Abstract
Trophic interaction between contiguous habitats is an understudied area despite their ecological importance. We tested four hypotheses related to the visit of foraging birds in temporary ponds inhabited by fish: the local characteristics of ponds influence the (1) abundance and (2) composition of foraging birds; (3) the number of foraging bird visits increases in higher fish abundance; and (4) pond characteristics lead to a non-random spatial structure of fish assemblage. We studied 18 temporary ponds in the floodplain area of Pantanal (center-west Brazil), where we measured environmental variables (pond size, depth, macrophyte coverage, and forest canopy coverage), recorded the number of foraging birds and sampled fish. Foraging birds’ abundance and composition were mainly influenced by forest canopy coverage and pond size, corroborating our first and second hypotheses. The hypotheses 3 and 4 were rejected. Fish abundance was not correlated with higher number of visits of foraging birds and fish distribution was random. Local environmental variables did not affect significantly fish richness and composition. Our results suggest that the abundance of foraging birds and fish presence are determined by different assembly processes (deterministic vs. random), which may limit ponds selection by birds due to the uncertainty in fish distribution and ephemeral nature of temporary ponds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The community assembly is governed by a complex network of processes that widely vary in the spatiotemporal scale (HilleRisLambers et al., 2012). In general, a given community structure is limited by colonization of species from a regional pool (Ricklefs, 2004). The success to colonize local habitat depends on the availability of resources, abiotic conditions, and the interaction with resident organisms (HilleRisLambers et al., 2012). This deterministic process, generally called environmental filtering, suggests that only species with particular phenotypes that tolerate these local limitations will establish and persist in a given habitat (Kraft et al., 2014). Consequently, environmental gradients influence the distribution of species on space, shaping particular metacommunity structures (e.g., checkerboard, nestedness or evenly spaced; Leibold & Mikkelson, 2002). On the other hand, stochasticity and randomness may play an important role in short timescales (Tilman, 2004), and, currently, it is a subject of intense debate in ecology (Rosindell et al., 2012). Despite most studies indicating that deterministic factors drive ecological community structures (Gotelli & McCabe, 2002), random species associations are not uncommon (Hubbel & Foster, 1986; Bellay et al., 2011; Ortega et al., 2015).
Trophic interactions are recognized as one of the strongest biotic interactions that may also shape community structure (Stevens, 2010). For instance, studies have suggested that predators may influence the number of rare species (Spiller & Schoener, 1998), patterns of co-occurrence (Maly & Maly, 1997) and behavior of preys (Fortin et al., 2005). However, the intensity and importance of predation depends on the nature where the interaction takes place. Complex and large environments generally could provide more refuges, weakening the antagonistic interaction strength (McCann, 2011; Bellmore et al., 2015) and, consequently, influencing population dynamics (Cooper & Frederick, 2007) and diversity of preys (Hixon & Menge, 1991). In this sense, landscape heterogeneity generates asynchrony in resource availability, which turns out to affect predator’s behavior (Eveleigh et al., 2007). Under these conditions, it is expected that large predators, which have higher mobility and capability to respond to complex environmental information, will move and explore more profitable habitats (Rooney et al., 2008).
Waterbirds are highly mobile organisms with high basal metabolic rates (Bicudo et al., 2010). To sustain the high energy requirements, waterbirds need to consume high levels of food. Searching for food, waterbirds generally explore a large area, connecting different habitats and ecosystems (Kameda et al., 2006; Bauer & Hoye, 2014; Kloskowski & Trembaczowski, 2015). The decision to where forage may depend on several factors linked with historical past events (Salewski & Bruderer, 2007), the foraging area detectability (Butler & Gillings, 2004), distribution and availability of prey type (Eveleigh et al., 2007; Benoit-Bird et al., 2013; Stanton et al., 2014), and the presence of predators (e.g., wild cats; Yasué et al., 2003). Since most waterbirds rely on visual cues to detect prey, environments with complex structure (e.g., tall grass, forest canopy coverage) may reduce prey accessibility and detectability in potential foraging areas (e.g., Nystrand & Granström, 1997; Froneman et al., 2001; Butler & Gillings, 2004; Whittingham et al., 2004; Pérez-García et al., 2014). Therefore, attack rate and prey handling could vary widely among environments and may also depend on prey type and thus may have strong effects on waterbirds’ foraging behavior and on waterbirds’ distribution (Benoit-Bird et al., 2013).
Fish are rich in proteins and lipids (Bone & Moore, 2008), being an important source of food for many waterbird species. However, the contrast between terrestrial and aquatic habitats lead waterbirds to face a complex task to catch fish. For instance, the foraging success of long-legged wading birds (e.g., Ardeidae and Ciconiidae members) depends on prey availability, which is limited by water depth as they cannot forage at waters deeper than their leg length (Powell, 1987; Bancroft et al., 2002; Gawlik, 2002). Then, shallow lentic water bodies, such as temporary ponds and wetlands, become an attractive foraging habitat for waterbirds due to their high abundance of resources and high capture rates (Weller, 1999). Shallow lakes, ponds and wetlands are abundant in floodplain areas (Mendonça et al., 2009), which are subjected to seasonal floods responsible for the input of nutrients, sediments, and organic matter that make floodplains one of the most productive habitats in the world (Junk et al., 1989). Numerous species of fish move to floodplains in the high water season seeking for food or suitable areas for spawning or protection (Fernandes, 1997; Winemiller & Jepsen, 1998; Osorio et al., 2011). In the falling water period, most fish return to the river main channel, while some get trapped by the desiccation and stay retained in lateral lakes or temporary ponds (Lowe-Mcconnell, 1987). The rapid hydrological dynamic made some authors to postulate that the species association and diversity in floodplains are generated randomly (Lowe-Mcconnell, 1987; Goulding et al., 1988). However, recent studies have recognized the effect of several variables on the structure of fish communities in temporary ponds, such as hydroperiod, depth, aquatic macrophytes occurrence, and connection to perennial water bodies (Baber et al., 2002; Pazin et al., 2006; Fernandes et al., 2010; Tondato et al., 2013).
