric power plants which can act as facilitators of species introduction (Júlio et al., 2009; Vitule et al., 2012). In Brazil, the construction of the Itaipu hydroelectric plant flooded natural geographic barriers (a set of waterfalls known as Sete Quedas Falls), linking two previously isolated eco-regions of the Paraná River (the Lower and Upper Paraná River; Abell et al., 2008; Vitule et al., 2012). Posteriorly to this event, many fish species previously endemic to the Lower Paraná River, including the piranha Serrasalmus marginatus Valenciennes, 1837 (Júlio et al., 2009), colonized the Upper Paraná River floodplain and some of its tributaries (Agostinho & Júlio, 2002; see Fig. 1). Some years after the construction of Itaipu, S. marginatus became the dominant species (in terms of abundance) over its congeneric Serrasalmus maculatus Kner, 1858, which is native from the Upper Paraná River floodplain (Agostinho et al., 1994; Agostinho & Júlio, 2002; Agostinho et al., 2003).
Illustrative model of the invasion of Serrasalmus marginatus from the Lower Paraná River to the Upper Paraná River after the construction of Itaipu dam in 1982 and consequent removal of a natural geographic barrier (Sete Quedas Falls). The fish pictures were modified from Fishbase (http://www.fishbase.org/search.php). (160 km) = distance between Itaipu dam and Sete Quedas Falls (data obtained from Google Earth Pro). The elements of the figure are not proportionate
The pattern of food resource use by S. marginatus and S. maculatus at the Upper Paraná River region was previously assessed by Agostinho et al. (2003). Their stomach content analysis revealed a high trophic niche overlap, attributed to the consumption of fish fragments. The analysis of stomach contents has been an effective tool to infer about feeding strategies and habits of fish species. However, for piranhas, this may not be the most appropriate analysis. Piranhas’ trophic behavior consists in biting other fish, taking small fragments of muscle or fins, which is almost impossible to identify through stomach content analysis (Almeida et al., 1998; Agostinho et al., 2003). Also, fish fragments may have been obtained from different fish species, which could occupy different trophic positions. Thus, the use of stable isotopes ratio (especially 12C/13C and 14N/15N) could provide a more robust analysis to infer about the trophic habits of native and non-native species of piranhas.
Stable isotope analysis (SIA) is based on the relationship between the isotopic composition of a consumer and its direct or indirect energy resources, allowing the investigation of the energy flux in food webs (Fry, 2006). Compared to other methodologies (i.e., stomach content analysis, direct observation, etc.), SIA has an efficient cost-benefit relationship, because this analysis integrates time in it and represent what is being assimilated in the consumer’s tissue (Fry, 2006). Metabolically more active tissues (with faster isotopic turnover) will reflect a recent diet, while tissues such as muscle (i.e., metabolically less active and with slower isotopic turnover) will reflect long-term measurement of isotopic incorporation, allowing to depict relatively long-term diets (Hobson & Clark, 1992; Martínez del Rio et al., 2009; Buchheister & Latour, 2010). Carbon stable isotope (δ13C) is typically used to trace the assimilated energy sources by a consumer, because it changes little (usually <1‰) through trophic levels, which allows the use of primary producers as baseline; the nitrogen stable isotope (δ15N) may be used to estimate the trophic position of a consumer, because it is enriched in about 3.4‰ per trophic level (Peterson & Fry, 1987; Vander-Zanden & Rasmussen, 1999; Post, 2002). Therefore, SIA is useful for understanding the functional role of organisms in food chains (Correa & Winemiller, 2014; Polačik et al., 2014) and is an important tool for elucidating trophic differences between phylogenetically related species (Faye et al., 2011; Le Loc’h et al., 2015). Thus, results provided by SIA can be used to define trophic niches (Layman et al., 2007), and have become an important predictor of the impacts of non-native species (Olsson et al., 2009; Monroy et al., 2014; Córdova-Tapia et al., 2015; Hill et al., 2015).
Serrasalmus species stably co-occur in other environments (as in Pantanal floodplain), such co-occurrence may be possible due to differences in trophic requirements of piranha species, which allows a long-term coexistence (Juncos et al., 2015). Here, we used simultaneous δ13C and δ15N values to evaluate the possible energy sources and the trophic position of the two species, and to understand what underlies the coexistence of two piranha species (the native Serrasalmus maculatus and the non-native S. marginatus) in the Upper Paraná River floodplain. We predicted that stable carbon and nitrogen isotope values from piranha species would not differ in the Upper Paraná River floodplain. Additionally, we evaluated the abundance (i.e., CPUE) of both piranha populations along the years in order to observe both population fluctuations and possible relations of dominance between piranha species, after the removal of a natural geographic barrier.
