Abstract
IPCC predictions indicate an increase in temperatures by 1.5–7°C in some Amazonian regions during the twenty-first century. These changes could disrupt the present distribution patterns of organisms, including wetland plant species. In this work, we determined in microcosms the effects of scenarios combining elevated temperature and atmospheric CO2 concentration on the germination and initial growth of the arborescent Amazonian aquatic macrophyte Montrichardia arborescens. Seeds were germinated, and seedlings produced were monitored over a 5-month period in four microcosms: Control: ambient temperature and CO2 level; Mild: Control + 1.5°C and + 200 ppm CO2; intermediate: control + 2.5°C and + 400 ppm CO2; Extreme: Control + 4.5°C and + 850 ppm of CO2. Rapid light response curves and Fv/Fm values taken in seedlings showed a decrease in electron transportation rate with CO2 and temperature elevation. Mild and Intermediate treatments stimulated biomass production; Extreme treatment and Control produced similar results. The severe climatic changes expected in the future may negatively influence carbon accumulation in M. arborescens. Since aquatic macrophytes in Amazonian wetlands and wetlands worldwide are key plant species, further studies are needed to predict their fate in a global change perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Average global surface temperatures are projected to increase by 1.5–5.8°C over the twenty-first century. In the Amazon Basin, an increase in temperatures by ca. 1.5–7.0°C is expected in some areas (IPCC, 2013). Over the decade 1984–1993, the rate of CO2 increase was on average 1.5 µmol/mol/year and that trend will probably continue over the current century, resulting in concentrations approaching 560 µmol/mol, twice the pre-industrial value by the middle or second half of the twenty-first century (Schimel et al., 2000). The steadily increasing human population and economic activities are likely to lead to even higher CO2 concentrations (approximately 700 µmol/mol) by the end of the XXI century. Projected increases in mean temperature are expected to greatly disrupt present patterns of plant and animal distributions. These climatic changes would reduce plant water availability and thereby increase drought stress for many Amazonian species (Piedade et al., 2013). Moreover, large changes in the patterns of warming, wind, and rainfall, which will entail extreme weather events, will directly affect the duration and intensity of flooding and the dry season and fire events in Amazonian wetlands (Cochrane & Laurance, 2002; IPCC, 2007, 2013; Nobre et al., 2007).
The Amazonian rainforest is one of the main storage compartments of terrestrial carbon (Turcq et al., 2002), with estimated values of 74–127 Mg C/ha/year in soil stock and 148–180 Mg C/ha/year in above ground biomass (Malhi et al., 2009a). The region is also responsible for the annual variation in carbon sequestration rates (Turcq et al., 2002), since it is influenced by climate variations, especially during the rainy season when the occurrence of El Niño may reduce the radiation quantity during dry years, due to the elevation of the temperature of the tropical North Atlantic Ocean, leading to the occurrence of more fire events and contributing to the increase in carbon emissions (Tian et al., 1998; Lewis et al., 2011; Vasconcelos et al., 2013). Extrapolated over the whole basin, CO2 flux from flooded areas was found to be of comparable magnitude to some estimates of carbon sequestration in upland forests (Hess et al., 2003). Net primary production (NPP) of aquatic herbaceous plants in Amazonian floodplains varies between 30 and 50 t/ha/year (Junk & Piedade, 1993), contributing substantially to the carbon budget of the region (Piedade et al., 2001). The decomposition of herbaceous plants promotes the enrichment of the aquatic system and associated floodplains, during both the aquatic and terrestrial phases of the hydrologic cycle (Piedade et al., 2001, 2010).
Temperature is one of the most important climatic factors affecting the distribution range of many species, including aquatic and wetland plants. It affects their physiology, seed germination, periodicity and rate of seasonal growth, and onset of dormancy (Short & Neckles, 1999). On the other hand, rising levels of atmospheric CO2 are predicted to have significant direct effects on global vegetation (Bowes, 1993), including aquatic plant communities (Wetzel & Grace, 1983; Edwards, 1995). Despite the fact that nature of the physiological adaptations may vary greatly between species according to the genetic load as a function of the availability of environmental resources (Pepper et al., 1998), typical responses to short-term CO2 enrichment include increases in photosynthesis, growth, total biomass, root/shoot ratio, and tissue carbon/nitrogen ratio (Short & Neckles, 1999). In Amazonian floodplain forest trees, the annual changes in water level owing to the flood pulse (Junk et al., 1989) influence parameters directly responsible for obtaining daily carbon, such as photosynthetic capacity and quantum efficiency and the uptake and use of nutrients in soil, which influences primary metabolism (Parolin, 2001; De Simone et al., 2002). Thus, the increase in CO2 concentration and temperature associated with changes in the flooding regime and precipitation are expected to affect the ecophysiological responses and carbon uptake of plant species colonizing floodplain ecosystems (Piedade et al., 2013).