Here, we verified the deterministic role of local characteristics of temporary ponds (forest canopy coverage, aquatic macrophyte coverage, depth, and size of ponds) on the foraging birds’ abundance and on the distribution of fish. Moreover, we examined the correlation between foraging birds and fish abundance. We tested four hypotheses: the local characteristics of ponds determine the abundance (hypothesis 1) and composition (hypothesis 2) of foraging birds; the number of foraging bird visits increases in higher fish abundance (hypothesis 3); and local characteristics of ponds determine a non-random spatial structure of fish assemblage (hypothesis 4). We also quantified the influence of ponds characteristics over fish richness and composition as a way to determine the main modelers of metacommunity structure.
Methods
Study area
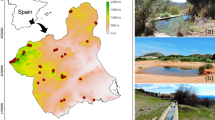
The study was carried out in temporary ponds of the floodplain area of Miranda River, middle-west of Brazil (Fig. 1). This region is part of the Pantanal, a savanna biome recognized as one of the largest floodplain areas in the world (Junk et al., 2011). The climate in the Miranda sub-basin is classified as tropical humid, with annual temperatures varying between 22.5 and 26.5°C (Gonçalves et al., 2011). The annual rain average varies between 1000 and 1200 mm, with pronounced seasonal variation (abundant rains between November–March). Consequently, the river water level varies significantly throughout the year. The highest water level occurs between December and February (Gonçalves et al., 2011), when the main river channel overflows to the adjacent floodplain. Then, the water level starts to decline up to September–October (Gonçalves et al., 2011), forming several temporary ponds in the floodplain. These temporary ponds are highly heterogeneous, varying in size, forest canopy coverage, depth, and the presence of aquatic macrophytes. In most cases, aquatic macrophytes cover the pond diffusely as a single multi-specific stand, which include floating, emergent, and submerged species. The terrestrial vegetation in the ponds’ surroundings varies typically between open grasslands and riparian forest (~4–15 m high). Additionally, this region has one of the highest concentrations of bird individuals in the world (Bini, 2014). Several resident and migratory birds use temporary ponds and other shallow waters to feed on fish, especially in the beginning of the dry season (Donatelli et al., 2014).
Sampling
We carried out fish samplings and foraging birds records in 18 temporary ponds (Fig. 1) in September of 2013 during four consecutive days. The temporary ponds studied ranged in size from 17.29 to 1500 m2 and in depth from ~7.4 to ~31 cm (Supplementary Material A). Fish were sampled along each temporary pond using a circular sieve (50 cm of diameter and mesh size of 0.5 cm). The sampling effort for fish assemblages was standardized in 20 min. The sampled fish were anesthetized with a lethal dose of benzocaine and fixed in a 10% formalin solution. Later in the laboratory, fish were identified (Britski et al., 2007), and the standard length (SL; mm) was measured.
Bird record was performed through 3 observations of 10 min in each pond, amounting 54 observations throughout the study. We kept a minimum distance of 20 m between the temporary pond and the observer. Two observations were realized in the morning, between 7:00 a.m. and 10:00 a.m., and one in the afternoon, between 4:00 p.m. and 5:30 p.m. Bird observations over time were conducted in different days to ensure sampling independency. Besides, fish sampling and bird observation were conducted in different days to avoid influence over birds’ behavior. Birds were identified in the field to species level using specialized literature (Erize et al., 2006; van Perlo, 2009). Later, these recorded birds were classified according to their feeding habit (Sick, 1997; Antas & Palo Jr., 2004), and only those species considered piscivores or potentially piscivores were included in further analyses. We also classified the foraging birds’ species according to foraging technique (wading, diving, still hunting and unconventional; Sick, 1997; Antas & Palo Jr., 2004; Bergmann et al., 2013) and main foraging habitat (terrestrial or aquatic; Sick, 1997) for descriptive purposes. It is worth noting that the annual flood pulse creates an open area in the understory close to the soil level, which allows the visual detection of birds independently of the forest structure (authors’ personal observation). Moreover, with the exception of Jabiru mycteria, which has a parental care longer than a year, the bird species found in the area were not breeding or feeding their offspring during the studied period.
Depth, macrophyte coverage, and forest canopy coverage were measured once in each temporary pond. Depth was estimated using a graduated stick through 3 measurements (near shores and center), whereas the percentage of macrophyte coverage (0–100) was estimated visually considering the whole pond. Forest canopy coverage was measured through 3 digital pictures mounted vertically (sky direction), located in the center and in the two extreme shores of each temporary pond, standardized in the breast height (1.6 m). These images were then analyzed in the Image J software (Rasband, 2015) to estimate the percentage of canopy coverage over the whole temporary pond. Since the studied ponds resembled to square and rectangular shapes, we opted to estimate the area of each system through the multiplication of its length (m) by its width (m). The width and length of each pond were determined through the mean of 3 measures using measuring tape. Detailed information about each studied ponds is shown in supplementary material A.
Data analysis
The frequency of occurrence (FO) and relative abundance (RA) of foraging birds and fish were calculated according to Amundsen et al. (1996). The FO and RA was used to construct diagrams according to Costello (1990); modified by Amundsen et al. (1996), which allows distinguishing the dominance of different bird and fish species in the studied temporary ponds. In this graphic analysis, the x axis represents the frequency of occurrence (FO), and the y axis represents the relative abundance (RA) of a specific taxa.