Methods
Study area
The isotopic composition (δ13C and δ15N) from S. maculatus and S. marginatus was evaluated in three floodplain ponds associated with the Upper Paraná River (Fig. 2). This floodplain is formed by a mosaic of aquatic habitats, including channels, tributaries, and floodplain ponds associated with these tributaries. The floodplain ponds are located on the right bank of the Upper Paraná River floodplain. Specifically, Patos pond and Ventura pond are associated with the Ivinhema River, and Fechada Pond is associated with the Baía River. All three floodplain ponds have similar dynamics and are classified as permanent ponds, with intermittent communication with the rivers or secondary channels during flood periods (Thomaz et al., 2004a). The flood regime of these floodplain ponds is characterized by low water phases from May to September, and high water from November to March (Thomaz et al., 2004b). The Upper Paraná River floodplain is the last stretch of the Paraná River that is free of dams (occupying an area of approximately 230 km long). The construction of the Itaipu dam submerged a set of waterfalls that acted as natural geographic barriers, allowing the invasion of a large number of fish species from the Lower to the Upper Paraná River (Agostinho & Júlio, 2002; Júlio et al., 2009; Agostinho et al., 2015).
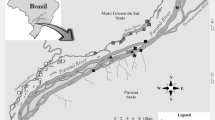
Map of the area of study, including the Upper Paraná River floodplain, and Itaipu dam and its reservoir. Points (black dots) in the highlighted box indicates the floodplain ponds in which the samples were taken (i.e., Fechada pond, Patos pond, and Ventura pond), where the non-native Serrasalmus marginatus have invaded. («) indicates the direction of river flow
Sampling design
Gill nets with different mesh sizes (ranging from 2.4 to 16 cm between opposite knots) were set in three floodplain ponds associated with the Upper Paraná River. The nets were exposed for a 24-h period and checked with an interval of 8 h (i.e.. at 8:00 AM, 4:00 PM, and 10:00 PM). In all three floodplain ponds, both fish species were captured for stable isotopes analysis. For abundance analysis, we used data from the same three floodplain ponds where the fish were found, using the same fishing design previously described. We selected only adult fish to control for ontogenetic variability in the isotopic signals. Adult fish were identified based on the standard length (SL) at first maturation (i.e., >9.0 cm for S. maculatus, and >9.2 cm for S. marginatus; Vazzoler et al., 1997).
Samples were taken during the low water phase (September 2011) because, during this period, fish are confined within the floodplain ponds, minimizing the chances of migration to the main channel and maximizing the probability of trophic interactions between individuals, as well as with their prey. Therefore, the individuals of each species have the same chances of obtaining the same amount and type of food resources. We highlight that the flood that preceded our study occurred in March 2011, which is six months before our sampling. This is enough time to promote the tissue renovation (i.e., tissue turnover; Sacramento et al., 2016) that allows stable isotopes signals to reflect the low water phase when the ponds have zero/low connection with the main channel (Thomaz et al., 2004a).
All sampled fish were immediately packed in ice coolers and subsequently frozen. The species were identified according to Graça & Pavanelli (2007). In total, we captured 36 specimens of both piranha species: seven in Fechada pond, 16 in Patos pond, and 13 in Ventura pond (see Table 1). From each specimen, we extracted a muscle sample from the base of the dorsal fin, which was used to determine the ratio of carbon and nitrogen stable isotopes. Voucher specimens were deposited in the Ichthyological Collection of Nupélia at the State University of Maringá, Brazil.
We sampled potential primary energy sources in each floodplain pond. Two to six replicates samples of each primary energy source were collected from each floodplain pond, where available. The primary energy sources consisted of phytoplankton, periphyton, particulate organic carbon (POC), predominant C3 aquatic macrophytes, and leaves from the predominant C3 trees surrounding the ponds (riparian vegetation). C4 plants were absent at the sampling locations; however, as the C4 signatures values do not have too much δ13C variability in freshwater ecosystems (usually with δ13C values between −13 and −12‰; Martinelli et al., 1991; Forsberg et al., 1993), we used values obtained by Hoeinghaus et al. (2007) in the same floodplain. Riparian vegetation and aquatic macrophytes consisted of multiple leaves of the most common and abundant vascular plants in each sample site, clipped directly from the plant, separated by species, dried, and grounded. Periphyton was obtained by gently scraping the stem of aquatic plants. Phytoplankton was sampled in the littoral and limnetic zones of each sample site using 15 µm plankton net horizontally dragged twice in each zone on the subsurface water, constituting 4 samples per lake. Each sample of phytoplankton was stored in a 500 ml bottle for further filtering. POC was obtained by filtering water collected directly from the subsurface of littoral and limnetic zones in each lake, in a 500 ml bottle. The periphyton and all the volume of water samples with phytoplankton and POC were filtered and retained on pre-combusted (400 °C for 4 h) 47 mm glass fiber filters (Whatman GFC).