Analyzing the combined effects of CO2 and temperature on plants is important because changes in CO2 concentrations are global, and different ecosystems in very different temperature regimes will be affected, irrespective of any past or future changes in temperature (Morison & Lawlor, 1999). The effect of the interaction of increased temperature and CO2 concentration in plants has been discussed by many authors (see Morison & Lawlor, 1999), but its effect on aquatic macrophytes has been poorly investigated. However, a huge variety of aquatic plants colonize the Amazonian floodplains, estimated at 388 species alone for central Amazonia (Junk & Piedade, 1997). Rooted aquatic macrophytes, such as Montrichardia arborescens (L.) Schott (Araceae), are subject to typical stresses of the aquatic environment, such as waves, oxygen shortage, instability of the substrate, and also the periodic variation of the water level which causes drastic changes in the environment (Piedade & Junk, 2000). Montrichardia arborescens occurs in the fertile white-water várzea and the poor acidic igapó flooded forests (Lopes et al., 2014). Although being an aquatic emergent macrophyte, M. arborescens occupies a position along the floodplain more related to the first succession stage of pioneer trees and shrubs with which it may compete due to its arboreal habit (Lopes et al., 2015). The climate change studies on emergent macrophytes are important owing to their crucial role in the ecosystem functioning in aquatic habitats (Ojala et al., 2002).
The aim of this study was to determine the responses of an Amazonian aquatic macrophyte, M. arborescens, in scenarios with substantial changes in temperature and atmospheric CO2 concentrations according to the predictions of the IPCC (2007). Our study was designed to evaluate the responses of this species to four different scenarios of CO2 concentration and temperature to characterize the potential effect of a CO2-enriched atmosphere on germination and seedling establishment. We aimed to determine whether climatic changes would interfere with responses of germination and growth parameters and photosynthesis. Our hypothesis was that moderate elevation of temperature and CO2 should stimulate seed germination and seedling growth, while high concentrations could inhibit germination and growth of seedlings.
Methods
Species investigated
Montrichardia arborescens, known as aninga, has a broad distribution along the river shores in the Amazon Basin, forming monospecific stands at the edges of floodplain forests (Lopes et al., 2015). The species can also be found in high densities in Central America and in isolated spots in the states of Minas Gerais and Bahia (Brazil) (http://splink.cria.org.br). In the Amazon Basin, M. arborescens may be found in nutritionally rich environments, such as the white-water rivers (várzeas), but especially in poor environments, such as black-water rivers (igapós) and in the brackish estuarine areas of the Amazon River (Lopes et al., 2015).
Mature infructescences of M. arborescens were collected in April 2012 from eight individuals (one infructescence/individual) approximately 100 m apart along the Negro River, the biggest black-water river in Central Amazonia (Sioli, 1984), in the locality of Praia Grande, Iranduba (Amazonas, Brazil). The pulp of the fruits was removed, and 200 similarly healthy seeds were chosen for the experiments in the microcosms. The seeds were placed in plastic pots (8 cm in height and 8 cm in diameter) with a vermiculite substrate, with one seed per pot. Trays with water were placed beneath the pots to maintain a high moisture level in the substrate. Each treatment had 50 replicates. The experiment was conducted from April 4 to September 13, 2012 (162 days).
Microcosm experiment
The microcosm was set up at the Laboratory of Genetics and Molecular Evolution—LEEM, INPA/Manaus (AM, Brazil). The microcosm itself consisted of four climatic chambers subjected to a daily 12-h photoperiod regime (12 h light and 12 h dark), and independently controlled humidity, temperature, and CO2 concentrations [Control (environmental), Mild, Intermediate and Extreme] (Table 1). The climatic conditions of the rooms as described above were programmed according to the scenarios forecasted for 2100 by the IPCC (2007). Since in the natural environment, temperature and CO2 vary throughout the day, the rooms were designed to simulate these fluctuations in the enriched rooms over 24 h. Therefore, the Control room mimicked in real time the current conditions of CO2 level, temperature, and humidity in the surrounding forested environment (Table 1).
Parameters measured
Germination, considered the protrusion of the radicle, was evaluated daily from the beginning of the experiment, and the number of seedlings produced was calculated for each treatment at the end of the experiment. The following parameters were evaluated and calculated: the percentage of germination (G%), initial time of germination (ITG), and final time of germination (FTG). Median time of germination (t) was calculated as \(\bar{t} = \sum\nolimits_{i = 1}^{k} {n_{i} t_{i} } /\sum\nolimits_{i = 1}^{k} {n_{i} },\) where t i is time from the start of the experiment to the ith observation (day); n i is number of seeds germinated in time i (not the accumulated number, but the number corresponding to the ith observation); and k is last time of germination (Labouriau, 1983). Germination speed index (GSI) was determined as GSI = (G1/N1) + (G2/N2) + (G3/N3) \(+\, {\cdots} \,+\) (Gn/Nn), where G1, G2, G3,…, Gn = number of germinated seeds in the first, second, third, and last count; and N1, N2, N3,…, Nn = number of days at first, second, third, and last count (Maguire, 1962) of number of seeds germinated. In the seedlings produced in the microcosms at the end of the experiment, morphological and physiological traits were measured as described below.
-
(1)
Morphological traits: number of leaves, length of roots and shoot (cm), total biomass (shoot plus root; g); seed biomass.
