Abstract
Edge populations are of conservation importance because of their roles as reservoirs of evolutionary potential and in understanding a given species’ ecological needs. Mainly due to loss of aquatic breeding sites, the great crested newt Triturus cristatus is amongst the fastest declining amphibian species in Europe. Focusing on the north-westerly limit of the T. cristatus range, in the Scottish Highlands, we aimed to characterise habitat requirements and conservation needs of an isolated set of edge populations. We recorded 129 breeding pond-related environmental parameters, and used a variable selection procedure followed by random forest analysis to build a predictive model for the species’ present occurrence, as well as for population persistence incorporating data on population losses. The most important variables predicting T. cristatus occurrence and persistence were associated with pond quality, pond shore and surrounding terrestrial habitat (especially mixed Pinus sylvestris–Betula woodland), and differed from those identified in the species’ core range. We propose that habitat management and pond creation should focus on the locally most favourable habitat characteristics to improve the conservation status and resilience of populations. This collaborative work, between conservation agencies and scientific researchers, is presented as an illustrative example of linking research, management and conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragmented peripheral populations are often reservoirs of genetic diversity, and play crucial roles in species’ persistence (Channell & Lomolino, 2000; Peterman et al., 2013). It is generally recognised that species’ geographic ranges are determined by the interplay between history, climate and habitat, as well as life history and physiology (summarised in e.g. Gaston, 2009). However, we still have only a poor understanding of the specific biotic and abiotic factors which influence the persistence of populations at the periphery of a geographic distribution (Sexton et al., 2009). From the view of biological conservation, range-edge populations are worthy of attention for a range of reasons. Such populations are often morphologically and genetically distinct, and therefore important for preserving the full evolutionary potential of a species (Lesica & Allendorf, 1995; Eckert et al., 2008). However, edge populations are often rather small and therefore exposed to high extinction risks through stochastic events, and a reduced amount of neutral genetic variation can further reduce their ability to persist (Sagarin & Gaines, 2002; Sexton et al., 2009). The combination of small population size and a peripheral location can also limit the potential of populations to adapt to changing local environmental conditions (Bridle & Vines, 2007; Kawecki, 2008). An important, but rather underreported, consideration for the conservation management of peripheral populations is that the ecological niche space occupied by a given species can vary across its range, with habitat requirements as quantified through species–environment relationships therefore depending on geographic location (Pearman et al., 2010).
Most Palaearctic amphibian species breed in small water bodies, such as ponds, using adjacent terrestrial areas as summer foraging habitat for hibernation, migration, and dispersal; the use of confined breeding foci makes them very amenable for studies at the level of populations (Petranka et al., 2004; Jehle et al., 2005a; Semlitsch, 2008). Whether an available pond is occupied largely depends on a species’ ecological requirements, as well as the degree of connectivity to other ponds (Halley et al., 1996; Marsh & Trenham, 2001; Ficetola & De Bernardi, 2004; Van Buskirk, 2005). In recent decades, significant attention has been devoted to the use of habitat parameters for predicting the suitability of ponds and their surroundings for specific species (e.g. Joly et al., 2001; Knapp et al., 2003; Denoël & Lehmann, 2006; Hartel et al., 2007). Habitat requirements, however, vary considerably across a species’ range (Gomez-Mestre & Tejedo, 2003; Arntzen & Themudo, 2008; Zanini et al., 2009), and such studies can therefore convey a view which is biased towards the core of a distribution, or to particular environments such as agricultural landscapes (Mazerolle et al., 2005; Hartel et al., 2010b). As a result, despite the importance of peripheral populations for conservation, predictive habitat models calibrated to landscapes typical of central populations might be of limited value elsewhere.
The great crested newt, Triturus cristatus (Laurenti, 1768), is protected under Annex II and Annex IVa of the European Habitats Directive. Although still widespread, T. cristatus is amongst the fastest declining amphibian species in Europe; its conservation status is assessed as favourable in only 2 out of 22 European countries (Luxembourg and Denmark), a fact which has been linked to habitat loss (Denoël, 2012; for a summary see Rannap et al., 2009; Jehle et al., 2011). At the core of its range, T. cristatus generally occupies both natural and artificial ponds in pastures and deciduous or mixed woodland, with pond macrophyte cover and connectivity being the most important parameters for predicting occurrence (Halley et al., 1996; Hartel et al., 2010a; Denoël et al., 2013; Hartel & von Wehrden, 2013). At the northern periphery of its range (e.g. Scandinavia), however, typical habitats for T. cristatus include acidic bog lakes surrounded by coniferous woodland (Dolmen, 1980; Skei et al., 2006; Vuorio et al., 2015). As current conservation management practices for T. cristatus are heavily based on habitat suitability models (Oldham et al., 2000; Unglaub et al., 2015), this raises the need to consider specific ecological, biogeographical and social contexts for assessing habitat requirements across its range (see also e.g. Sjögren-Gulve, 1994; Cayuela et al., 2016 for other amphibian species).