All samples were properly identified and dried in an oven at 60 °C for 72 h. The fish muscles and leaves from the aquatic macrophytes and riparian vegetation were ground to obtain a fine, homogeneous powder. Approximately 1.5 mg (fish) and 4 mg (plant leaves) of each sample were stored in tin capsules for subsequent stable isotope analysis. Filters containing phytoplankton, periphyton, and POC were cut in half and stored in tin capsules. Samples were sent to the University of California at Davis Stable Isotope Facility Center for determination of carbon and nitrogen isotope ratios. The determination of isotope ratios was performed on an Isotope Ratio Mass Spectrometer from PDZ Europe ANCA-GSL with a PDC Europe 20-20 interface (Sercon Ltd., Cheshire, UK). The results were expressed in delta notation (parts per thousand deviation from a standard material): δ13C or δ15N = [(R sample/R standard) − 1] × 1000, where R = 13C/12C or 15N/14N. The standard material for carbon was the international Vienna Pee Dee Belemnite (V-PDB) limestone, and the nitrogen standard was atmospheric nitrogen. Standard deviations of δ13C and δ15N for five different replicate analyses of internal standards were between 0.04 and 0.13‰ and 0.09 and 0.22‰, respectively.
To characterize each floodplain pond, one measurement of temperature, dissolved oxygen concentration, turbidity, conductivity, and pH was taken using handheld probes (see Electronic Supplemental Material 1). Transparency was measured with Secchi disk. Water samples were collected at subsurface limnetic region and packaged in polyethylene gallon for laboratory analysis of phosphorous (total phosphorous and PO4 3−) and nitrogen (total nitrogen, NO3 − and NH4 + concentrations, with the methodology described in Roberto et al. (2009). Isotopic values variability of primary energy sources is related to local limnological conditions (as observed in a previous study in several floodplains; Alves et al., in press) and may influence the consumers’ stable isotope signals, biasing the results. Hence, we decided to interpret the stable carbon and nitrogen isotopes data separately for each pond, in order to account for isotopic variability among different floodplain habitats.
Data analysis
An exploratory δ15N and δ13C bi-plot graph was constructed to visualize the possible primary energy sources for both piranha species in each floodplain pond. Considering that δ13C fractionates from 0.4 to 1‰ between the diet of a consumer and its energy source (usually with consumers having tissues enriched in up to 1‰ of δ13C in relation to their food; Post, 2002; McCutchan et al., 2003), the relative importance of an assimilated primary energy source is indicated by the relative positions of the consumer and its potential primary energy sources in the x-axis of the bi-plot. For the interpretation of the bi-plots, we assumed 0.4‰ of trophic fractionation for the carbon stable isotope per trophic level (Post, 2002; Reid et al., 2008). Therefore, we used 0.8‰ between the primary energy source and our fish species (secondary consumer; see ‘Results’ for trophic level). Different from carbon, nitrogen stable isotopes fractionate 2–3.4‰ between consumers and their food (Post, 2002; McCutchan et al., 2003). In addition, the δ15N may be used as an indicator of trophic position in the so-called delta space when combined with the estimative of primary energy source contribution to consumer (given by δ13C). We decided not to run the SIAR mixing models (Parnell & Jackson, 2013) because we had only collected primary energy sources and not potential preys (i.e., small bodied omnivores or invertivores fish) of piranhas. Also, some of our primary energy sources had a low number of samples (e.g., we were able to collect only 2 samples of periphyton in each pond), which would be insufficient to run SIAR models. Thus, our graphical analysis provides a general assessment of primary energy source contributions to piranhas in each sampled pond. Trophic position (TP) was estimated using the δ15N values of consumers and primary energy sources, for each fish in each sampled pond, given by the expression proposed by Vander-Zanden et al. (1997): TP = [(δ15Nconsumer − δ15Nsource)/3.4) + 1], where Nconsumer is the mean δ15N value of each fish, δ15Nsource is the mean δ15N value of all sources sampled in each pond, and 3.4‰ is the mean trophic fractionation (Post, 2002). We added +1 to represent one trophic level above the producers because our baseline is in the first trophic level (i.e., primary energy sources).
We argue that the number of samples for interpretation was reduced for each site. However, the influence of low abundance of individuals on results involving stable isotopes is related to the kind of statistical analysis that is going to be performed. In this regard, we used the non-parametric Mann–Whitney U test separately for δ15N and δ13C to test possible differences in stable isotope values (δ15N and δ13C) between the two piranha species in each floodplain pond. Differences in δ15N values give us an insight of consuming preys in different trophic levels, while differences in δ13C values allow us to infer that both piranha species are consuming different energy resources (i.e., from different preys). Therefore, differences in stable isotope values (δ15N and δ13C) may be used as a surrogate of trophic segregation. The Mann–Whitney U test was performed in the SSPS Statistics® software. The significance level was set at α = 0.05.
Aiming to evaluate the relationship between the abundance of both species in the Upper Paraná River floodplain, we calculated the Catch per Unit Effort (CPUE; individuals/1000 m2 gillnet/24 h) in a non-continuous time series from 1986 to 2015 for Patos, Fechada, and Ventura floodplain ponds. Data from 1989 to 1991 and from 1995 to 1999 were absent because of missing samples. For each year, it was calculated the average CPUE from the values of CPUE obtained in the quarterly samples in each year and in each pond.