-
(2)
Physiological traits:
-
2.1
The content of chlorophyll a, chlorophyll b, and total chlorophyll of leaves was measured with a portable Clorofilog (Falker, Brazil). Chlorophyll was measured on the abaxial and adaxial surfaces of one leaf per plant. The chlorophyll index was converted to chlorophyll (µmol/mm2) by the equations: total chlorophyll = 1.64 X Clorofilog index + 17.00, R 2 = 0.88; chlorophyll a = y = 0.81 X Clorofilog index—11.65, R 2 = 0.72; and chlorophyll b = 0.25 X Clorofilog index +1.38, R 2 = 0.90 (Lopes et al., 2015)
-
2.2
The maximal photochemical yield of PSII was measured in ten plants in each room. The ‘current photochemical capacity’ of PSII (Bolhár-Nordenkampf & Öquist, 1993) or ‘intrinsic efficiency’ (Maxwell & Johnson, 2000) was assessed with a Mini-PAM (Walz, Effeltrich, Germany). The maximal photochemical yield (Y = Fv/Fm) was measured in dark-adapted leaves (10 min), when a saturation pulse induces maximal fluorescence yield (Fm) and maximal variable fluorescence (Fv), which are considered reliable measures of the potential quantum yield of photosystem II. In dark-adapted leaves (all reaction centers of the electron transport chain oxidized), Fv/Fm is a parameter for measuring the maximum relative electron transport rate of PSII (Krause & Weis, 1991). Healthy leaves usually show Fv/Fm values between 0.66 and 0.80, while lower values indicate intracellular or physiological changes (calculated according to Bolhár-Nordenkampf & Götzl, 1992 and Waldhoff et al., 2000 for several common floodplain species).
-
2.3
Rapid light curves (RLC) in three plants in each treatment were additionally carried out. This measure shows the photosynthetic electron transport rate (ETR). Responses to short-term irradiance were measured using increasing irradiation in nine steps within 3 min, with each irradiation period lasting 10 s.
-
2.1
Statistical analyses
ANCOVA was used to evaluate the effect of the treatments on morphological traits, with the time available for plant’s growth as co-variable. ANOVA was used to evaluate the effect of the treatments on physiological traits and germination parameters. The differences between the factors were estimated a posteriori by the Tukey test. The non-parametric Kruskal–Wallis test was applied when at least one of the assumptions of ANOVA was not met, followed by of the Dunnett test. The statistical analyses were performed with Systat 12 (SYSTAT, 2007).
Results
During the experimental period, the climatic conditions in the microcosms varied in response to outside-room ambient changes. The CO2 concentration varied between 350 and 500 ppm in the control treatment, 550–680 in the Mild treatment, 770–890 in the Intermediate treatment, and 1200–1330 in the Extreme treatment (Table 2). The temperature varied between 26 and 28°C in Control, 28–30°C in Mild, 29–31°C in Intermediate, and 31–33°C in Extreme treatments (Table 2). The humidity varied between 69 and 77% in all treatments during the experiment period (Table 2).
The elevation in CO2 concentration together with increased temperature did not exert significant effects on any of the germination parameters analyzed (Table 3). The median time of germination tended to be shorter with increasing CO2 and temperature in treatments, but differences were not significant (F 15 3 = 2.82, P = 0.075). The maximum time for germination was 163 days in the control treatment, and the minimum was 44 days in the control, intermediate, and extreme treatments.
The seedling morphology did not change with CO2 and temperature elevation. The number of leaves was similar between the treatments with averages ranging from 1.7 to 2.0 leaves. A higher number of leaves occurred in the control, 4 leaves, and the other treatments showed a maximum of 3 leaves, but this difference occurred in response to the time of growth of each individual. There was an effect on elongation of the roots in response to the elevation in CO2 and temperature and the time of growth (Table 4; Fig. 1), although this effect was not reflected in root biomass (Table 4). There were no effects of the treatments on shoot length (Table 4), but there was an increase in shoot biomass with increase in CO2 and temperature (Table 4). The root/shoot ratio did not change with treatments (Table 4). Total biomass was influenced by the treatments and time (Table 4; Fig. 2). The treatments with mild and intermediate conditions showed higher values of total biomass than did the control (P < 0.05), whereas the treatment with extreme conditions showed values similar to the control (P > 0.05).
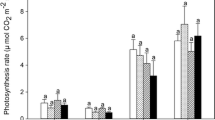
The physiological parameters were affected by CO2 and temperature. Fv/Fm was lower in the Extreme than Intermediate treatment (Table 4; Fig. 3); the time of growth did not influence this parameter. RLC of leaves in the control, Mild, and Intermediate treatments showed a maximum ETR of 6.3, 6.5, and 5.0 µmol, respectively, at approximately 200 PPFD, and the Extreme treatment showed a maximum ETR of 4.5 at approximately 100 PPFD and slightly decreasing ETR at higher light intensities (Fig. 4). ETR was related to treatment (ANCOVA; F = 6.16, P < 0.001), with the Extreme treatment showing lower values than the other treatments.
