Abstract
White-spotted charr (Salvelinus leucomaenis) typically occupy the upstream reaches of Japanese streams, whereas masu salmon (Oncorhynchus masou) are generally found downstream. Temperature varies predictably with altitude; thus, it is thought to be an important determinant of the altitudinal niche partitioning. We examined (i) the species composition and habitat availability (e.g., water temperature and velocity) in reaches along an altitudinal gradient (elevation: 0–270 m, gradient: 0.6–11%), (ii) microhabitat use at the individual level, and (iii) swimming stamina against a fixed water flow velocity using stamina tunnel tests in the Ohkamaya River, Hokkaido, Japan. The proportion of white-spotted charr increased in an upstream direction from 5 to 95%, whereas summer water temperature and average velocity increased downstream (temperature: 15–18°C, velocity: 17–40 cm s−1). Underwater observations revealed that white-spotted charr used slow velocity microhabitat more than masu salmon under sympatric and allopatric conditions (charr: 7–13 cm s−1, salmon: 15–23 cm s−1). Masu salmon swam twice as long as white-spotted charr against a fixed-velocity (66 cm s−1). Our results suggest that velocity was an important determinant of the observed altitudinal distribution patterns of masu salmon and white-spotted charr.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animal and plant species coexist but their compositions often change spatially along altitudinal/latitudinal gradients (Tokeshi, 1999). Because temperature notably changes with altitude/latitude, it has often been suggested as a major determinant of the altitudinal/latitudinal distributions of organisms. However, other abiotic factors (e.g., productivity and humidity) can also co-vary with altitudinal/latitudinal gradients. Therefore, it is important to identify species habitat requirements for a comprehensive understanding of the altitudinal/latitudinal distribution of a species.
Riverine animal species also exhibit longitudinal changes in composition (Rahel & Hubert, 1991; Growns & Davis, 1994; Taniguchi et al., 1998; Torgersen et al., 2006; Ogitani et al., 2011; Kimoto et al., 2015). For example, Dolly Varden (Salvelinus malma) inhabit upstream areas and white-spotted charr (Salvelinus leucomaenis) inhabit downstream areas in the rivers of northern Japan (Fausch et al., 1994). Taniguchi & Nakano (2000) demonstrated that water temperature affects the outcome of inter-specific competition, suggesting that altitude-related temperature is a major determinant of altitudinal distribution of Dolly Varden and white-spotted charr.
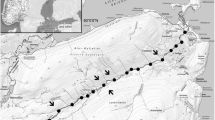
White-spotted charr and masu salmon (Oncorhynchus masou) are common in Japanese mountain streams (Fig. 1). White-spotted charr primarily occupy the upstream reaches, whereas masu salmon are typically found downstream (Imanishi, 1951; Miyasaka et al., 2003; Tsuboi et al., 2013). Competitive relationships between white-spotted charr and masu salmon are well recognized based on behavioral observations and diet analyses between allopatric and sympatric populations (Furukawa-Tanaka, 1988; Nakano, 1995; Morita & Suzuki, 1999; Miyasaka et al. 2003). Imanishi (1951) suggested that water temperature is a key factor causing the distribution shift and that the maximum summer water temperature for the transition is 15°C.
Although the altitudinal shifts from masu salmon to white-spotted charr toward upstream reaches are widely accepted, the temperature hypothesis is often criticized because temperature alone does not explain this altitudinal distribution pattern. Maruyama (1977, 2005) and Yamamoto (1991) suggested that gradient-related habitat features (e.g., water velocity, shelter availability, and food availability) are key factors affecting the relative abundance of white-spotted charr and masu salmon. A higher gradient has been hypothesized to induce lower water velocity areas, abundant shelters, and decreased food availability, all of which benefit charr in comparison with salmon (Maruyama, 1977, 2005; Yamamoto, 1991). In fact, the probability of white-spotted charr occurrence increases significantly with gradient and is independent of altitude (Morita & Yamamoto, 2002). However, few attempts have been made to relate riverine environmental data with the altitudinal distributions of white-spotted charr and masu salmon.
The objectives of this study were to identify proximate factors that influence altitudinal niche partitioning between white-spotted charr and masu salmon and to address whether the temperature or gradient hypothesis explains their altitudinal distribution in nature.