We estimated the importance of each predictor (forest canopy coverage, aquatic macrophyte coverage, pond depth, and size) to the foraging birds’ abundance (hypothesis 1) and to the fish richness by the model averaging approach (Burnham & Anderson, 2002). We also included fish and foraging birds’ abundances as predictors of each other in the analysis to test our hypothesis 3. Before conducting the model averaging analysis, we verified multicollinearity in our data using the variance inflation factor (VIF; Nakazawa, 2014), which indicated low correlation among the predictors (VIF < 2.2). The model averaging method consisted of a sequence of steps. First, we carried out all possible models with a maximum of two predictors per model to each dataset (foraging birds and fish). For the foraging birds’ dataset, we conducted generalized linear mixed models (GLMM) using the ponds ID as a random variable, while for the fish dataset, we used generalized linear models (GLM). Considering that fish richness and foraging birds’ abundance are discrete variables and do not have normal error distribution, we used Poisson distribution using the inverse link function. Second, the second-order Akaike information criterion with a correction for small sample sizes (AICc) was used to measure the plausibility of each candidate model. Third, the Akaike weight was calculated for each model (WI) normalized across the set of candidate models to sum to one. Fourth, the Akaike weights were used to obtain averaged estimates for each parameter. Finally, the relative importance of each predictor variable was calculated by summing all Akaike weights over all models that included each predictor. The relative importance (I) ranges from 0 to 1, and the larger the value of the relative importance of a predictor, the more important it is compared to the others (Burnham & Anderson, 2002). We also evaluated the significance of models with delta AICc lower than 2 using a parametric bootstrap comparison (1000 randomizations; Halekoh & Højsgaard, 2014) with a null model (without variables). For the forest canopy coverage, we also carried out quantile regressions (5th, 25th, 50th, 75th, and 95th) to deal with an over-dispersion of foraging birds in areas with open canopy. In this last case, we think that the forest canopy coverage may set the upper limit for the foraging bird abundance rather than affecting the mean values, driving a relationship with triangular shape in graphs (Gotelli & Ellison, 2004). The model averaging procedure was conducted in the R package “MuMIn” (Barton, 2015), the parametric bootstrap comparison was conducted in the R package “pbkrtest” (Halekoh & Højsgaard, 2014), and the quantile regression was conducted in the R package “quantreg” (Koenker, 2015).
The non-metric multidimensional scaling (NMDS) ordination based on Bray–Curtis dissimilarity was used to assess the variation in fish and birds structure among the 18 sampled ponds. Both the fish and bird data (abundance values) were square root transformed before NMDS ordinations. The pond characteristics (depth, size, forest canopy coverage, fish abundance, foraging birds’ abundance and aquatic macrophyte coverage) were correlated onto the birds (hypothesis 2) and fish (hypothesis 4) NMDS ordination using the vector fitting procedure (Oksanen, 2009; Keppel et al., 2010). The significance (P value) between the ordination and the predictors was assessed after 1000 permutations. Only the forest canopy coverage was correlated with bird species composition (see Results), and in this case, we also carried out a similarity percentage breakdown procedure (SIMPER) to investigate the difference in foraging birds’ composition along the forest canopy coverage gradient. Given that SIMPER analysis discriminates species among levels of categorical variables, we separated the temporary ponds in two main groups: ponds with low forest canopy coverage (<20% of forest canopy coverage) and ponds with high forest canopy coverage (>30% of forest canopy coverage). The probability of getting equal or larger average contribution for each species (P value) was accessed through 1000 random permutations of the group factor. To guarantee that both groups (ponds with low forest canopy coverage and high forest canopy coverage) are legitimate, we also tested their difference regarding foraging birds composition through a Permutational Multivariate Analysis of Variance (PERMANOVA; 1000 randomizations). The vector fitting, NMDS, SIMPER, and PERMANOVA were carried out in the R package “vegan” (Oksanen et al., 2009).
We tested the non-random distribution of fish in temporary ponds (hypothesis 4) using the elements of metacommunity structure (EMS). This method allows classifying metacommunities through a unique framework according to multiple patterns of species distribution, such as checkerboards, nestedness, and Gleasonian distribution (Henriques-Silva et al., 2013). The EMS is based on the evaluation of three main metrics: coherence (level in which species respond to the same environmental gradient), turnover (number of times a species replaced another between sites), and boundary clumping (dispersion of species occurrences among sites) (Leibold & Mikkelson, 2002). The coherence, turnover, and boundary clumping were calculated from a presence/absence matrix (sites as rows and species occurrences as columns) ordinate via reciprocal averaging (Gauch, 1982). More specifically, the coherence was calculated by counting the number of embedded absences, while the turnover was measured by calculating the number of times one species replaced another between sites (Dallas, 2014). The coherence and turnover were then compared with an appropriated null model to identify non-random patterns (positive or negative). In this study, we used the algorithm “swap” (sum of rows and columns is maintained) to create the null models due to its best performance on the reduction of type I and II errors (Gotelli & Entsminger, 2001; Ulrich & Gotelli, 2007). On the other hand, we calculated the boundary clumping using the Morisita’s index, which is a measure of the dispersion of species occurrences among sites (Morisita, 1971). Boundaries are considered not clumped if the Morisita’s index is one, clumped when values are greater than one and hyperdispersed boundaries when the values are less than one. We expected that coherence should be different from the null model (1000 simulations, P < 0.05), which indicates a non-random distribution of species independently from turnover and boundary clumping results (Leibold & Mikkelson, 2002; Presley et al., 2010). The EMS procedure was carried out in the R package “Metacom” (Dallas, 2015).
Results
Overall, foraging birds were abundant in the study area, being absence in just three ponds. A total of 230 birds distributed among 14 families and 37 species were observed foraging in the temporary ponds. From these birds species, 13 (147 individuals) were known as piscivores (25.1%) or facultative piscivores (74.9%) and were used in data analysis (Table 1). The majority (79.6%) of the foraging birds recorded were wading foragers (Fig. 2), while 17% were opportunistic terrestrial birds with unconventional foraging methods, 2% were diving birds, and 1.4% were still hunting birds (Table 1). Phimosus infuscatus (Lichtenstein, 1823) (FO = 50, RA = 21.7; Fig. 3a), Jacana jacana (Linnaeus, 1766) (FO = 27.8, RA = 14.9; Fig. 3a), and Pitangus sulphuratus (Linnaeus, 1766) (FO = 61.1, RA = 12.9; Fig. 3a) were the most frequent bird species in the studied temporary ponds.