The total chlorophyll measured on the abaxial and adaxial sides of the leaf did not show significant differences between the treatments (P > 0.05), as well as the ratio Chl a/Chl P (P > 0.05). The minimum value of total chlorophyll on the adaxial side was 19.9 μmol/mm2 in the intermediate treatment, and the maximum was 42.7 μmol/mm2 in the control (Table 5).
Discussion
Higher CO2 concentrations and increased temperature influenced the initial growth of M. arborescens in our experiments. Plants exposed to the highest CO2 concentrations and temperatures (extreme treatment) showed a lower accumulation of biomass and lesser electron transportation in the leaves compared to the Mild and Intermediate treatments.
The faster development of plants at warmer temperatures may result in substantial shortening of the growth period in some annual species. This results in less time for carbon fixation and biomass accumulation (Rawson, 1992; Wheeler et al., 1996; Bowes, 1996). Most studies of terrestrial plants have revealed that an increase in CO2 level generally causes greater increases in growth at higher temperatures, beyond the optimal temperatures (Chen et al., 1994). In the present study, the exposure of M. arborescens to the Extreme treatment with the highest conditions of temperature and CO2 showed no differences in germination parameters and total biomass when compared with the Control treatment. However, plants in the Mild and Intermediate treatments showed higher values of biomass compared to the control, indicating that the temperature and CO2 concentration in the Extreme treatment were higher than optimal for M. arborescens.
Studies with aquatic macrophytes analyzing the effects of the interaction of elevated CO2 and temperature are still scant. Idso et al. (1987) demonstrated that the relative increase in net photosynthesis of Azolla pinnata var. pinnata and Eichhornia crassipes due to CO2 enrichment was higher during the spring and fall than during winter. Allen et al. (1990) reported that there were significant interactive effects of CO2 and temperature on photosynthesis in Nymphaea marlic. Chen et al. (1994) showed that the relative increase in Hydrilla verticillata growth by 700 ppm of CO2 was less at 32°C than 15 and 25°C. In our study, M. arborescens showed similar results with a decline in total biomass at 31–33°C and around 1200 ppm of CO2 compared to treatments with lower temperature and CO2 concentration, indicating that the analyses of the scenario considering both CO2 and temperature are more appropriate, since these effects in nature are combined. Indeed, Idso et al. (1987) and Chen et al. (1994) demonstrated that the effect of CO2 enrichment is strongly temperature-dependent.
Working with seedlings of Hymenaea courbaril, L. Aidar et al. (2002) noted that when reserves were mobilized and growth occurred primarily at the expense of an internal source of carbon (cotyledons), the effect of elevated CO2 in the external environment was significantly less. In M. arborescens, like in other monocotyledons, cotyledons are highly modified leaves with a scutellum specialized to absorb stored reserves from the endosperm, and a coleoptile, which serves as a protective cap that covers the part that will become the stem and leaves of the plant. This could influence the effect of CO2 on the growth of the species. Since the seed remained fixed to the seedlings in all plants germinated in the microcosms, this may eventually mitigate the effect of the external elevation of CO2 level. Another explanation for the absence of effect of treatment on germination parameters could be the combined effect of CO2 and temperature. The rise in temperature leads to an increase in respiration rate, affecting the germination capacity, since temperature accelerates biochemical reactions and metabolism of seeds (Perez-Garcia & Gonzalez-Benito, 2006). This accelerates the breakdown of reserves stored in the supporting tissues, which are more rapidly transported and assimilated in the embryonic axis. As a result, the increase in CO2 concentration reduces metabolic activity and favors the conservation of seeds of tropical forest species and crops for longer periods (Khurana & Singh, 2001). This occurs because increasing CO2 levels reduce metabolic activity and sharply lower the deterioration and aging of seeds, favoring the maintenance of quality during storage as stated for soybean seeds by several authors (Ainsworth et al., 2002; Schmidt, 2007). On the other hand, higher temperatures increase metabolic rates in early germination (Carvalho & Nakagawa, 2000).
In contrast to reports in the literature of higher CO2 concentrations causing an increase in plant height in comparison to the control (Poorter, 1993), in the present study, M. arborescens did not show differences in this parameter between treatments with different CO2 levels. Ojala et al. (2002) found a reduction in shoot length of the emergent macrophyte Equisetum fluviatile in response to CO2 elevation, and an increase in response to temperature elevation, but a non-significant effect when evaluating the interaction between temperature and CO2 concentration. Therefore, it is important to consider the joint effect of factors such as increased temperature and CO2 enrichment on plant growth in future climate scenarios.
Effects of elevated CO2 on carbon allocation between roots and shoots are variable (Bazzaz, 1990; Rogers et al., 1983), but the general conclusion of studies on root growth under elevated CO2 concentration is that the root mass increases (Norby, 1994). As described for E. fluviatile during 5 months of observation (Ojala et al., 2002), in M. arborescens, only a discrete increase in root growth took place when CO2 concentration was 600–800 ppm, coupled with elevated temperature.