Results
The SL of S. maculatus ranged from 16.0 to 23.0 cm, while SL of S. marginatus was between 9.5 and 17.0 cm. The primary energy sources showed high variability in δ13C and δ15N values across ponds in Upper Paraná River floodplain (Fig. 3). The above-mentioned variability also reflected in piranha’s tissue, and native and non-native piranhas rely on different primary energy sources in all sampled ponds (P < 0.05; Table 1). For example, in Ventura pond, the mean δ13C values for consumers were −28.62 ± 0.19‰ for S. maculatus and −30.63 ± 0.40‰ for S. marginatus. Considering the trophic fractionation, the most probable primary energy source in Ventura pond for S. maculatus was a mixture of the autochthonous primary energy sources (i.e., C3 aquatic macrophytes, periphyton, and POC) with values ranging from −29.06‰ (C3 aquatic macrophytes) to −26.56‰ (POC) and for S. marginatus was riparian vegetation (ranging from −31.32 to −29.53‰; Fig. 3). In contrast, in Patos pond, the δ13C values of S. maculatus (ranging from −29.16 to −27.50‰) seems to be related to the assimilation of C3 aquatic macrophytes (average δ13C = −28.79 ± 0.46‰), while S. marginatus δ13C values (ranging from −31.05 to 28.15‰) seems to be related to a mixture of riparian vegetation (average δ13C = −29.15 ± 0.73‰), C3 aquatic macrophytes, and an unknown primary energy source (Fig. 3).
Bi-plot of δ13C and δ15N of primary energy sources (open squares; mean ± SD), Serrasalmus maculatus (gray triangles) and S. marginatus (black circles) in three ponds where one piranha species (S. marginatus) have recently invaded (Upper Paraná River floodplain): Pato pond, Ventura pond, and Fechada pond. Phyto Phytoplankton, Peri Periphyton, POC Particulate organic carbon, Rip. Veg Riparian vegetation. The black arrows are used to indicate the primary energy sources in the delta space. C4 plants data were obtained from Hoeinghaus et al. (2007)
We only found significant different values of δ15N between the two species of piranhas from Patos pond (P = 0.01; Table 1). However, in general, both piranha species were positioned around the third trophic level (i.e.. secondary consumers) in all environments, with slight variation across the floodplain ponds (Table 1).
Regarding the abundance of both piranha species over time in the Upper Paraná River floodplain ponds, there was a clear dominance of the native S. maculatus over its congeneric, the non-native S. marginatus, in the first sampling (three years after the geographic barrier removal; Fig. 4). In the following years (i.e., 1987/88), we observed an abrupt population decline of the native piranha species. Subsequently, the native population stabled (with low abundance), while the population of the non-native piranha increased and became always dominant in the following years (Fig. 4).
Mean abundance (CPUE) and standard error (SE) of the non-native Serrasalmus marginatus (black triangles and solid line) and native S. maculatus (gray circles and dashed line) piranha species over a non-continuous time series in three ponds grouped (Fechada, Ventura, and Patos floodplain ponds) of the Upper Paraná River floodplain, after the removal of a geographic barrier
Discussion
We found that native and non-native Serrasalmus species have different δ13C signatures in the newly invaded ponds, allowing us to conclude (allied to the relative position of consumers/primary energy sources in the isotopic space) that their carbon signatures are from distinct preys that incorporated different primary energy sources. Such findings go against the high trophic niche overlap between native and non-native piranha species found by Agostinho et al. (2003) in the same environment, analyzing the stomach content of both piranha species (in two periods: 1986–1988 and 1992–1994), which guided us to propose the initial predictions. Moreover, it contrasts with our predictions that the species would use the same primary energy resources and that δ13C and δ15N would not differ between species, leading us to reject them. However, any interpretation should be provided with caution because we performed just one sampling in each pond and have a limited number of individuals in each environment, thus our results (even using a ‘time integrator’ analysis like stable isotope analysis) could represent a “snapshot” of the entire complex dynamic between piranha species. Also, we used stable isotopes data from thirty years after the invasion had happened in the Upper Paraná River floodplain, and any character displacement could naturally have been selected during such stable coexistence time, as a result from a competitor coevolution (Chesson, 2000). Nevertheless, the robustness of the data could be confirmed by observing a similar pattern (trophic segregation) in the delta space comparing the isotopes values of S. maculatus and S. marginatus in three areas with completely different environmental conditions (see Online Resource 1).