Triturus cristatus reaches its north-westerly limit in the Scottish Highlands, where a set of populations is separated from the remainder of the British range by over 80 km of unfavourable habitat. Due to this spatial isolation, it was previously assumed that these populations stem from introductions, and their native status was only recently demonstrated using genetic means (O’Brien & Hall, 2012; O’Brien et al., 2015). The aim of the present study is to employ the case of T. cristatus in the Scottish Highlands as a model to describe the habitat requirements of, and the effect of human activities on, a European flagship wetland species at the edge of its distribution. In order to achieve this, we used a detailed dataset of 129 ecological variables to compare occupied with unoccupied ponds, also considering ponds with reported disappearance events. Our approach to establishing local habitat requirements differs, for example, from the existing Habitat Suitability Index for this species (Oldham et al., 2000) by incorporating a much larger (>10 fold) number of variables, which should enable the description of local ecological needs of T. cristatus in more detail. Besides serving as an example for elucidating different ecological niches across the range of a species of considerable conservation importance, the study has already been used to inform habitat creation and management measures instigated by the local government agencies.
Materials and methods
Study area and field survey
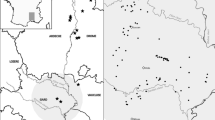
Field work for this study built upon volunteer surveys started in the late 1980s, as well as ad hoc records extending back to 1896 (NBN, 2014). We considered 88 ponds in total, which encompassed all 33 known ponds in the Scottish Highlands with T. cristatus records since 1990 (excluding known introductions), seven ponds with populations found during the present study, and 48 control ponds without T. cristatus occurrence. Control ponds were located within the same group of 10 × 10 km2 as the known T. cristatus ponds, chosen using a random number generator to select grid references (4°35′–3°35′W, 57°38′–57°11′N; Fig. 1). The studied ponds represented glacial and man-made sites, the age of the latter ranging from prior to the earliest detailed maps (surveyed c. 1870) to ponds created within the last 10 years. Altitudes ranged from 10 m to 248 m a.s.l. (median 91.5 m).
We surveyed each pond to determine the presence of T. cristatus at least three times per year, using four techniques following the British National Amphibian and Reptile Recording scheme (NARRS) protocol: egg searching, dip netting, torching, and trapping (Langton et al., 2001; Griffiths & Langton, 2003; ARG-UK, 2013). Egg searching involved looking for folded leaves, containing eggs, amongst the submerged vegetation. Dip netting was carried out from the shore using a net with a 2 mm mesh, sweeping the whole perimeter of ponds smaller than 3,000 m2, and at least 300 m of shoreline, including all habitats present, for ponds larger than 3,000 m2. Torching (Cluson Clulite CB2, 1 million C/P) was conducted from shortly after dusk to shortly after midnight, walking around the entire pond perimeter. Trapping was carried out using up to 20 46 × 21 × 21 cm funnel traps for each pond (4 mm nylon mesh with 6 cm diameter openings at each end, see Madden & Jehle, 2013). Funnel traps were installed amongst aquatic plants shortly before sunset and checked within 10 h. Data from surveys were pooled to determine the presence or absence of T. cristatus at given ponds.
All surveying followed Scottish Natural Heritage guidance, to ensure welfare of newts and non-target species, and the disease and non-native species control measures advised for amphibian field workers (ARG-UK, 2008).
Habitat descriptors and data analysis
To investigate which habitat features were most important to predict the presence of T. cristatus, we collected data from 129 variables, 88 derived through field work and 41 through desk study after the field sampling period. Topographical features were obtained from GIS using 1:25,000 maps from the British mapping agency Ordnance Survey. Water-associated variables were gathered in the field by handheld devices or estimated using semi-quantitative scales. Anthropogenic activities, the aquatic macroinvertebrate community, the vegetation communities, and other habitat characteristics of the ponds and their surroundings were assessed using percentages or semi-quantitative scales. Given the conservation management context of the study and the proximity of occupied and non-occupied control ponds, we did not include spatial autocorrelation variables to avoid unnecessary complexity and collinearity in the models. For further details on habitat descriptors see Online Resource 1.
We assessed the habitat requirements of T. cristatus applying two successive statistical approaches: variable selection by individual binomial tests and variable exploration by principal component analysis (PCA), followed by establishing the relative importance of the selected predictor variables by non-parametric random forest analyses (see below). Each statistical procedure was performed separately to investigate either T. cristatus presence or absence (occurrence analyses), as well as population persistence (persistence analyses). We defined lack of persistence as failure to record T. cristatus since 2010 despite previous records, based on annual surveys consisting of at least three visits each.
Individual binomial tests were made on each of the 129 variables to determine their relevance to T. cristatus occurrence and persistence. Mann–Whitney U tests and Chi square (χ 2) tests were used for numerical and categorical variables, respectively. Individual Generalized Additive Models (GAM) for every original variable were also produced with the same objective. Significant variables (P < 0.05) were grouped and examined for collinearity. We excluded numerical and categorical variables which had a Variance Inflation Factor (VIF) > 3.0, and numerical variables which showed Pearson pairwise correlations >0.6 (Tables 2–7 in Online Resource 1, Zuur et al., 2009). The remaining significant predictor variables were represented by PCAs, where categorical variables were used directly as dummy (1/0) transformed variables.
We then investigated the relative importance of the selected predictor variables using random forest classification analyses (Breiman, 2001; Cutler et al., 2007). Random forest analysis generates multiple classification (or regression) trees, using a predefined number of random variables for each split. At the end of the process, the importance of the variables is estimated based on the frequency at which each variable has been chosen as the best in all trees (or the average value for regression trees). Specifically, we produced non-parametric unbiased recursive random forests (Hothorn et al., 2006), where the selection of the best split is based on conditional inference tests, to avoid bias in favour of continuous variables and variables with many categories. The number of trees was specified as 500, and the number of random preselected variables in each split was the square root of the total number of available variables (e.g. Hapfelmeier & Ulm, 2013). Individual variable importance measures (VIM) were computed through an algorithm which uses the area under the curve (AUC) as a measure of accuracy, which is robust against class imbalance of the response variable (Janitza et al., 2013).