The measurements of RLC in leaves exposed to different levels of CO2 and temperature showed that the potential for photosynthetic activity of PSII decreased when the CO2 concentration and temperature were very high, as occurred in the Extreme treatment. Relative ETR is an approximation of the rate of electrons pumped through the photosynthetic chain (Beer et al., 2001), and can provide a reliable assessment of photosynthetic activity, by integrating the leaf’s ability to tolerate light fluctuation, as well as reflecting its immediate short-term light history (Schreiber et al., 1997; White & Critchley, 1999). Therefore, it can be assumed that the rates of M. arborescens photosynthesis were also reduced in the higher CO2 and temperature treatments. Wang et al. (2012) concluded from a meta-analysis study that the increase in Fv/Fm of herbaceous and crop species with elevated CO2 concentration only occurs at an elevated temperature, but no effect on woody and non-crop species was reported. The Fv/Fm values of M. arborescens were between 0.69 and 0.86, indicating that the leaves were healthy, using the standard values established for aquatic macrophytes (Koch et al., 2007). However, the Extreme treatment showed a significant reduction of approximately 3.0% in Fv/Fm values together with a reduction in ETR of about 60%, indicating that the photosynthetic apparatus may be damaged under such conditions. This tendency probably could be confirmed with increasing time of exposure of plants to Extreme treatment.
Although seedlings of M. arborescens responded by increasing the parameters evaluated when subjected to the Mild and Intermediate treatments (600–800 ppm CO2), when in the Extreme treatment (1200 ppm), there was a decline in carbon incorporation. These results are consistent with observations by Hartz-Rubin & DeLucia (2001) who recorded an increase in biomass in 14 species of herbaceous plants enriched with CO2 between 370 and 800 ppm. However, as found for wheat by Grotenhuis & Bugbee (1997) and also in M. arborescens in this study, when CO2 enrichment was above 900 ppm, a decrease in plant biomass takes place. At higher CO2 concentrations, the photosynthesis rate also decreases as demonstrated in cotton (Reddy et al., 1995) and rice (Baker et al., 1990). However, this response is in contrast to that of the emergent macrophyte E. fluviatile, in which maximum biomass was influenced by increase in temperature and not by higher CO2 levels (600–700 ppm) or by increase in temperature together with CO2. The different responses of M. arborescens and E. fluviatile may be explained by the morphology of these species. While M. arborescens is an arborescent plant, tall and with big leaves, E. fluviatile has slender side branches with scale leaves.
In the Amazon floodplain, with low nutrient levels, the quantity of floating plants and herbaceous shoreline vegetation is low (Piedade et al., 2010). In extremely nutrient-poor and acidic rivers, aquatic macrophytes may only be represented by small patchy stands of M. arborescens along the margins of the floodplain forest (Junk & Howard-Williams, 1984). Climate models predict that from the middle of this century on, the Amazon rainforest may give way to a cerrado-type vegetation (Oyama & Nobre, 1999; Jenkins et al., 2005) or a semi-deciduous forest (Malhi et al., 2009b). Under such conditions, the Amazonian wetlands may become a refuge for species of rain forests, minimizing the effects of decreased precipitations (Piedade et al., 2013). Our results with M. arborescens indicate that when CO2 level and temperature increased in the range of 600–900 ppm and 28–30°C, plant biomass was stimulated; however, in the Extreme treatment (ca. 1200 ppm and 33°C), biomass values declined due to physiological limitations. Over time, this could lead to smaller populations of the species, which is one of the most abundant aquatic macrophytes in Amazonian floodplains, especially in the igapó system (Lopes et al., 2014). The composition of aquatic macrophytes in the black-water igapó is much lower than that in the white-water várzea counterpart (Piedade et al., 2010). Therefore, if other aquatic plants in the igapó would also decline in growth and biomass under climate change, severe reductions in the number of species and floristic composition of aquatic macrophytes may be expected in these areas. This may lead to an impoverishment of food chains and the carbon budget of this already fragile ecosystem.
Conclusions
No germination parameters of M. arborescens were affected by temperature and CO2 concentration in a microcosm study. However, the initial growth of M. arborescens was influenced, resulting in a higher accumulation of biomass in the seedling subjected to CO2 concentrations in the range of 600–900 ppm and temperatures between 28 and 30°C, but in the Extreme treatment, biomass accumulation was in the same range as that of the control, owing to physiological stress confirmed by Fv/Fm and ETR rates. Therefore, long-term investigations of interactions of CO2 and temperature effects, like the proposed IPCC scenarios, are needed to clarify their effects on Amazonian aquatic macrophytes and the whole ecosystem.
References
Aidar, M. P. M., C. A. Martinez, A. C. Costa, P. M. F. Costa, S. M. C. Dietrich & M. S. Buckeridge, 2002. Effect of atmospheric CO2 enrichment on the establishment of seedlings of jatobá, Hymenaea courbaril L. Biota Neotropica 2: 1–10.
Ainsworth, E. A., P. A. Davey, C. J. Bernacchi, O. C. Dermody, E. A. Heaton, D. J. Moore & S. P. Long, 2002. A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Global Change Biology 8: 695–709.