The hypothesis that sympatric populations of competing species have reduced niche overlap compared with allopatric ones (Dayan & Simberloff, 2005) assumes that long-term coexistence is just possible when species differ in their trophic requirements (one niche dimension; Gause, 1934; Chesson, 2000). Although the piranha species co-occur in the Upper Paraná River floodplain for a short period of time (i.e.. approximately 30 years) compared to evolutionary time, they have different stable isotopes signatures, which are a strong evidence of trophic segregation. However, we cannot be sure when this segregation started, because we do not have stable isotopes data of fish and their food resources from periods following the geographic barrier removal. Non-native species, such as S. marginatus, usually have high trophic plasticity and the capacity of narrow their trophic requirements reducing the trophic niche overlap (e.g., Guzzo et al., 2013; Ferreira et al., 2014; Eloranta et al., 2015; Juncos et al., 2015), which could result in trophic segregation between species of piranhas.
Most studies using an isotopic approach have demonstrated high trophic overlap between native and non-native species in several environments (Mercado-Silva et al., 2009; Olsson et al., 2009; Ruokonen et al., 2012; Monroy et al., 2014; Córdova-Tapia et al., 2015), including in the Upper Paraná River floodplain (Philippsen et al., 2015). Nevertheless, our data show the opposite: assimilation of different primary energy resources in the newly invaded environment between congeneric species. We believe that the successful establishment of the non-native S. marginatus in the Upper Paraná River floodplain could be due to the higher aggressiveness of non-native piranha than native one (Sazima & Machado, 1990; Agostinho et al., 2003). Also, the non-native piranha has a more pronounced ontogenetic feeding shift than its congeneric. According to Agostinho et al. (2003), small native piranha (i.e.. individuals with the length between 4 and 8 cm) already have ‘fish muscle’ as the main food item, which overlaps with the feeding habits of adult non-native piranhas (i.e.. individuals with the length between 14 and 24 cm), giving them competitive strength.
The primary energy sources that support each piranha population varies pond by pond and seems to be determined by the inherent limnological characteristics of each pond. As a consequence (and considering the sedentary habit of piranhas), consumers also vary their δ13C values, suggesting that the fish living in a determined pond depend on the resources therein produced. For example, in Ventura pond, S. maculatus seems to be supported by a mixture of the autochthonous primary energy sources, while in Patos pond, the δ13C values of the same species seem to be related to C3 aquatic macrophytes. Despite such variability in the assimilated primary energy source by S. maculatus, its isotope values never overlapped with its sympatric congeneric piranha. Thus, the pattern of trophic segregation was maintained in all studied floodplain ponds. Additionally, sedentary fish reflecting the limnological conditions of their habitats in their muscle tissue (i.e.. δ13C values) is a welcome result, because this among-pond difference in carbon stable isotope enables to create a regional fine-scale isotopic landscape (i.e., isoscape; Bowen, 2010). Therefore, for further investigations, it would be possible to use this fine-scale isoscape to track organisms (Hobson et al., 2010) that migrate small distances (i.e.. among ponds) as well as track and monitor new invaders along the floodplain.
Sete Quedas falls acted as an important natural barrier separating two different ichthyofaunas (Bonetto, 1986; Vitule et al., 2012). The flooding of these falls put these two fish community together, forcing them to interact. One expected issue for the establishment of the new species in the Upper Paraná River was that the environmental filters would act negatively upon the non-native species populations (Ernandes-Silva et al., 2016). However, for the invasive piranha, the new environment did not seem to be an obstacle as an environmental filter (i.e.. invasibility of the new environment). As suggested by ‘Pre-adaptation hypothesis,’ the invasion could be more likely to succeed where a close phylogenetic-related species already dwell the environment because close relatives share similar traits (Ricciardi & Mottiar, 2006; Li et al., 2015).
The non-native piranha species appears to demonstrate a competitive advantage over the native one. Evidence to this advantage is suggested by the inversion of dominance observed after the non-native species establishment, in which S. marginatus became dominant over S. maculatus, a pattern also observed in the relation between Nile tilapia over the native pearl cichlid population in Brazilian river systems (Linde et al., 2008; Sanches et al., 2012). Agostinho & Júlio (2002) were the pioneers in observing this shift in abundance of the piranha species from 1986 to 1994. Such authors suggested that S. maculatus should behave as a fugitive competitor until patterns in reproduction and resources use began to differ, in order to stably coexist with its congeneric. Our data suggest that the trophic niche dimension seems not to be the reason of the observed inversion in the dominant species. Anywise, despite the population of S. maculatus had been extensively depleted over the past 30 years, after the introduction of S. marginatus (Agostinho & Júlio, 2002), it is possible to observe a stabilization tendency in abundance between both piranha populations. Although depleted, S. maculatus population was not excluded, as postulated by Competitive Exclusion principle (Gause, 1934). In fact, the native piranha managed to survive, and one of the plausible explanations for that is the trophic niche segregation, observed herein.