Heron (Pilherodius pileatus), a wading forager, preying the annual fish Trigonectes balzanii in one of the temporary ponds studied in the Pantanal, middle-west of Brazil. To our knowledge, this is the first picture evidencing the role of birds as a predator of annual fish, fact attested indirectly by several authors (Costa, 1998; Lanés et al., 2014)
Local characteristics influenced the numerical abundance and composition of foraging birds, suggesting a deterministic effect of local characteristics on the visit of birds in temporary ponds. The numerical abundance of foraging birds was mainly influenced by pond size (I = 0.72; Tables 2, 3; Fig. 4a) and forest canopy coverage (I = 0.62; Table 2, 3; Fig. 4b). Besides, the models with pond size and forest canopy coverage were significant (P < 0.05; Table 3) in the best models selected by AICc. On the other hand, fish abundance (hypothesis 3), pond depth, and macrophyte coverage had low values of relative importance (Table 2) and were related to models with high delta AICc (>2; Table 3), suggesting that these variables are weak predictors. We also found significant negative relationship between the numerical abundance of foraging birds and forest canopy coverage for the 50° (P = 0.04), 75° (P = 0.009), and 95° (P < 0.001) quantiles, while the 5° and 25° were not significant (P > 0.05; Fig. 4b). These results from the quantile regressions indicated that forest canopy cover caused an upper limit for the abundance of foraging birds rather than affecting the mean values. In relation to foraging birds composition, forest canopy coverage was the only variable that was significant (R 2 = 0.40 P = 0.04; Fig. 5a). Large birds, such as members of Ardeidae and Ciconiidae family, were more related to open areas (Fig. 5a). Similarly, the PERMANOVA test also showed that ponds with high forest canopy coverage differed in terms of foraging bird composition from ponds with low forest canopy coverage (F 1,12 = 3.43, P = 0.007, R 2 = 0.22). Seven foraging birds species accounted for a little more than 70% of the dissimilarities among ponds with high forest canopy coverage and ponds with low forest canopy coverage (Table 4), but Aramides cajanea (P = 0.04), Theristicus caerulescens (P = 0.09), and Egretta thula (P = 0.08) were the only species that contributed significantly or marginally significantly to distinguish between both pond groups. Aramides cajanea was more related with ponds with high forest canopy coverage, while T. caerulescens and E. thula were more related with ponds with low forest canopy coverage (Table 4; Fig. 5a).
A total of 473 fish, divided into 8 families and 23 species, were found in the studied temporary ponds (Table 5). Fish abundance varied from 5 to 72 among the temporary ponds, and Characidae (59%), Callichthyidae (18%), and Lebiasinidae (10%) were the most abundant families. According to the EMS, the coherence (embedded absences observed = 106, embedded absences expected = 129.91 ± SE 17.53, z = 1.36, P = 0.17) and turnover (replacements observed = 2139, replacements expected = 1816.9 ± SE 556.58, z = −0.57, P = 0.56) were not affected negatively from what was expected by the null models, while the boundary clumping was significantly higher than expected (Morisita’s index = 1.90, DF = 20, P = 0.0002). These results suggest a random distribution of fish species in the studied temporary ponds. Similarly, we did not find any interpretable pattern in fish ordination (Fig. 5b) nor significant influence of pond characteristics over fish composition (P > 0.05; Fig. 5b). In relation to fish richness, aquatic macrophyte coverage was the most important predictor (I = 0.44; Table 2) and was present in the best model according to AICc criterion (Table 3). However, all models, including the one with aquatic macrophyte coverage parameter, were not significant (P > 0.05, Table 3). Besides, the second best model, which had a delta AICc of 0.41, was the one without any predictor, indicating that in general the pond variables measured were weak predictors for fish richness.
Discussion
Deterministic processes drive bird abundance and composition in temporary ponds as expected in our first and second hypotheses, respectively. Forest canopy coverage and pond size affected numerical abundance of foraging birds. Open large ponds have higher foraging bird visitation than small ponds with high forest canopy coverage. These results reflect the detectability and accessibility of the ponds by birds. Prey detectability and accessibility decrease with the increasing habitat structure for birds that rely on visual cues to detect their prey (Butler & Gillings, 2004). All the birds found foraging in the studied temporary ponds are first oriented by their visual system (Sick, 1997). Therefore, small ponds in forest areas with large canopy cover should affect negatively pond detectability and also the mobility of birds, decreasing the number of visits and the attack rate over aquatic organisms. It is noteworthy that the forest canopy coverage created a limiting response in bird abundance instead of a classic linear relationship. The over-dispersion found in ponds with low forest canopy coverage, which created a triangular shape in the relation between forest canopy coverage and foraging bird abundance, was probably a consequence of the heterogeneity in pond sizes. Even in open areas, the chance of find birds in small ponds is much lower than in the large ones. Indeed, we observed foraging birds in all ponds that were located in open areas with no forest canopy coverage (foraging birds were absent in three ponds under high forest canopy coverage).
Foraging decisions, such as the choice of where to feed, are usually portraiture of the balance between energetic costs and gains (Pyke, 1984). Following this idea, several studies have suggested that prey availability is the main constraint of the numerical abundance of wading birds in wetlands (Kushlan, 1976; Butler, 1994; Hafner, 1997; Gawlik, 2002; Master et al., 2005). Kushlan (1976) observed that wading birds forage more intensively when the wetland is shallow and with high density of fish, increasing their success of capture. Our results suggest that pond depth and fish abundance were weak predictors of foraging birds’ abundance, which led us to reject our third hypothesis. The shorter period of water permanence in temporary ponds compared to wetlands may have affected this lack of relation between fish concentration and bird abundance. During the dry season, the high solar intensity in the region increase water temperature causing fast evaporation (Junk et al., 2011). Two days after the end of the study, 3 ponds had already dried completely. Therefore, we suggest that the decreasing water level was fast enough to create a short window of time, which could prevent bird’s learning and foraging adaptation. On the other hand, the similarity in depth and fish abundance among the ponds may have caused the absence of the expected variation in foraging birds’ abundance. The maximum difference found in terms of depth was ~23.6 cm, while for fish abundance was 67 individuals. Although these differences are significant for small temporary ponds, the limitations of depth and fish abundance over the foraging of birds may not apply, leading to the absence of pond’s selection by birds. Either way, studies in large spatial areas, comparing ponds with different hydroperiods, depths, and fish abundances are needed to clarify these hypotheses.