Given the high number of modelled variables in comparison to the number of ponds in our dataset, the statistical procedure we followed offered more accuracy and was easier to interpret than other methods such as generalized linear or additive models (GLM/GAM). We refrained from performing occupancy modelling, since the comprehensive surveys to confirm the presence of T. cristatus provided consistent results across the six breeding seasons.
All analyses were performed with R statistical software (R Development Core Team, 2014) using the basic functions and the packages MASS to compute χ 2 tests (Venables & Ripley, 2002), mgcv to perform and plot individual GAMs (Wood, 2011), and party to produce random forests (Hothorn et al., 2015). Numerical variables were normalised before being introduced in random forest analyses when necessary.
Results
General characteristics of the ponds
The investigated ponds had a maximum water depth ranging from 0.1 to 4.5 m (median 1.0 m), surface areas of 2–164,500 m2 (median 1,615 m2), and perimeters ranging between 5 and 3,127 m (median 221.5 m). Conductivity ranged between 8.8 and 441 µS/cm (median of 100.6 µS/cm), and pond water pH was between 3.42 and 9.52 (median 6.34). Dissolved oxygen concentrations ranged from 1.0 to 18.3 mg/l (median 9.4 mg/l), leading to oxygen saturations of 10–180% (median 87.5%). None of these characteristics showed statistical differences between ponds with and without T. cristatus (Fig. 4 in Online Resource 1).
Triturus cristatus presence
We confirmed the presence of T. cristatus in 24 of the 33 ponds with post-1990 records, and found seven new T. cristatus ponds, resulting in a total of 31 ponds where T. cristatus was present. All newly discovered populations were within 1 km of at least one previously known, occupied pond. Triturus cristatus could not be detected from the remaining 57 study ponds, which comprised 48 unoccupied ponds (where T. cristatus has never been recorded) and nine ponds where T. cristatus was previously recorded but not found over the 6 years of our study.
Occurrence analyses
After accounting for collinearity, 12 predictor variables (eight numerical and four categorical) were selected as significantly related to T. cristatus occurrence (Table 1; Fig. 5 and 6 in Online Resource 1). Individual GAMs identified nine habitat quality variables that were positively related to a high probability of T. cristatus presence. These were: adjacent mixed Pinus sylvestris–Betula woodland (EUNIS category G4.4, European Environment Agency, 2014), substrate of organic mud, macroinvertebrate richness, slightly sloping bank, aquatic macrophytes (except aquatic mosses and Lemna sp.), soils with humus-rich iron podzols, terrestrial habitat diversity, underlying geology of sand and gravel, and moss coverage within 1 m of the water’s edge (Fig. 6 in Online Resource 1). Presence of fish and underlying geology of boulder clay decreased the probability of occurrence of T. cristatus. The probability of T. cristatus presence also decreased when ponds frequently dried up (more than twice in ten years), although occurrence was highest in ponds drying once every 10 years (Fig. 6 in Online Resource 1).
PCA representation showed that the variables associated with habitat quality were strongly correlated with the first principal component, which represented 18.7% of the explained variance and allowed a good discrimination between ponds with and without T. cristatus (Fig. 2a). Presence of fish was the best predictor variable of the second principal component (16.4% of the explained variance), combining most of the ponds where T. cristatus was absent (Fig. 2a). Although only 35.1% of variance is explained by the two principal components, the graphic accurately represents the relationship between pond characteristics and significant explanatory variables.
Distance PCA for a the 12 selected variables for the occurrence analyses and for b the eight selected variables for the persistence analyses. Colour of symbols indicates presence (light grey), absence (white) and disappearance (dark grey) of T. cristatus. Circles indicate fish absence and diamonds indicate fish presence. Categorical variables were used directly as dummy (1/0) variables. Abbreviations used are: number of years in 10 when the pond dries up (DRYING), fish presence (Fish), macroinvertebrate richness (MINVRICH), coverage of the pond occupied by submerged and emergent macrophytes except aquatic mosses and Lemna sp. (MACROPH), pond substrate comprising organic mud (ORMUD), proportion of slightly sloping banks (LITTLE), moss coverage of the pond shore (MOSS), coverage of adjacent mixed Pinus sylvestris–Betula woodland (MIXEDWOOD), adjacent terrestrial habitat diversity (TERRSHAN), dominant geological category Sand and Gravel over middle old red sandstone in the pond area (SandGravelC2), dominant geological category Boulder Clay over middle old red sandstone in the pond area (BoulderClayC2), dominant humus-rich iron podzols around the pond (Soil97), grass coverage of the pond shore (GRASS), tree coverage of the pond shore (TREE_WOOD) and second principal component for all anthropogenic stressor types (STRESPC2). Note that high values of STRESPC2 are strongly correlated with low presence of adjacent roads (Table 9 in Online Resource 1). Abbreviations of numerical variables are written with upper case and abbreviations of categorical dummy transformed variables are written with lower case
The random forest model had a misclassification error of 12.5%, an out-of-bag mean error of 28.4% and a 10-fold cross-validation mean error of 26.2%. In general, the variables most associated with the principal components of the PCA showed the highest values of VIM. In addition to fish presence, variables related to habitat quality were most important: adjacent mixed Pinus sylvestris–Betula woodland, substrate of organic mud, macroinvertebrate richness, years when the pond dries up and slightly sloping bank. However, fish presence was the second most important variable. VIM are shown in Fig. 3a and in Table 8 of Online Resource 1.