With stable isotope analysis, it was possible to observe that trophic segregation was evident among congeneric species and that competition for feeding resources does not seem to be a limiting factor to the coexistence of the two studied species. The establishment of S. marginatus was probably driven by access to those resources not used by the native species, by other niche dimensions not evaluated in this study (e.g., reproductive aspects, competition among juveniles, etc.), or by other biotic interactions. Thus, for a complete description of the coexistence of these species, and that of various other native species influenced by competitive invaders, it is necessary to conduct additional studies that will integrate other niche dimensions and spatiotemporal analysis on the dynamic availability of trophic resources. In addition, the population decline of native piranha (almost leading it to local extinction) is further evidence of the ecological problem provoked by the construction of reservoirs, which may promote the removal of natural geographic barriers and connect different regions with distinct biota.
References
Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. C. Balderas, W. Bussing, M. L. J. Stiassny, P. Skelton, G. R. Allen, P. Unmack, A. Naseka, R. Ng, N. Sindorf, J. Robertson, E. Armijo, J. V. Higgins, T. J. Heibel, E. Wikramanayake, D. Olson, H. L. López, R. E. Reis, J. G. Lundberg, M. H. S. Pérez & P. Petry, 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58: 403–414.
Agostinho, C. S. & H. F. Júlio Jr., 2002. Observation of an invasion of the piranha Serrasalmus marginatus, Serrasalmidae) into the Upper Paraná River. Brazil. Acta Scientiarium. Biological Sciences 24: 391–395.
Agostinho, C. S., N. S. Hahn & E. E. Marques, 2003. Patterns of food resource use by tow congeneric species of piranhas (Serrasalmus) on the Upper Paraná River floodplain. Brazilian Journal of Biology 63: 177–182.
Agostinho, A. A., H. F. Júlio Jr. & M. Petrere-Junior, 1994. Itaipu reservoir: impacts of the impoundment on fish fauna and fisheries. In Cowx, I. G. (ed.), Rehabilitation of Freshwaters Fisheries. Blackwell Scientific Publications, Oxford: 171–184.
Agostinho, A. A., H. I. Suzuki, R. Fugi, D. C. Alves, L. H. Tonella & L. A. Espindola, 2015. Ecological and life history traits of Hemiodus orthonops in the invasion process: looking for clues at home. Hydrobiologia 746: 415–430.
Almeida, V. L. L., N. S. Hahn & C. S. Agostinho, 1998. Stomach content of juvenile and adult of piranhas (Serrasalmus marginatus) in the Paraná floodplains, Brazil. Studies in Neotropical Fauna and Environment 33: 100–105.
Alves, G. H. Z., D. J. Hoeinghaus, G. I. Manetta & E. Benedito, in press. Dry season limnological conditions and basin geology exhibit complex relationships with δ13C and δ15N of carbon sources in four Neotropical floodplains. PLoS ONE.
Bøhn, T., P. A. Amundsen & A. Sparrow, 2008. Competitive exclusion after invasion? Biological Invasions 10: 359–368.
Bonetto, A. A., 1986. The Paraná river system. In Davies, B. R. & K. F. Walker (eds), The ecology of river systems. Junk Publishers, Dordrecht, Dr. W: 541–555.
Bowen, G. J., 2010. Isoscapes: spatial pattern in isotopic biogeochemistry. Annual Review of Earth and Planetary Sciences 38: 161–187.
Buchheister, A. & R. Latour, 2010. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Canadian Journal of Fisheries and Aquatic Sciences 67: 445–461.
Chesson, P., 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology, Evolution, and Systematics 31: 343–366.
Córdova-Tapia, F., M. Contreras & L. Zambrano, 2015. Trophic niche overlap between native and non-native fishes. Hydrobiologia 746: 291–301.
Correa, S. C. & K. O. Winemiller, 2014. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 95: 210–224.
Daga, V. S., F. Skóra, A. A. Padial, V. Abilhoa, E. A. Gubiani & J. R. S. Vitule, 2015. Homogenization dynamics of the fish assemblages in Neotropical reservoirs: comparing the roles of introduced species and their vectors. Hydrobiologia 746: 327–347.
Dayan, T. & D. Simberloff, 2005. Ecological and community-wide character displacement the next generation. Ecology Letters 8: 875–894.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews Cambridge Philosophical Society 81: 163–182.
Eloranta, A. P., P. Nieminen & K. K. Kahilainen, 2015. Trophic interactions between introduced lake trout (Salvelinus namaycush) and native Arctic charr (S. alpinus) in a large Fennoscandian subarctic lake. Ecology of Freshwater Fish 24: 181–192.
Ernandes-Silva, J., F. H. Ragonha, L. C. Rodrigues & R. P. Mormul, 2016. Freshwater invasibility level depends on the population age structure of the invading mussel species. Biological Invasions 18: 1421–1430.
Faye, D., L. T. Morais, J. Raffray, O. Sadio, O. T. Thiaw & F. Le Loc’h, 2011. Structure and seasonal variability of fish food webs in an estuarine tropical marine protected area (Senegal): evidence from stable isotope analysis. Estuarine, Coastal and Shelf Science 92: 607–617.