Interestingly, Haas et al. (2007) observed that the presence of fish in ponds influence negatively the occurrence of waterbirds. More specifically, fish compete with birds for invertebrate preys, and thus, birds select ponds without fish (Haas et al., 2007). It seems unlikely that this process occurred in our studied sites, since fish species inhabiting the temporary ponds were small sized (see Table 3) and are also preys of birds, as attested by several attacks witnessed by us. Indeed, in our study, we captured, to our knowledge, the first picture evidencing the role of birds as a predator of annual fish (Fig. 2). However, many bird species found are not strictly piscivorous and their diet also consists of macroinvertebrates (see Table 1). For instance, P. infuscatus, J. jacana, and P. sulphuratus represent almost half of the foraging birds recorded and all of them are facultative piscivorous. Therefore, it is possible that macroinvertebrates availability also influenced the numerical abundance of bird’s foraging, which was not measured in our study.
Foraging birds’ composition varied among the temporary ponds as expected in our second hypothesis. Large birds, such as herons and storks, were not found in pond covered by forest vegetation. On the other hand, ponds in open areas included a wide range of species, such as the small Jacana jacana (~23 cm) and the large J. mycteria (Linchtenstein, 1819) (~1.5 m). This result supports the modeler role of habitat structural complexity on the composition and morphology of biological assemblages through filtering effect (Gibb & Parr, 2013). The structure and spatial grain of vegetation cover may determine the presence of species with certain body sizes over others (Gunnarsson, 1992; Telleria & Carrascal, 1994; McAbendroth et al., 2005). A structural complex habitat may affect large body birds, reducing their mobility and, consequently, increasing their energetic demands (Brodmann et al., 1997). Alternatively, large slow birds may avoid forested areas due to a higher risk of predation (Whittingham & Evans, 2004). Either way, in a savanna biome, such as the Pantanal, where the landscape is flat and composed by a mosaic of forest and grassland, the vegetation cover play an important role on bird predation dynamics in ponds.
Unlike the deterministic structure of foraging birds’ assemblage, fish varied between temporary ponds randomly. This result rejects our fourth hypothesis and supports the importance of flood pulse to the random colonization and distribution of fish in temporary ponds. In general, harsh abiotic conditions strongly limit the number of species that can inhabit temporary ponds (Jocque et al., 2010). With the exception of those fish that are adapted to live in seasonal habitats, such as annual fish (Costa, 1998), the recruitment of species in temporary ponds occur by those species that are incapable to return to perennial habitats and end up retained in these temporary habitats (Pazin et al., 2006). In the 23 fish species sampled, just 2 are strictly adapted to survive the dry period via drought-tolerant embryos (annual fish from Rivulidae family; Wourms, 1972). Some species, such as members from the Callichthyidae family, are capable to bury into the mud or move small distance creeping or leaping to others water bodies (Magoulick & Kobza, 2003). However, most species occurring in these temporary habitats are fated to desiccation or aerial predation without produce offspring for the next generation.
Although the harsh conditions and low life expectancy for fish in temporary ponds, recent studies suggest that fish assemblages may be deterministic and highly predictable in these habitats (Baber et al., 2002; Pazin et al., 2006; Fernandes et al., 2010; Tondato et al., 2013). Pazin et al. (2006) observed a nested subset structure and the influence of local factors (e.g., hydroperiod, canopy cover, depth) on fish richness and composition in ponds from Amazon. However, our results indicate that fish species do not exhibit a coherent range along the sampled environmental gradient, which breach an important assumption of all non-random metacommunities distributions (nestedness, checkboard, evenly spaced, Gleasonian, Clementsian, and other quasi structures; Presley et al., 2010). Furthermore, none local variable measured was correlated to fish richness and composition, suggesting a random species assembly in temporary ponds from Pantanal. Despite these strong evidences, we should be cautious and do not assume that fish assemblage structure in temporary ponds is purely random. Community structures that are identified as random may be caused by the interaction among deterministic processes (Boschilia et al., 2008; Caruso et al., 2011). Our data are snapshots of fish community composed by small sized individuals (less than 7 cm), and therefore, deterministic process of long-time scale may have been not identified. Long-time deterministic process could be more prone to be observed during the falling water period, when large body fish may be less likely to be trapped into temporary ponds due to their high mobility and higher capacity to respond to complex environmental information (Rooney et al., 2008).
Predator–prey interactions are strongly affected by how individuals distribute themselves into space (Hammond et al., 2007). Our study suggests that the uncertainty in fish distribution may have limited pond selection by foraging birds. More specifically, the random organization of fish in temporary ponds and the absence of pond characteristics that serve as surrogate for resources availability may have hampered bird recognition and selection of profitable sites. In this scenario, physical characteristics of ponds, such as accessibility and detection, may become the strongest factors influencing the intensity of visits of foraging birds. Additionally, the cost of diet specialization in animals that forage in temporary ponds may be high and disadvantageous due to the wide inter-annual and spatial variation in prey availability (Polacik & Reichard, 2010). Indeed, most wetland birds are generalist predators (Sick, 1997; Eastman, 1999), changing diet according to season and availability of preys (Weller, 1999; Jakubas & Manikowska, 2011). Thus, the dynamic of bird and fish assemblage in temporary ponds is another good example that food webs are dynamical systems subjected to predators’ behavior and preys’ fluctuation, instead of just static entities (Polis & Winemiller, 1996; McCann, 2011).
In summary, trophic interaction between contiguous habitats is still an understudied area despite their importance for the maintenance of communities in ecological landscapes (Nakano & Murakami, 2001). In this study, we provide information about the predator–prey relationship between foraging birds and fish in temporary ponds from Pantanal, highlighting the roles of deterministic factors and stochasticity in the structure of both assemblages. These results could help elucidate the energy pathways between aquatic and terrestrial environments in savanna like biomes subjected to seasonal floods, providing basic information for ecological modelling and conservation management.
References
Antas, P. T. Z. & H. Palo Jr., 2004. Pantanal—Guia de aves. Sesc. http://www.avespantanal.com.br/paginas/index.htm].
Amundsen, P. A., H. M. Gabler & F. J. Staldvik, 1996. A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. Journal of Fish Biology 48: 607–614.
Baber, M. J., D. L. Childers, K. J. Babbitt & D. H. Anderson, 2002. Controls on fish distribution and abundance in temporary wetlands. Canadian Journal of Fisheries and Aquatic Sciences 59: 1441–1450.