Importance of variables based on the non-parametric random forests for T. cristatus a occurrence and b persistence analyses. Area under the curve (AUC) was used to generate the variable importance measure (VIM) for each variable. Abbreviations of numerical variables are written with upper case and abbreviations of categorical dummy transformed variables are written with lower case. Abbreviations are: number of years in 10 when the pond dries up (DRYING), fish presence (Fish), macroinvertebrate richness (MINVRICH), coverage of the pond occupied by submerged and emergent macrophytes except aquatic mosses and Lemna sp. (MACROPH), pond substrate coverage comprising organic mud (ORMUD), proportion of slightly sloping banks (LITTLE), moss coverage of the pond shore (MOSS), coverage of adjacent mixed Pinus sylvestris–Betula woodland (MIXEDWOOD), adjacent terrestrial habitat diversity (TERRSHAN), dominant geological category Sand and Gravel over middle old red sandstone in the pond area (SandGravelC2), dominant geological category Boulder clay over middle old red sandstone in the pond area (BoulderClayC2), dominant humus-rich iron podzols around the pond (Soil97), grass coverage of the pond shore (GRASS), tree coverage of the pond shore (TREE_WOOD) and second principal component for all anthropogenic stressor types (STRESPC2). Note that STRESPC2 is strongly correlated with low presence of adjacent roads (Table 9 in Online Resource 1)
Persistence analyses
Through the individual tests, and after accounting for collinearity, eight predictor variables (seven numerical and one categorical) were selected as significantly related to T. cristatus persistence (Table 1; Figs. 7 and 8 in Online Resource 1), with individual GAMs highlighting five habitat quality variables positively related to T. cristatus persistence: grass coverage of the shore, macroinvertebrate richness, the second principal component for the set of human activities, adjacent mixed Pinus sylvestris–Betula woodland, and moss coverage of the shore. The second principal component of the stressor structure was positively correlated with the level of shooting (Spearman’s rho = 0.352, P = 0.026), and negatively correlated with the high presence of roads surrounding the ponds (rho = −0.711, P < 0.001) as well as noise (rho = −0.580, P < 0.001), which was itself strongly correlated with the presence of surrounding roads (Spearman’s rho = 0.37, P = 0.009; Table 9 in Online Resource 1). Presence of fish also lowered the probability of T. cristatus persistence, whereas there was no clear relationship with pond drying (Fig. 8 in Online Resource 1).
The PCA revealed that the first axis represented 30.8% of the explained variance, with a good discriminatory ability between ponds with permanent occupancy and ponds where T. cristatus had disappeared (Fig. 2b). The variables connected with shore habitat quality were most strongly correlated with the second principal component (18.9% of the explained variance), which also identified the ponds where T. cristatus has disappeared. Presence of fish was correlated with both axes (Fig. 2b).
The random forest model produced for T. cristatus persistence had a misclassification error, an out-of-bag mean error and a 10-fold cross validation mean error of 22.5% each. Grass coverage of the shore was the most important habitat feature, linked to macroinvertebrate richness, pond drying and tree coverage of the shore. Fish presence was the second most important variable, followed by the second principal component for the stressor structure (mainly correlated with the high presence of roads surrounding the pond). VIM are shown in Fig. 3b; and in Table 10 of Online Resource 1.
Discussion
Management and conservation of Triturus cristatus peripheral populations
We have developed a detailed ecological model to illuminate the occurrence and persistence of a flagship species at the edge of its distribution. We showed that habitat characteristics favouring T. cristatus in the Scottish Highlands noticeably differ from its core range, while previously described negative predictors for occurrence, such as fish presence, exert similar adverse effects regardless of local habitat preferences (see also e.g. Sjögren-Gulve, 1994; Cayuela et al., 2016 for other amphibian species). Our inferences are informing habitat creation and population management.
Our model, based on 129 habitat parameters, is amongst the most comprehensive datasets applied to predicting the occurrence or persistence of any amphibian species (compare e.g. Joly et al., 2001; Knapp et al., 2003). Such studies rely on accurate information on the presence or absence of a given species, which is a function of sampling effort and detection probability (e.g. MacKenzie et al., 2003; Schmidt, 2005). Employing a nationally recognised protocol to record the presence of T. cristatus ensured comparability with other UK studies, but does not guarantee accurate detection across all ponds (see discussion in Griffiths et al., 2015). However, our presence/absence findings at individual ponds were consistent across the six-year period of the study, suggesting that our records of population loss represent true demic extinctions. Another potential confounding factor when describing habitat relationships is spatial autocorrelation (e.g. Ficetola et al., 2015). Our predictive models did not explicitly include, for example, information on the distance to the nearest occupied ponds, since our analysis was geared towards active habitat management for conservation. The confined range of our study area further suggests that spatial autocorrelation is likely to have had little effect on our inferences (e.g. Griffiths et al., 2010). Previous studies on T. cristatus have also shown that, at similar spatial scales to those studied herein, demographic properties of populations play a more important role in shaping patterns of gene flow than terrestrial habitat characteristics (un)favourable for migration (Jehle et al., 2005b). We nevertheless plan to incorporate inter-pond terrestrial habitat variables more explicitly in future studies.