Allen, S. G., S. B. Idso & B. A. Kimball, 1990. Interactive effects of CO2 and environment on net photosynthesis of water-lily. Agriculture, Ecosystems & Environment 30: 81–88.
Baker, J. T., L. H. Jr & K. J. Boote Allen, 1990. Growth and yield responses of rice to carbon dioxide concentration. Journal of Agricultural Science 115: 313–320.
Bazzaz, F. A., 1990. The response of natural ecosystems to the rising global CO2 levels. Annual Review of Ecology and Systematic 21: 67–96.
Beer, S., M. Björk, R. Gademann & P. J. Ralph, 2001. Measurement of photosynthesis in seagrasses. In Short, F. T. & R. Coles (eds), Global Seagrass Research Methods. Elsevier Publishers, Amsterdam: 183–198.
Bolhár-Nordenkampf, H. R. & M. Götzl, 1992. Chlorophyllfluoreszenz als Indikator der mit Seehöhe zunehmenden Streßbelastung von Fichtennadeln. FBVA Berichte Schriftenreihe der Forstlichen Bundesveranstaltungen 67: 119–131.
Bowes, G., 1993. Facing the inevitable: plants and increasing atmospheric CO2. Annual Review of Plant Physiology and Plant Molecular Biology 44: 309–332.
Bowes, G., 1996. Photosynthetic responses to changing atmospheric carbon dioxside concentration. In Baker, N. R. (ed.), Photosynthesis and the Environment. Kluwer Academic Publisher, Dordrecht: 387–407.
Carvalho, N. M. & J. Nakagawa, 2000. Sementes: Ciência, Tecnologia e Produção. FUNEP, Jaboticabal: 588.
Chen, D. X., M. B. Coughenour, D. Eberts & J. S. Thullen, 1994. Interactive effects of CO2 enrichment and temperature on the growth of dioecious Hydrilla verticillata. Environmental and Experimental Botany 34: 345–353.
Cochrane, M. A. & W. F. Laurance, 2002. Fire as a large-scale edge effect in Amazonian forests. Journal of Tropical Ecology 18: 311–325.
De Simone, O., E. Müller, W. J. Junk & W. Schmidt, 2002. Adaptations of Central Amazon tree species to prolonged flooding: root morphology and leaf longevity. Plant Biology 4: 515–522.
Edwards, A. J., 1995. Impact of climatic change on coral reefs, mangroves, and tropical seagrass ecosystems. In Eisma, D. (ed.), Climate Change: impact on Coastal Habitation. CRC Press, Boca Raton: 209–234.
Grotenhuis, T. P. & B. Bugbee, 1997. Super-optimal CO2 reduces seed yield but not vegetative growth in wheat. Crop Science 37: 1215–1222.
Hartz-Rubin, J. S. & E. H. DeLucia, 2001. Canopy development of a model herbaceous community exposed to elevated atmospheric CO2 and soil nutrients. Physiologia Plantarum 113: 258–266.
Hess, L. L., J. M. Melack, E. M. Novo, C. C. Barbosa & M. Gastil, 2003. Dual-season mapping of wetland inundation and vegetation for the central Amazon basin. Remote Sensing of Environment 87: 404–428.
Idso, S. B., B. A. Kimball, M. G. Anderson & J. R. Mauney, 1987. Effects of atmospheric CO2 enrichment on plant growth: the interactive role of air temperature. Agriculture, Ecosystems & Environment 20: 1–10.
IPCC, 2007: Climate Change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Core Writing Team, Pachauri, R. K & A. Reisinger. (eds), IPCC, Geneva.
IPCC, 2013: Climate change 2013: The physical science basis. In Stocker T. F, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex & P. M. Midgley (eds), Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate change. Cambridge University Press, Cambridge, New York
Jenkins, G., R. Betts, M. Collins, D. Griggs, J. Lowe & R. Wood, 2005. Stabilizing climate to avoid dangerous climate change—a summary of relevant research at the Hadley Centre: Department for Environment Food a Rural Affairs, Met Office Hadley Centre, Exeter: 16
Junk, W. J. & C. Howard-Williams, 1984. Ecology of aquatic macrophytes in Amazonia. In Sioli, H (ed.), The Amazon: limnology and Landscape Ecology of a Mighty Tropical River and its Basin. Dr. W. Junk Publishers, Dordrecht 10: 269–293.
Junk, W. J. & M. T. F. Piedade, 1997. Plant life in the floodplain with special reference to herbaceous plants. In Junk, W. J. (ed.), The Central Amazon Floodplain. Springer, Berlin: 147–185.
Junk, W. J. & M. T. F. Piedade, 1993. Biomass and primary production of herbaceous plants communities in the Amazon floodplain. Hydrobiologia 263: 155–162.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Khurana, E. & J. S. Singh, 2001. Ecology of seed and seedling growth for conservation and restauration of tropical dry forest: a review. Conservation Biology 28: 39–52.
Koch, M. S., S. A. Schopmeyer, C. Kyhn-Hansen, C. J. Madden & J. S. Peters, 2007. Tropical seagrass species tolerance to hypersalinity stress. Aquatic Botany 86: 14–24.