Bancroft, G. T., D. E. Gawlik & K. Rutchey, 2002. Distribution of wading birds relative to veg-etation and water depths in the northern Everglades of Florida, USA. Waterbirds 25: 265–277.
Barton, K., 2015. MuMIn: Multi-model inference. R package version 1.15.1. http://CRAN.R-project.org/package=MuMIn].
Bauer, S. & B. J. Hoye, 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344: 1242552.
Bellay, S., D. P. Lima, R. M. Takemoto & J. L. Luque, 2011. A host-endoparasite network of Neotropical marine fish: are there organizational patterns? Parasitology 138: 1945–1952.
Bellmore, J. R., C. V. Baxter & P. J. Connolly, 2015. Spatial complexity reduces interaction strengths in the meta-food web of a river floodplain mosaic. Ecology 96: 274–283.
Benoit-Bird, K. J., B. C. Battaile, S. a. Heppell, B. Hoover, D. Irons, N. Jones, K. J. Kuletz, C. A. Nordstrom, R. Paredes, R. M. Suryan, C. M. Waluk & A. W. Trites, 2013. Prey patch patterns predict habitat use by top marine predators with diverse foraging strategies. Plos One 8: e53348.
Bergmann, F. B., H. L. Amaral, D. P. Pinto, C. C. Chivittz & A. M. Tozetti, 2013. Foraging activity of the snail kite, Rostrhamus sociabilis (Aves: Accipitridae) in wetlands of southern Brazil. Brazilian Journal of Biology 73: 245–252.
Bicudo, J. E. P. W., W. A. Buttemer, M. A. Chappell, J. T. Pearson & C. Bech, 2010. Ecological and environmental physiology of birds. Oxford University Press, Oxford.
Bini, E., 2014. Birds of Brazil—Pantanal. HP Publicações.
Bone, Q. & R. H. Moore, 2008. Biology of Fishes. Taylor & Francis, New York.
Boschilia, S. M., E. F. Oliveira & S. M. Thomaz, 2008. Do aquatic macrophytes co-occur randomly? An analysis of null models in a tropical floodplain. Oecologia 156: 203–214.
Britski, H., K. Z. Silimon & B. S. Lopes, 2007. Peixes do Pantanal: manual de identificação. Embrapa, Brasília.
Brodmann, P. A., H. U. Reyer & B. Baer, 1997. The relative importance of habitat structure and of prey characteristics for the foraging success of water pipits (Anthus spinoletta). Ethology 103: 222–235.
Burnham, K. P. & D. R. Anderson, 2002. Model selection and multimodel inference: an information theoretic approach. Springer, New York.
Butler, R. W., 1994. Population regulation of wading ciconiiform birds. Colonial Waterbirds 17: 189–199.
Butler, S. J. & S. Gillings, 2004. Quantifying the effects of habitat structure on prey detectability and accessibility to farmland birds. Ibis 146: 123–130.
Caruso, T., Y. Chan, D. C. Lacap, M. C. Y. Lau, C. P. McKay & S. B. Pointing, 2011. Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. The ISME Journal 5: 1406–1413.
Cooper, W. E. & W. G. Frederick, 2007. Optimal time to emerge from refuge. Biological Journal of the Linnean Society 91: 375–382.
Costa, W. J. E. M., 1998. Phylogeny and classification of Rivulidae revisited: origin and evolution of annualism and miniaturization in rivulid fishes (Cyprinodontiformes: Aplocheiloidei). Journal of Comparative Biology 3: 33–94.
Costello, M. J., 1990. Predator feeding strategy and prey importance: a new graphical analysis. Journal of Fish Biology 36: 261–263.
Dallas, T., 2014. Metacom: an R package for the analysis of metacommunity structure. Ecography 37: 402–405.
Dallas, T., 2015. Metacom: analysis of the “elements of metacommunity structure.” http://cran.r-project.org/package=metacom].
Donatelli, R. J., S. R. Posso & M. C. B. Toledo, 2014. Distribution, composition and seasonality of aquatic birds in the Nhecolândia sub-region of South Pantanal, Brazil. Brazilian Journal of Biology 74: 844–853.
Eastman, J., 1999. Birds of lake pond and marsh: water and wetland birds of eastern North America. Stackpole Books, Mechanicsburg.
Erize, F., J. R. R. Mata & M. Rumboll, 2006. Birds of South America. Princeton University Press, Oxford.
Eveleigh, E. S., K. S. McCann, P. C. McCarthy, S. J. Pollock, C. J. Lucarotti, B. Morin, G. A McDougall, D. B. Strongman, J. T. Huber, J. Umbanhowar & L. D. B. Faria, 2007. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proceedings of the National Academy of Sciences of the United States of America 104: 16976–16981.
Fernandes, C. C., 1997. Lateral migration of fishes in Amazon floodplains. Ecology of Freshwater Fish 6: 36–44.
Fernandes, I. M., F. A. Machado & J. Penha, 2010. Spatial pattern of a fish assemblage in a seasonal tropical wetland: effects of habitat, herbaceous plant biomass, water depth, and distance from species sources. Neotropical Ichthyology 8: 289–298.
Fortin, D., H. L. Beyer, M. S. Boyce, D. W. Smith, T. Duchesne & J. S. Mao, 2005. Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86: 1320–1330.
Froneman, A., M. J. Mangnall, R. M. Little & T. M. Crowe, 2001. Waterbird assemblages and associated habitat characteristics of farm ponds in the Western Cape, South Africa. Biodiversity & Conservation 10: 251–270.
Gauch, H. G., 1982. Multivariate Analysis in Community Ecology. Cambridge University Press, Cambridge.
Gawlik, D. E., 2002. The effects of prey availability on the numerical response of wading birds. Ecological Monographs 72: 329–346.
Gibb, H. & C. L. Parr, 2013. Does structural complexity determine the morphology of assemblages? An experimental test on three continents. Plos One 8: e64005.
Gonçalves, H. C., M. A. Mercante, E. T. Santos, J. R. Godoy, G. Petts & J. Salo, 2011. Hydrological cycle. Brazilian Journal of Biology 71: 241–253.
Gotelli, N. J. & A. M. Ellison, 2004. A Primer of Ecological Statistics. Sinauer Associates, Sunderland.