The vulnerability of T. cristatus in the Scottish Highlands stems from the small number of occupied ponds and their isolation from the species’ core range, and its recent recognition as a native species makes its conservation a priority for government agencies (O’Brien et al., 2015). Long-term effectiveness of conservation interventions, such as pond creation and habitat management, relies on a thorough understanding of the species’ habitat requirements at this part of its range. The relationship between population loss and fish presence is a particular concern, due to local pressure from recreational angling. New pond creation is focusing on sites where landowners support amphibian conservation and understand the dangers of fish, and on locations with low risk of fish introduction (e.g. avoiding roads and established fishing lakes). Whilst fish eradication has been apparently successful at one of the Highland ponds (O’Brien unpublished data), this may not always be practical.
Pond creation is a relatively inexpensive form of habitat management (Baker et al., 2011), although it utilises otherwise productive farmland or forest. An understanding of favourable habitat characteristics informs better pond design and creation. In the Scottish Highlands, we recommend pond creation close to mixed Pinus sylvestris–Betula woodland, on humus-rich iron podzols with underlying sand and gravel and away from busy roads. Surrounding terrestrial habitat should be managed to favour grassy and mossy shores. Informed by the data presented here, Scottish Natural Heritage and Forestry Commission Scotland created or modified 25 ponds in 2014 and 2015, of which three were colonised by T. cristatus within 14 months (in preparation).
Predicting Triturus cristatus occurrence
Our empirical data confirmed that habitat preferences of edge populations in the Scottish Highlands differ from the core of the species’ range. Elsewhere (excluding Scandinavia) Triturus cristatus is associated with deciduous woodland and arable land, along with artificial breeding sites such as ponds dug for livestock or associated with mineral extraction (Swan & Oldham, 1993; Latham et al., 1996; Beebee & Griffiths, 2000; Jehle et al., 2011). Our study showed a strong link with mixed Pinus sylvestris–Betula woodland. Use of pine forest in northern regions was also shown by Skei et al. (2006), but was somewhat unexpected in our study as sizeable areas of deciduous woodland are present. The locally thermophilic T. cristatus may benefit from the relatively high ground level incident solar radiation afforded by the open canopy of Pinus sylvestris–Betula woodland, compared to the denser canopy of the dominant local deciduous woodland types acidophilus Quercus-dominated woodland and meso- and eutrophic Quercus, Fraxinus, Acer, Tilia, Ulmus and related woodland (EUNIS codes G1.8 and G1.A, respectively, European Environment Agency, 2014). If the Scottish Highland population represents an isolated group of colonists (O’Brien & Hall, 2012), then it seems likely that they are adapted to the dominant British habitat at the time of colonisation (Edwards & Whittington, 2003). Thus, their phenotypic traits may differ from the main population (as observed for other amphibians, e.g. Rollins et al., 2015).
In contrast to previous evidence (Klinge, 2001), we found a negative association between T. cristatus presence and clay, and a corresponding positive association with humus-rich iron podzols, substrates which are both common in the study area. Organic mud, an important breeding area for potential food species, was also positively associated with T. cristatus presence. The prominent role of substrate in our models contrasts with a study from north-eastern Europe (Rannap et al., 2009). The strong negative relationship with fish presence agrees with previous studies, although we could not confirm a negative association with waterfowl (Oldham et al., 2000).
The frequency of pond drying proved important in predicting T. cristatus occurrence, although the relationship appears complex; drying in 1/10 years was most favourable (confirming Griffiths, 1997; Oldham et al., 2000). Triturus cristatus bred in ponds with pH between 4.9 and 9.3, demonstrating the use of more acidic ponds than elsewhere in Britain (Denton, 1991, found adults in ponds with pH 4.7, but did not observe breeding), and a wider range than found at other northern limits (Dolmen, 1980; Skei et al., 2006). Other factors positively correlated with T. cristatus presence were macroinvertebrate richness, slightly sloping bank, aquatic macrophytes (except mosses and Lemna sp.), moss coverage of the shore, and terrestrial habitat diversity (largely confirming Green, 1984; Gustafson et al., 2006). In contrast to studies from other areas pond shading had no influence on the presence or absence (Filoda, 1981; Oldham et al., 2000), and negative effects from agriculture and forestry (presumed anthropogenic stressors) were not observed. Agricultural runoff and grazing pressure may be less of an issue in our region, where land management is less intensive than elsewhere in Western Europe. Potential negative impacts of commercial forestry may have been mitigated, or even reversed, through collaborative work with forestry agencies to manage habitat for amphibians (e.g. Forestry Commission Scotland & Scottish Natural Heritage, 2009). Water abstraction and other artificial changes in water levels, which have strong negative effects on amphibians and other aquatic biodiversity elsewhere (Miró, 2016), are uncommon in the study area, as is mineral extraction. Whilst shooting had been considered a possible stressor, it was positively correlated with presence (P < 0.03). This may reflect both habitat management for quarry species that also favour T. cristatus, and deer culling in woodland for wider conservation and commercial benefit. As hunting is economically important across the species’ range, this finding may merit further investigation.