Krause, G. H. & E. Weis, 1991. Chlorophyll fluorescence and photosynthesis: The basics. Annual Review of Plant Biology 42: 313–349.
Labouriau, L. G., 1983. A germinação das sementes. Organização dos Estados Americanos. Programa Regional de Desenvolvimento Científico e Tecnológico. Série de Biologia. Monografia 24.
Lewis, S. L., P. M. Brando, O. L. Phillips, G. M. van der Heijden & D. Nepstad, 2011. The 2010 Amazon drought. Science 331: 554.
Lopes, A., P. Parolin & M. T. F. Piedade, 2015. Morphological and physiological traits of aquatic macrophytes respond to water chemistry in the Amazon Basin: an example of the genus Montrichardia Crueg (Araceae). Hydrobiologia. doi:10.1007/s10750-015-2431-x.
Lopes, A., F. Wittmann, J. Schöngart & M. T. F. Piedade, 2014. Herbáceas aquáticas em seis igapós na amazônia central: composição e diversidade de gêneros. Revista Geográfica Acadêmica 8: 5–17.
Maguire, J. D., 1962. Speed of germination-aid in selection and evaluation of seedling emergence and vigour. Crop Science 2: 176–177.
Malhi, Y., L. E. O. Aragão, D. B. Metcalfe, R. Paiva, C. A. Quesada, S. Almeida & L. M. Teixeira, 2009a. Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Global Change Biology 15: 1255–1274.
Malhi, Y., L. E. O. Aragão, D. Galbraith, C. Huntingford, R. Fisher, P. Zelazowski & P. Meir, 2009b. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proceedings of the National Academy of Sciences 106: 20610–20615.
Maxwell, K. & G. N. Johnson, 2000. Chlorophyll fluorescence-a practical guide. Journal of Experimental Botany 5: 659–668.
Morison, J. & D. Lawlor, 1999. Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell and Environment 44: 659–682.
Nobre, C. A., G. Sampaio & L. Salazar, 2007. Mudanças climáticas e Amazônia. Ciência e Cultura 59: 22–27.
Norby, R. J., 1994. Issues and perspectives for investigating root responses to elevated atmospheric carbon dioxide. Plant and Soil 165: 9–20.
Nordenkampf, H. R. & G. Öquist, 1993. Chlorophyll fluorescence as a tool in photosynthesis research. In Hall, D. O., J. M. O. Scurlock, H. R. Nordenkampf, R. C. Leegood & S. P. Long (eds), Photosynthesis and Production in a Changing Environment. Chapman and Hall, London: 193–206.
Ojala, A., P. Kankaala & T. Tulonen, 2002. Growth response of Equisetum fluviatile to elevated CO2 and temperature. Environmental and Experimental Botany 47: 157–171.
Oyama, M. D. & C. A. Nobre, 1999. A new climate-vegetation equilibrium state for tropical South America. Geophysical Research Letters 30: 2199–2203.
Parolin, P., 2001. Morphological and physiological adjustments to waterlogging and drought in seedlings of Amazonian floodplain trees. Oecologia 128: 326–335.
Pepper, W. J., W. Barbour, A. Sankovski & B. Braaz, 1998. No-policy greenhouse gas emission scenarios: revisiting IPCC 1992. Environmental Science & Policy 1: 289–311.
Perez-Garcia, F. & M. E. Gonzalez-Benito, 2006. Seed germination of five Helianthemum species: Effect of temperature and presowing treatments. Journal of Arid Environments 65: 688–693.
Piedade, M. T. F. & W. J. Junk, 2000. Natural herbaceous plant communities in the Amazon floodplains and their use. In Junk, W. J., J. Ohly, M. T. F. Piedade & M. G. M. Soares (eds), The Central Amazon Floodplain: actual Use and Options for a Sustainable Management. Backhuys Publishers, Leiden: 269–290.
Piedade, M. T. F., W. Junk, S. A. D’Ângelo, F. Wittmann, J. Schöngart, K. M. D. N. Barbosa & A. Lopes, 2010. Aquatic herbaceous plants of the Amazon floodplains: state of the art and research needed. Acta Limnologica Brasiliensia 22: 165–178.
Piedade, M. T. F., J. Schöngart, F. Wittmann, P. Parolin & W. J. Junk, 2013. Impactos da inundação e seca na vegetação de áreas alagáveis amazônicas. In Borma, L. S. & C. Nobre (eds), Secas na Amazônia: causas e Consequências. Oficina de Textos, São Paulo: 268–305.
Piedade, M. T. F., M. Worbes & W. J. Junk, 2001. Geo-ecological controls on elemental fluxes in communities of higher plants in Amazonian floodplains. In McClain, M. E., R. L. Victoria & J. Richey (eds), The Biogeochemistry of the Amazon Basin. Oxford University Press, Oxford: 209–233.
Poorter, H., 1993. Interspecific variation in the growth reduction to long-term elevated CO2 in winter wheat. Vegetatio 104(105): 77–97.