Gotelli, N. J. & G. L. Entsminger, 2001. Swap and fill algorithms in null model analysis: rethinking the Knight’s Tour. Oecologia 129: 281–291.
Gotelli, N. J. & D. J. McCabe, 2002. Species co-occurrence: A meta-analysis of J. M. Diamond’s assembly rules model. Ecology 83: 2091–2096.
Goulding, M., M. L. Carvalho & E. G. Ferreira, 1988. Rio Negro, Rich Life in Poor Water. SPB Academic Publishing, The Hague.
Gunnarsson, B., 1992. Fractal dimension of plants and body size distribution in spiders. Functional Ecology 6: 636–641.
Haas, K., U. Köhler, S. Diehl, P. Köhler, S. Dietrich, S. Holler, A. Jaensch, M. Niedermaier & J. Vilsmeier, 2007. Influence of fish on habitat choice of water birds: a whole system experiment. Ecology 88: 2915–2925.
Hafner, H., 1997. Ecology of wading birds. Colonial Waterbirds 20: 115–120.
Halekoh, U. & S. Højsgaard, 2014. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models–the R package pbkrtest. Journal of Statistical Software 59: 1–32.
Hammond, J. I., B. Luttbeg & A. Sih, 2007. Predator and prey space use: dragonflies and tadpoles in an interactive game. Ecology 88: 1525–1535.
Henriques-Silva, R., Z. Lindo & P. R. Peres-Neto, 2013. A community of metacommunities: exploring patterns in species distributions across large geographical areas. Ecology 94: 627–639.
HilleRisLambers, J., P. B. Adler, W. S. Harpole, J. M. Levine & M. M. Mayfield, 2012. Rethinking community assembly through the lens of coexistence theory. Annual Review of Ecology, Evolution, and Systematics 43: 227–248.
Hixon, M. A. & B. A. Menge, 1991. Species diversity: prey refuges modify the interactive effects of predation and competition. Theoretical Population Biology 39: 178–200.
Hubbel, S. P. & R. B. Foster, 1986. Biology, chance and history and the structure of tropical rain forest tree communities. In Diamond, J. M. & T. J. Case (eds), Community Ecology. Harper & Row, New York: 314–329.
Jakubas, D. & B. Manikowska, 2011. The response of grey herons Ardea cinerea to changes in prey abundance. Bird Study 58: 487–494.
Jocque, M., B. Vanschoenwinkel & L. Brendonck, 2010. Anostracan monopolisation of early successional phases in temporary waters? Fundamental and Applied Limnology/Archiv für Hydrobiologie 176: 127–132.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Junk, W. J., C. J. Silva, C. N. Cunha & K. M. Wantzen, 2011. The Pantanal: Ecology, biodiversity and sustainable management of a large neotropical seasonal wetland. Pensoft Publishers, Sofia.
Kameda, K., K. Koba, S. Hobara, T. Osono & M. Terai, 2006. Pattern of natural 15 N abundance in lakeside forest ecosystem affected by cormorant-derived nitrogen. Hydrobiologia 567: 69–86.
Keppel, G., Y. M. Buckley & H. P. Possingham, 2010. Drivers of lowland rain forest community assembly, species diversity and forest structure on islands in the tropical South Pacific. Journal of Ecology 98: 87–95.
Kloskowski, J. & A. Trembaczowski, 2015. Fish reduce habitat coupling by a waterbird: evidence from combined stable isotope and conventional dietary approaches. Aquatic Ecology 49: 21–31.
Koenker, R., 2015. quantreg: Quantile regression. R package version 5.11. http://CRAN.R-project.org/package=quantreg].
Kraft, N. J. B., P. B. Adler, O. Godoy, E. C. James, S. Fuller & J. M. Levine, 2014. Community assembly, coexistence and the environmental filtering metaphor. Functional Ecology 29: 592–599.
Kushlan, J. A., 1976. Wading bird predation in a seasonally fluctuating pond. The Auk 93: 464–476.
Lanés, L. E. K., F. W. Keppeler & L. Maltchik, 2014. Abundance variations and life history traits of two sympatric species of Neotropical annual fish (Cyprinodontiformes : Rivulidae) in temporary ponds of southern Brazil. Journal of Natural History 48: 1971–1988.
Leibold, M. A. & G. M. Mikkelson, 2002. Coherence, species turnover, and boundary clumping: elements of meta-community structure. Oikos 97: 237–250.
Lowe-Mcconnell, R., 1987. Ecological Studies in Tropical Fish Communities. Cambridge University Press, London/New York.
Magoulick, D. D. & R. M. Kobza, 2003. The role of refugia for fishes during drought: a review and synthesis. Freshwater Biology 48: 1186–1198.
Maly, E. J. & M. P. Maly, 1997. Predation, competition, and co-occurrences of Boeckella and Calamoecia (Copepoda: Calanoida) in Western Australia. Hydrobiologia 354: 41–50.
Master, T. L., J. K. Leiser, K. A. Bennett, J. K. Bretsch & H. J. Wolfe, 2005. Patch selection by snowy egrets. Waterbirds 28: 220–224.
McAbendroth, L., P. M. Ramsay, A. Foggo, S. D. Rundle & D. T. Bilton, 2005. Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 111: 279–290.
McCann, K. S., 2011. Food Webs. Princeton University Press, New Jersey.
Mendonça, L. B., E. V. Lopes & L. Anjos, 2009. On the possible extinction of bird species in the Upper Paraná River floodplain, Brazil. Brazilian Journal of Biology 69: 747–755.
Morisita, M., 1971. Composition of the I-index. Researches on Population Ecology 13: 1–27.
Nakano, S. & M. Murakami, 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences 98: 166–170.
Nakazawa, M. 2014. fmsb: Functions for medical statistics book with some demographic data. R package version 0.5.1. http://CRAN.R-project.org/package=fmsb.
Nystrand, O. & A. Granström, 1997. Post-dispersal predation on Pinus sylvestris seeds by Fringilla spp: ground substrate affects selection for seed color. Oecologia 110: 353–359.
Oksanen, J., 2009. Ordination and analysis of dissimilarities: Tutorial with R and vegan. http://www.bio.utk.edu/fesin/msa2009/R/veganbird.pdf.