Predicting Triturus cristatus population losses
Conservation of a species found in a small number of sites depends on an understanding of the reasons for its disappearance from previously occupied sites. Fish presence, or introduction, has been linked to T. cristatus disappearance (for a review see Jehle et al., 2011), as has presence of roads for amphibians in general, through direct mortality and population isolation (for a review see Beebee, 2013). While noise was associated with disappearance linked to road presence, ponds with high noise levels under the flight path of Inverness airport were readily used, as were sites where shooting takes place, suggesting that noise in itself may not be problematic. As expected, macroinvertebrate richness was positively related to population persistence. Significant relationships were found with shore habitat within 1 m of the water’s edge; while high coverage of grass and moss was associated with persistence, high density of trees was linked to disappearance. Together with the lack of a significant impact of shading, this may suggest that, at least at high latitudes, shore vegetation is important for shading as well as for foraging and shelter. Other factors showing a significant relationship with persistence were adjacent mixed Pinus sylvestris–Betula woodland (within 500 m of the pond), and the likelihood of pond drying, agreeing with the statistical inferences based on presence data. Many of these biotic and abiotic factors are amenable to conservation intervention which enhances the resilience of populations to stochastic events and adverse effects, for example by planting appropriate tree species or taking steps to manage desiccation frequency.
Conclusion
Triturus cristatus habitat requirements at the edge of its range differ from those at the core. Most of the habitat characteristics which we found to be significant may be managed (mainly those associated with the quality of the pond and surrounding habitat), and the results are already being used to inform the design of conservation interventions. The results of this study provide practical criteria for managing existing ponds and creating new ones, thus mitigating risks to the conservation of T. cristatus peripheral populations. We are currently working with landowners to create new ponds within the dispersal range of existing ponds and within habitat types most strongly associated with presence and persistence. The management of existing ponds focuses on the maintenance and enhancement of features associated with T. cristatus persistence.
References
ARG-UK, 2008. Amphibian disease precautions: a guidance for UK fieldworkers, version 1: February 2008. Amphibian and Reptile Groups of the UK. Advice Note 4.
ARG-UK, 2013. NARRS Amphibian Survey Protocols (v. 2013). Amphibian and Reptile Groups of the United Kingdom.
Arntzen, J. W. & G. E. Themudo, 2008. Environmental parameters that determine species geographical range limits as a matter of time and space. Journal of Biogeography 35: 1177–1186.
Baker, J., T. Beebee, J. Buckley, A. Gent & D. Orchard, 2011. Amphibian Habitat Management Handbook. Amphibian and Reptile Conservation, Bournemouth.
Beebee, T. J. C., 2013. Effects of road mortality and mitigation measures on amphibian populations. Conservation Biology 27: 657–668.
Beebee, T. J. C. & R. A. Griffiths, 2000. Amphibians and Reptiles: A Natural History of the British Herpetofauna. Harper Collins, London.
Breiman, L., 2001. Random forests. Machine Learning 45: 5–32.
Bridle, J. R. & T. H. Vines, 2007. Limits to evolution at range margins: when and why does adaptation fail? Trends in Ecology & Evolution 22: 140–147.
Cayuela, H., D. Arsovski, J.-M. Thirion, E. Bonnaire, J. Pichenot, S. Boitaud, A.-L. Brison, C. Miaud, P. Joly & A. Besnard, 2016. Contrasting patterns of environmental fluctuation contribute to divergent life histories among amphibian populations. Ecology 97: 980–991.
Channell, R. & M. V. Lomolino, 2000. Dynamic biogeography and conservation of endangered species. Nature 403: 84–86.
Cutler, D. R., T. C. Edwards Jr., K. H. Beard, A. Cutler & K. T. Hess, 2007. Random forests for classification in ecology. Ecology 88: 2783–2792.
Denoël, M., 2012. Newt decline in Western Europe: highlights from relative distribution changes within guilds. Biodiversity and Conservation 21: 2887–2898.
Denoël, M. & A. Lehmann, 2006. Multi-scale effect of landscape processes and habitat quality on newt abundance: implications for conservation. Biological Conservation 130: 495–504.
Denoël, M., A. Perez, Y. Cornet & G. F. Ficetola, 2013. Similar local and landscape processes affect both a common and a rare newt species. PLoS ONE 8: e62727.
Denton, J. S., 1991. The distribution and breeding site characteristics of newts in Cumbria, England. Herpetological Journal 1: 549–554.
Dolmen, D., 1980. Distribution and habitat of the smooth newt, Triturus vulgaris (L.) and the warty newt, Triturus cristatus (Laurenti), in Norway. In Coburn, J. (ed.), Proceedings of the European Herpetological Symposium, Oxford: 127–139.
Eckert, C. G., K. E. Samis & S. C. Lougheed, 2008. Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molecular Ecology 17: 1170–1188.
Edwards, K. J. & G. Whittington, 2003. Vegetation change. In Edwards, K. J. & I. B. M. Ralston (eds), Scotland After the Ice Age: Environment, Archaeology and History 8000 BC–AD 1000. Edinburgh University Press, Edinburgh.
European Environment Agency, 2014. EUNIS habitat type hierarchical view. http://eunis.eea.europa.eu/habitats-code-browser.jsp. Accessed 16 February 2014.
Ficetola, G. F. & F. De Bernardi, 2004. Amphibians in a human-dominated landscape: the community structure is related to habitat features and isolation. Biological Conservation 119: 219–230.
Ficetola, G. F., C. Rondinini, A. Bonardi, D. Baisero & E. Padoa-Schioppa, 2015. Habitat availability for amphibians and extinction threat: a global analysis. Diversity and Distributions 21: 302–311.
Filoda, H., 1981. Amphibien im östlichsten teil Lüchow-Dannenbergs – eine siedlungsbiologische bestandsaufnahme. Beiträge zur Naturkunde Niedersachsens 34: 125–136.
Forestry Commission Scotland & Scottish Natural Heritage, 2009. Forest operations and great crested newts. FCS Guidance Note 35b. www.forestry.gov.uk.