Ferreira, F. S., W. Vicentin, F. E. S. Costa & Y. R. Súarez, 2014. Trophic ecology of two piranha species, Pygocentrus nattereri and Serrasalmus marginatus (Characiformes, Characidae), in the floodplain of the Negro River, Pantanal. Acta Limnologica Brasiliensia 26: 381–391.
Forsberg, B. R., C. A. R. M. Araujo-Lima, L. A. Martinelli, R. L. Victoria & J. A. Bonassi, 1993. Autotrophic carbon sources for fish of the central Amazon. Ecology 74: 643–652.
Fry, B., 2006. Stable Isotope Ecology. Springer, New York.
Gause, G. F., 1934. The Struggle for Existence. Williams & Wilkins, Baltimore.
Graça, W. J. & C. S. Pavanelli, 2007. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Eduem, Maringá.
Guzzo, M. M., G. D. Haffner, N. D. Legler, S. A. Rush & A. T. Fisk, 2013. Fifty years later: trophic ecology and niche overlap of a native and non-indigenous fish species in the western basin of Lake Erie. Biological Invasions 15: 1695–1711.
Hill, J. M., R. W. Jones, M. P. Hill & O. L. F. Weyl, 2015. Comparisons of isotopic niche widths of some invasive and indigenous fauna in a South African river. Freshwater Biology 60: 893–902.
Hobson, K. A. & R. G. Clark, 1992. Assessing avian diets using stable isotopes I: turnover of 13C in tissues. The Condor 94: 181–188.
Hobson, K. A., R. Barnett-Johnson & T. Cerling, 2010. Using isoscapes to track animal migration. In West, J. B., G. J. Bowen, T. E. Dawson & K. P. Tu (eds), Isoscapes. Springer, Dordrecht: 273–298.
Hoeinghaus, D. J., K. O. Winemiller & A. A. Agostinho, 2007. Landscape-scale hydrologic characteristics differentiate patterns of carbon flow in large-river food webs. Ecosystems 10: 1019–1033.
Júlio Jr., H. F., C. D. Tós, A. A. Agostinho & C. S. Pavanelli, 2009. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotropical Ichthyology 7: 709–718.
Juncos, R., D. Milano, P. J. Macchi & P. H. Vigliano, 2015. Niche segregation facilitates coexistence between native and introduced fishes in a deep Patagonian lake. Hydrobiologia 747: 53–67.
Keppeler, F. W., L. E. K. Lanés, A. S. Rolon, C. Stenert, P. Lehmann, M. Reichard & L. Maltchik, 2014. The morphology-diet relationship and its role in the coexistence of two species of annual fishes. Ecology of Freshwater Fish 24: 77–90.
Latini, A. O. & M. Petrere Jr., 2004. Reduction of a native fish fauna by alien species: an example from Brazilian freshwater tropical lakes. Fisheries Management and Ecology 11: 71–79.
Layman, C. G., D. A. Arrington, C. G. Montaña & D. M. Post, 2007. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88: 42–48.
Le Loc’h, F., J. Durand, K. Diop & J. Panfili, 2015. Spatio-temporal isotopic signatures (δ13C and δ15N) reveal that two sympatric West African mullet species do not feed. Journal of Fish Biology 86: 1444–1453.
Li, S. P., M. W. Cadotte, S. J. Meiners, Z. S. Hua, H. Y. Shu, J. T. Li & W. S. Shu, 2015. The effects of phylogenetic relatedness on invasion success and impact: deconstructing Darwin’s naturalisation conundrum. Ecology Letters 18: 1285–1292.
Linde, A. R., J. I. Izquierdo, J. Costa-Moreira & E. Garcia-Vazquez, 2008. Invasive tilapia juveniles are associated with degraded river habitats. Aquatic Conservation 18: 891–895.
Martinelli, L. A., A. H. Devol, R. L. Victoria & J. E. Richey, 1991. Stable carbon isotope variation in C3 and C4 plants along the Amazon River. Nature 353: 57–59.
Martínez del Rio, C., N. Wolf, S. A. Carleton & L. Z. Gannes, 2009. Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews 84: 91–111.
McCutchan Jr., H. J., W. M. Lewis Jr., C. Kendall & C. C. McGrath, 2003. Vatiation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulphur. Oikos 102: 378–390.
Mercado-Silva, N., M. R. Helmus & M. J. Vander-Zanden, 2009. The effects of impoundment and non-native species on a river food web in Mexico’s Central plateau. River Research and Application 25: 1090–1108.
Monroy, M., A. Maceda-Veiga, N. Caiola & A. De Sostoa, 2014. Trophic interactions between native and introduced fish species in a littoral fish community. Journal of Fish Biology 85: 1693–1706.
Olsson, K., P. Stenroth, P. Nystrom & W. Graneli, 2009. Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology 54: 1731–1740.
Parnell. A. C. & A. Jackson, 2013. Siar: Stable Isotope Analysis in R. R package version 4.2. http://CRAN.R-project.org/package=siar.