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2009. Vegan: community ecology package. http://CRAN.R-project.org/package=vegan.
Ortega, J. C. G., R. M. Dias, A. C. Petry, E. F. Oliveira & A. A. Agostinho, 2015. Spatio-temporal organization patterns in the fish assemblages of a neotropical floodplain. Hydrobiologia 745: 31–41.
Osorio, D., J. Terborgh, A. Alvarez, H. Ortega, R. Quispe, V. Chipollini & L. C. Davenport, 2011. Lateral migration of fish between an oxbow lake and an Amazonian headwater river. Ecology of Freshwater Fish 20: 619–627.
Pazin, V. F. V., W. E. Magnusson, J. Zuanon & F. P. Mendonça, 2006. Fish assemblages in temporary ponds adjacent to “terra-firme” streams in Central Amazonia. Freshwater Biology 51: 1025–1037.
Pérez-García, J. M., E. Sebastián-González, K. L. Alexander, J. A. Sánchez-Zapata & F. Botella, 2014. Effect of landscape configuration and habitat quality on the community structure of waterbirds using a man-made habitat. European Journal of Wildlife Research 60: 875–883.
Polacik, M. & M. Reichard, 2010. Diet overlap among three sympatric African annual killifish species Nothobranchius spp. from Mozambique. Journal of Fish Biology 77: 754–768.
Polis, G. A. & K. O. Winemiller (eds), 1996. Food Webs: integration of Patterns and Dynamics. Chapman and Hall, New York.
Powell, G. V. N., 1987. Habitat use by wading birds in a subtropical estuary: implications of hy-drography. The Auk 104: 740–749.
Presley, S. J., C. L. Higgins & M. R. Willig, 2010. A comprehensive framework for the evaluation of metacommunity structure. Oikos 119: 908–917.
Pyke, G. H., 1984. Optimal foraging theory: a critical review. Annual Review of Ecology Evolution and Systematics 15: 523–575.
Rasband, W.S., 2015. ImageJ. U. S. National Institutes of Health, Maryland. http://imagej.nih.gov/ij/.
Ricklefs, R. E., 2004. A comprehensive framework for global patterns in biodiversity. Ecology Letters 7: 1–15.
Rooney, N., K. S. McCann & J. C. Moore, 2008. A landscape theory for food web architecture. Ecology Letters 11: 867–881.
Rosindell, J., S. P. Hubbell, F. He, L. J. Harmon & R. S. Etienne, 2012. The case for ecological neutral theory. Trends in Ecology and Evolution 27: 203–208.
Salewski, V. & B. Bruderer, 2007. The evolution of bird migration–A synthesis. Naturwissenschaften 94: 268–279.
Sick, H., 1997. Ornitologia Brasileira. Editora Nova Fronteira, Rio de Janeiro.
Spiller, D. A. & T. W. Schoener, 1998. Lizards reduce spider species richness by excluding rare species. Ecology 79: 503–516.
Stanton, R. A., D. C. Kesler & F. R. Thompson, 2014. Resource configuration and abundance affect space use of a cooperatively breeding resident bird. The Auk 131: 407–420.
Stevens, A. N. P., 2010. Dynamics of predation. Nature Education Knowledge 3(10):46. http://www.nature.com/scitable/knowledge/library/dynamics-of-predation13229468].
Telleria, J. L. & L. M. Carrascal, 1994. Weight-density relationships between and within bird communities: implications of niche space and vegetation structure. The American Naturalist 143: 1083–1092.
Tilman, D., 2004. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proceedings of the National Academy of Sciences of the United States of America 101: 10854–10861.
Tondato, K. K., I. Fantin-cruz, O. C. Pedrollo & Y. R. Súarez, 2013. Spatial distribution of fish assemblages along environmental gradients in the temporary ponds of Northern Pantanal, Brazil. Journal of Limnology 72: 95–102.
Ulrich, W. & N. J. Gotelli, 2007. Disentangling community patterns of nestedness and species co-occurrence. Oikos 116: 2053–2061.
Van Perlo, B., 2009. A field Guide to the Birds of Brazil. Oxford University Press, New York.
Weller, M. W., 1999. Wetland Birds: habitat Resources and Conservation Implications. Cambridge University Press, Cambridge.
Whittingham, M. J. & K. L. Evans, 2004. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 146: 210–220.
Whittingham, M. J., S. J. Butler, J. L. Quinn & W. Cresswell, 2004. The effect of limited visibility on vigilance behaviour and speed of predator detection: implications for the conservation of granivorous passerines. Oikos 106: 377–385.
Winemiller, K. O. & D. B. Jepsen, 1998. Efects of seasonality and fish movement on tropical river food webs. Journal of Fish Biology 53: 267–296.
Wourms, J. P., 1972. The developmental biology of annual fishes III: pre embryonic and embryonic diapause of variable duration in the eggs of annual fishes. Journal of Experimental Zoology 182: 389–414.
Yasué, M., J. L. Quinn & W. Cresswell, 2003. Multiple effects of weather on the starvation and predation risk trade-off in choice of feeding location in Redshanks. Functional Ecology 17: 727–736.
Acknowledgment
This study was designed and developed during the Pantanal Ecology course (Ecologia do Pantanal—ECOPAN) of 2013. Thus, we would like to thank all organizers, professors, sponsors (UFMS, MMX, Fundect, Capes, CNPq, Instituto Homem Pantaneiro, Embrapa Pantanal, PPG Ecologia e Conservação), and participants of the ECOPAN 2013 for field logistics, suggestions, and team play. We also thank Elaine Corrêa for all help in the field work and Paulo Henrique Araujo and Francisco Severo Neto for bird and fish identification. The first author would like to thank CAPES/UFRGS for the master’s degree scholarship, and CNPq/UEM and CAPES/Texas A&M for PhD scholarships. We also thank the editor and the anonymous reviewers for improving the manuscript with comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Ivan Nagelkerken
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keppeler, F.W., Cruz, D.A., Dalponti, G. et al. The role of deterministic factors and stochasticity on the trophic interactions between birds and fish in temporary floodplain ponds. Hydrobiologia 773, 225–240 (2016). https://doi.org/10.1007/s10750-016-2705-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2705-y