Gaston, K. J., 2009. Geographic range limits: achieving synthesis. Proceedings of the Royal Society B – Biological Sciences 276: 1395–1406.
Gomez-Mestre, I. & M. Tejedo, 2003. Local adaptation of an anuran amphibian to osmotically stressful environments. Evolution 57: 1889–1899.
Green, D. M., 1984. A Study of The Great Crested Newt (Triturus cristatus) in Durham and Tyne and Wear South. DCCT, Durham.
Griffiths, R. A., 1997. Temporary ponds as amphibian habitats. Aquatic Conservation – Marine and Freshwater Ecosystems 7: 119–126.
Griffiths, R. A. & T. Langton, 2003. Catching and handling. In Gent, T. & S. Gibson (eds), Herpetofauna Workers Manual. Joint Nature Conservation Committee (JNCC), Peterborough: 33–44.
Griffiths, R. A., D. Sewell & R. S. McCrea, 2010. Dynamics of a declining amphibian metapopulation: survival, dispersal and the impact of climate. Biological Conservation 143: 485–491.
Griffiths, R. A., J. Foster, J. W. Wilkinson & D. Sewell, 2015. Science, statistics and surveys: a herpetological perspective. Journal of Applied Ecology 52: 1413–1417.
Gustafson, D. H., C. J. Pettersson & J. C. Malmgren, 2006. Great crested newts (Triturus cristatus) as indicators of aquatic plant diversity. Herpetological Journal 16: 347–352.
Halley, J. M., R. S. Oldham & J. W. Arntzen, 1996. Predicting the persistence of amphibian populations with the help of a spatial model. Journal of Applied Ecology 33: 455–470.
Hapfelmeier, A. & K. Ulm, 2013. A new variable selection approach using Random Forests. Computational Statistics & Data Analysis 60: 50–69.
Hartel, T. & H. von Wehrden, 2013. Farmed areas predict the distribution of amphibian ponds in a traditional rural landscape. PLoS ONE 8: e63649.
Hartel, T., S. Nemes, D. Cogălniceanu, K. Öllerer, O. Schweiger, C.-I. Moga & L. Demeter, 2007. The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 583: 173–182.
Hartel, T., S. Nemes, K. Oellerer, D. Cogalniceanu, C. Moga & J. W. Arntzen, 2010a. Using connectivity metrics and niche modelling to explore the occurrence of the northern crested newt Triturus cristatus (Amphibia, Caudata) in a traditionally managed landscape. Environmental Conservation 37: 195–200.
Hartel, T., O. Schweiger, K. Oellerer, D. Cogalniceanu & J. W. Arntzen, 2010b. Amphibian distribution in a traditionally managed rural landscape of Eastern Europe: probing the effect of landscape composition. Biological Conservation 143: 1118–1124.
Hothorn, T., K. Hornik & A. Zeileis, 2006. Unbiased recursive partitioning: a conditional inference framework. Journal of Computational and Graphical Statistics 15: 651–674.
Hothorn, T., K. Hornik, C. Strobl & A. Zeileis, 2015. A laboratory for recursive partytioning. Party R package version 1.0-20. http://CRAN.R-project.org/package=party.
Janitza, S., C. Strobl & A.-L. Boulesteix, 2013. An AUC-based permutation variable importance measure for random forests. BMC Bioinformatics 14(119): 1–11.
Jehle, R., T. Burke & J. W. Arntzen, 2005a. Delineating fine-scale genetic units in amphibians: probing the primacy of ponds. Conservation Genetics 6: 227–234.
Jehle, R., G. A. Wilson, J. W. Arntzen & T. Burke, 2005b. Contemporary gene flow and the spatio-temporal genetic structure of subdivided newt populations (Triturus cristatus, T. marmoratus). Journal of Evolutionary Biology 18: 619–628.
Jehle, R., B. Thiesmeier & J. Foster, 2011. The Crested Newt: A Dwindling Pond-Dweller. Laurenti, Bielefeld.
Joly, P., C. Miaud, A. Lehmann & O. Grolet, 2001. Habitat matrix effects on pond occupancy in newts. Conservation Biology 15: 239–248.
Kawecki, T. J., 2008. Adaptation to marginal habitats. Annual Review of Ecology Evolution and Systematics 39: 321–342.
Klinge, A., 2001. Zur Situation des Kammolchs (Triturus cristatus Laurenti, 1768) in Schleswig-Holstein. Rana 4: 41–50.
Knapp, R. A., K. R. Matthews, H. K. Preisler & R. Jellison, 2003. Developing probabilistic models to predict amphibian site occupancy in a patchy landscape. Ecological Applications 13: 1069–1082.
Langton, T., C. Beckett & J. Foster, 2001. Great Crested Newt Conservation Handbook. Froglife, Halesworth.
Latham, D. M., R. S. Oldham, M. J. Stevenson, R. Duff, P. Franklin & S. M. Head, 1996. Woodland management and the conservation of the great crested newt (Triturus cristatus). Aspects of Applied Biology 44: 451–459.
Lesica, P. & F. W. Allendorf, 1995. When are peripheral populations valuable for conservation? Conservation Biology 9: 753–760.
MacKenzie, D. I., J. D. Nichols, J. E. Hines, M. G. Knutson & A. B. Franklin, 2003. Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84: 2200–2207.
Madden, N. & R. Jehle, 2013. Farewell to the bottle trap? An evaluation of aquatic funnel traps for great crested newt surveys (Triturus cristatus). Herpetological Journal 23: 241–244.