Pelicice, F. M., J. R. S. Vitule, D. P. Lima-Junior, M. L. Orsi & A. A. Agostinho, 2014. A serious new threat to Brazilian freshwater ecosystems: the naturalization of nonnative fish by decree. Conservation Letters 7: 55–60.
Peterson, B. J. & B. Fry, 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology Evolution and Systematics 18: 293–320.
Philippsen, J. S., M. Hauser & E. Benedito, 2015. Isotopic niches of sympatric native and exotic fish species in a Neotropical floodplain. Anais da Academia Brasileira de Ciências 87: 825–833.
Polačik, M., C. Harrod, R. Blažek & M. Reichard, 2014. Trophic niche partitioning in communities of African annual fish: evidence from stable isotopes. Hydrobiologia 721: 99–106.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
R Core Team, 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
Reid, D. J., G. P. Quinn, P. S. Lake & P. Reich, 2008. Terrestrial detritus supports the food webs in lowland intermittent streams of south-eastern Australia: a stable isotope study. Freshwater Biology 53: 2036–2050.
Ricciardi, A. & M. Mottiar, 2006. Does Darwin’s naturalization hypothesis explain fish invasions? Biological Invasions 8: 1403–1407.
Roberto, M. C., M. F. Santana & S. M. Thomaz, 2009. Limnology in the Upper Paraná River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Brasilian Journal of Biology 69: 717–725.
Ruokonen, T. J., J. Karjalainen, M. Kiljunen, M. Pursiainen & H. Hämäläinene, 2012. Do introduced crayfish affect benthic fish in stony littoral habitats of large boreal lakes. Biological Invasions 14: 813–825.
Sacramento, P. A., G. I. Manetta & E. Benedito, 2016. Diet-tissue discrimination factors (Δ13C and Δ15N) and turnover rate in somatic tissues of a neotropical detritivorous fish on C3 and C4 diets. Journal of Fish Biology 89: 213–219.
Sagouis, A., J. Cucherousset, S. Villéger, F. Santoul & S. Boulêtreau, 2015. Non-native species modify the isotopic structure of freshwater fish communities across the globe. Ecography 38: 979–985.
Sanches, F. H. C., C. A. Miyai, T. M. Costa, R. A. Christofoletti, G. L. Volpato & R. E. Barreto, 2012. Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. Plos ONE 7: e29746.
Sazima, I. & F. A. Machado, 1990. Underwater observations of piranhas in western Brazil. Environmental Biology of Fish 28: 17–31.
Thomaz, S. M., T. A. Pagioro, L. M. Bini, M. C. Roberto & R. R. A. Rocha, 2004. Limnological characterization of the aquatic environments and the influence of hydrometrics levels. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds) The Upper Paraná River and Its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden 75:102.
Thomaz, S. M., T. A. Pagioro, L. M. Bini, M. C. Roberto & R. R. A. Rocha, 2004. Limnology of the Upper Paraná River Floodplain: Patterns of Spatio-temporal Variations and Influence of the Water Levels. In Agostinho, A. A., L. Rodrigues, L. C. Gomes, S. M. Thomaz & L. E. Miranda (eds) Structure and Functioning of the Paraná River and Its Floodplain: LTER – site 6. EDUEM, Maringá 37: 42.
Vander-Zanden, M. J. & J. B. Rasmussen, 1999. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80: 1395–1404.
Vander-Zanden, M. J., G. Cabana & J. B. Rasmussen, 1997. Comparing the trophic position of litoral fish estimated using stable nitrogen isotopes (δ15N) and dietary. Canadian Journal of Fisheries and Aquatic Sciences 54: 1142–1158.
Vazzoler, A. E. A. M., H. I. Suzuki, E. E. Marques & M. L. A. P. Lizama, 1997. Primeira maturação gonadal, períodos e áreas de reprodução. In Vazzoler, A. E. A. M., A. A. Agostinho & N. S. Hahn (eds), A planície de inundação do alto rio Paraná: Aspectos físicos, biológicos e socioeconômicos. EDUEM, Maringá: 248–265.
Vitule, J. R. S., F. Skóra & V. Abilhoa, 2012. Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Diversity and Distributions 18: 111–120.
Acknowledgements
The authors thank Nupélia/UEM, the CNPq/SISBIOTA project, the Graduate Program in Ecology of Continental Aquatic Environments (Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais - PEA); CNPq/PELD for financial support and infrastructure to develop the study; and CNPq and CAPES for the scholarship granted to Gustavo H. Z. Alves, Bruno R. S. Figueiredo, Raffael M. Tófoli, and Patrícia A. Sacramento. We are also grateful to three anonymous referees for providing valuable suggestions. This work was partially supported by CAPES, an organ of the Brazilian Government for the training of human resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Michael Power
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alves, G.H.Z., Figueiredo, B.R.S., Manetta, G.I. et al. Trophic segregation underlies the coexistence of two piranha species after the removal of a geographic barrier. Hydrobiologia 797, 57–68 (2017). https://doi.org/10.1007/s10750-017-3159-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3159-6