Rawson, H. M., 1992. Plant responses to temperature under conditions of elevated CO2. Australian Journal of Botany 40: 473–490.
Reddy, V. R., K. R. Reddy & H. F. Hodges, 1995. Carbon-dioxide enrichment and temperature effects on cotton canopy photosynthesis, transpiration, and water-use efficiency. Field Crops Research 41: 13–23.
Rogers, H. H., W. W. Heck & A. S. Heagle, 1983. A field technique for the study of plant responses to elevated carbon dioxide concentrations. Journal of the Air Pollution Control Association 33: 42–44.
Schimel, D., I. G. Enting, M. Heimann, T. M. L. Wigley, D. Raynaud, D. Alves & U. Siegenthaler, 2000. CO2 and the carbon cycle (extracted from the intergovernmental panel on climate change (IPCC) report, “climate change, 1994”. In Wigley, T. M. L. & D. S. Schimel (eds), The Carbon Cycle. Cambridge University Press, Cambridge: 1–37.
Schmidt, L., 2007. Tropical Forest Seed. DFSC, Dinamarca: 421.
Schreiber, U., R. Gademann, P. J. Ralph & A. W. D. Larkum, 1997. Assessment of photosynthetic performance of Prochloron in Lissoclinum patella by in situ and in hospite chlorophyll fluorescence measurements. Plant and Cell Physiology 38: 945–951.
Short, F. & H. Neckles, 1999. The effects of global climate change on seagrasses. Aquatic Botany 63: 169–196.
Sioli, H., 1984. The Amazon and its main affluents: hydrography, morphology of the river courses, and river types. In The Amazon. Springer, Dordrecht 127–165.
Systat Inc. 2007.SYSTAT 12 SYSTAT Software Inc.
Tian, H., J. M. Mellilo, D. W. Kicklighter, A. D. McGuire, J. V. K. Helfrich, B. Moore & C. J. Vörösmarty, 1998. Effect of interannual climate variability on carbon storage in Amazonian ecosystems. Nature 396: 664–667.
Turcq, B., R. C. Cordeiro, A. Sifeddine, F. F. Simoes-Filho, J. J. Abrão, F. B. O. Oliveira, A. O. Silva, J. L. Capitaneo & F. A. K. Lima, 2002. Carbon storage in Amazonia during the LGM: data and uncertainties. Chemosphere 49: 821–835.
Vasconcelos, S. S. D., P. M. Fearnside, P. M. L. D. A. Graça, E. M. Nogueira, L. C. D. Oliveira & E. O. Figueiredo, 2013. Forest fires in southwestern Brazilian Amazonia: estimates of area and potential carbon emissions. Forest Ecology and Management 291: 199–208.
Waldhoff, D., W. Junk, & B. Furch, 2000. Fluorescence measurements as indicator of adaptation strategies in an abundant tree species from central amazonian floodplain forests. In Lieberei, R., H. Bianchi, V. Boehm & C. Reisdorff (eds), Neotropical Ecosystems: proceedings of the German-Brazilian Workshop, Geesthacht: GKSS-Forschungszentrumpp, Hamburg 573–577.
Wang, D., S. A. Heckathorn, X. Wang & S. M. Philpott, 2012. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169: 1–13.
Wetzel, R. G. & J. B. Grace, 1983. Aquatic plant communities. In Lemon, E. R. (ed.), CO2 and Plants: the Response of Plants to Rising Levels of Atmospheric Carbon Dioxide. AAAS Selected Symposium 83, Boulder, Colorado 223–280.
Wheeler, T. R., G. Batts, R. Ellis, P. Hadley & J. Morison, 1996. Growth and yield of winter wheat (Triticum aestivum) crops in response to CO2 and temperature. The Journal of Agricultural Science 127: 37–48.
White, A. J. & C. Critchley, 1999. Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynthesis Research 59: 63–72.
Acknowledgments
This work was supported by INCT ADAPTA—Brazilian Ministry of Science, Technology and Innovation (CNPq/FAPEAM), Universal CNPq (14/2009; 14/2011), PELD MAUA (CNPq/FAPEAM) and FAPEAM EDITAL N. 017/2014—FIXAM/AM Nº Processo: 062.01174/2015 to Dr. Aline Lopes. The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Doctorate Grant awarded to Aline Lopes and MAUA Research Group, Laboratório de Ecofisiologia e Evolução (LEEM) for logistical and technical support. Dr. A. Leyva helped with English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
We dedicate this manuscript to the memory of Dra. Mônica Elisa Bleich a great and beloved colleague from the MAUA Group who left us early and suddenly.
Guest editors: Helmut Habersack & Roswitha Samek / Multifunctionality of large rivers
Rights and permissions
About this article
Cite this article
Lopes, A., Ferreira, A.B., Pantoja, P.O. et al. Combined effect of elevated CO2 level and temperature on germination and initial growth of Montrichardia arborescens (L.) Schott (Araceae): a microcosm experiment. Hydrobiologia 814, 19–30 (2018). https://doi.org/10.1007/s10750-015-2598-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2598-1