Marsh, D. M. & P. C. Trenham, 2001. Metapopulation dynamics and amphibian conservation. Conservation Biology 15: 40–49.
Mazerolle, M. J., A. Desrochers & L. Rochefort, 2005. Landscape characteristics influence pond occupancy by frogs after accounting for detectability. Ecological Applications 15: 824–834.
Miró, A., 2016. Fish as local stressors of Pyrenean high mountain lakes: arrival process and impact on amphibians and other organisms. PhD Thesis, University of Barcelona.
NBN, 2014. The National Biodiversity Network’s Gateway. https://data.nbn.org.uk/. Accessed 14 February 2014.
O’Brien, C. D. & J. E. Hall, 2012. A hypothesis to explain the distribution of the great crested newt Triturus cristatus in the Highlands of Scotland. Herpetological Bulletin 119: 9–14.
O’Brien, C. D., J. E. Hall, D. Orchard, C. D. Barratt, J. W. Arntzen & R. Jehle, 2015. Extending the natural range of a declining species: genetic evidence for native great crested newt (Triturus cristatus) populations in the Scottish Highlands. European Journal of Wildlife Research 61: 27–33.
Oldham, R. S., J. Keeble, M. J. S. Swan & M. Jeffcote, 2000. Evaluating the suitability of habitat for the great crested newt (Triturus cristatus). Herpetological Journal 10: 143–155.
Pearman, P. B., M. D’Amen, C. H. Graham, W. Thuiller & N. E. Zimmermann, 2010. Within-taxon niche structure: niche conservatism, divergence and predicted effects of climate change. Ecography 33: 990–1003.
Peterman, W. E., S. M. Feist, R. D. Semlitsch & L. S. Eggert, 2013. Conservation and management of peripheral populations: spatial and temporal influences on the genetic structure of wood frog (Rana sylvatica) populations. Biological Conservation 158: 351–358.
Petranka, J. W., C. K. Smith & A. F. Scott, 2004. Identifying the minimal demographic unit for monitoring pond-breeding amphibians. Ecological Applications 14: 1065–1078.
R Development Core Team, 2014. R: A language and environment for statistical computing, version 3.1.2. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Rannap, R., A. Lohmus & L. Briggs, 2009. Niche position, but not niche breadth, differs in two coexisting amphibians having contrasting trends in Europe. Diversity and Distributions 15: 692–700.
Rollins, L. A., M. F. Richardson & R. Shine, 2015. A genetic perspective on rapid evolution in cane toads (Rhinella marina). Molecular Ecology 24: 2264–2276.
Sagarin, R. D. & S. D. Gaines, 2002. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecology Letters 5: 137–147.
Schmidt, B. R., 2005. Monitoring the distribution of pond-breeding amphibians when species are detected imperfectly. Aquatic Conservation – Marine and Freshwater Ecosystems 15: 681–692.
Semlitsch, R. D., 2008. Differentiating migration and dispersal processes for pond-breeding amphibians. The Journal of Wildlife Management 72: 260–267.
Sexton, J. P., P. J. McIntyre, A. L. Angert & K. J. Rice, 2009. Evolution and ecology of species range limits. Annual Review of Ecology Evolution and Systematics 40: 415–436.
Sjögren-Gulve, P., 1994. Distribution and extinction patterns within a northern metapopulation of the pool frog, Rana lessonae. Ecology 75: 1357–1367.
Skei, J. K., D. Dolmen, L. Ronning & T. H. Ringsby, 2006. Habitat use during the aquatic phase of the newts Triturus vulgaris (L.) and T. cristatus (Laurenti) in central Norway: proposition for a conservation and monitoring area. Amphibia-Reptilia 27: 309–324.
Swan, M. J. S. & R. S. Oldham, 1993. National amphibian survey. English Nature. Research Report No. 38.
Unglaub, B., S. Steinfartz, A. Drechsler & B. R. Schmidt, 2015. Linking habitat suitability to demography in a pond-breeding amphibian. Frontiers in Zoology 12: 9.
Van Buskirk, J., 2005. Local and landscape influence on amphibian occurrence and abundance. Ecology 86: 1936–1947.
Venables, W. N. & B. D. Ripley, 2002. Modern Applied Statistics with S. Springer, New York.
Vuorio, V., O.-P. Tikkanen, L. Mehtatalo & J. Kouki, 2015. The effects of forest management on terrestrial habitats of a rare and a common newt species. European Journal of Forest Research 134: 377–388.
Wood, S. N., 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society Series B – Statistical Methodology 73: 3–36.
Zanini, F., J. Pellet & B. R. Schmidt, 2009. The transferability of distribution models across regions: an amphibian case study. Diversity and Distributions 15: 469–480.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
Acknowledgements
This project was carried out under licence 29609 by Scottish Natural Heritage. The authors would like to acknowledge all people who helped us during the field and the desk work, especially Karen Frake, who prepared the maps. We also want to thank Scottish Natural Heritage for the use of a pool car and fuel, and our families for their continuing support. Most importantly, we would like to thank the landowners of the survey sites for granting access and for their enthusiasm for the protection of these animals. We thank the three anonymous reviewers and Prof Chris Joyce for their valuable comments which helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Chris Joyce
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miró, A., O’Brien, D., Hall, J. et al. Habitat requirements and conservation needs of peripheral populations: the case of the great crested newt (Triturus cristatus) in the Scottish Highlands. Hydrobiologia 792, 169–181 (2017). https://doi.org/10.1007/s10750-016-3053-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-3053-7