Methods
Study site
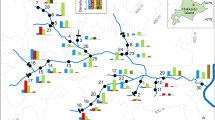
A field study was undertaken in the Ohkamaya River, Hokkaido, the northernmost island of the Japanese archipelago (Fig. 2). Ten 70-m-long study reaches were established from the river mouth to an impassable waterfall (Fig. 2). This river is relatively pristine because no dams have been built, no records of salmonid hatchery releases have been found, and only one private house lies within the watershed. White-spotted charr and masu salmon are the dominant fish species in the river, but the river also contains river sculpins (Cottus nozawae, C. amblystomopsis, and C. hangiongensis), freshwater gobies (Chaenogobius urotaenia and Rhinogobius brunneus), dace (Tribolodon hakonensis), ayu (Plecoglossus altivelis), and chum salmon (O. keta). All species are diadromous, except C. nozawae.
Altitudinal distributions
White-spotted charr and masu salmon were collected using a backpack electrofishing unit (300–400 V DC, model 12B, Smith-Root, Inc., Vancouver, WA, USA) and a 3-mm mesh dipnet (width, 30 cm) in the 10 study reaches during August 2010. A numbers of each species within a reach were estimated using the two-pass removal method (model Pollock and Otto’s M(bh), program CAPTURE, available at http://www.mbr-pwrc.usgs.gov/software/index.html). The captured fish were anesthetized with phenoxyethanol (ca. 0.5 ml/l water), fork length was measured to the nearest 1 mm, and body weight was measured to the nearest 0.1 g. Condition factor [(body weight)·(fork length)−3·104] was calculated for each fish. Young-of-the-year charr and salmon were distinguished as ≤67 and ≤81 mm fork lengths, respectively, based on length-frequency histograms (Electronic Supplementary Material Appendix). A total of 514 charr, 362 salmon and six hybrids (charr × salmon) were captured in the 10 study reaches. The hybrids were easily distinguished externally (Kato, 1977; Sato et al., 2008; Miyazawa et al., 2010) and were excluded from the analysis. Some early run anadromous adults were collected (26 anadromous charr and four anadromous salmon), and these fish were also excluded from the analysis. Only parr were used in this study.
Abiotic environmental variables of the 10 study reaches were measured during August 2010. Stream width was measured and substrate composition was determined at 15 transects spaced at 5-m intervals. Substrate composition was numericalized (Bain et al., 1985). Water depth and velocity available for fish were measured at three evenly spaced points (1/4, 1/2, and 3/4) along the 15 transects (i.e., 3 × 15 points in each study reach). Water velocity was measured at 60% of the depth from the surface to the bed using a propeller-type meter (VR-301; Kenek Co., Tokyo, Japan). The study reaches were located using a portable GPS receiver (GPSMAP60CSx, Garmin Ltd., Olathe, KS, USA), and the altitude and gradient were determined using 1:25000 scale topographic maps (http://maps.gsi.go.jp). Water temperature was measured at each study reach at hourly intervals using data loggers (Stow-Away TidbiT, Onset Computer Corp., Bourne, MA, USA) deployed on the stream beds during August 1–22, wherein the maximum temperature was registered. Mean (averaged for all study periods) and maximum temperatures were obtained for analysis.
Kendall’s rank correlation analysis was used to examine the relationships between the altitudinal distributions of charr and salmon and the abiotic environmental variables. Because most of these variables covaried with altitude, the residuals of the regression on altitude were calculated for each variable for the Kendall’s rank correlation analysis.
Microhabitat use
The foraging microhabitats of the charr and salmon parr were compared by underwater observations during July 2011. One observer equipped with a wetsuit, mask, and snorkel entered the stream at the lower end of study reach #2 (downstream) or #8 (upstream) and crawled slowly upstream in a zigzag pattern between 10:00 and 17:00 h. Fish encountered without a disturbance were observed for up to 5 min to determine their primary holding position. The holding position was marked with a ribboned peg. Water velocity at the holding position was measured using the propeller-type meter. The height of the holding position above the bottom was measured using a ruler. Water temperature at the holding position was measured using an electric thermometer. A total of 54 charr and 79 salmon were observed under natural sympatric conditions. A digital video of the foraging microhabitat during the underwater observations is available in Supplementary Material (Video A).
Allopatric experiments was conducted during July 2013 to evaluate the intrinsic microhabitat preferences of the two species. First, all fish were removed by intensive electrofishing in a pool and adjoining riffles in study reach #6, and individual charr or salmon were allowed to enter the pool alternately. The foraging microhabitat of the charr or salmon parr under allopatric conditions were observed underwater using the same protocol described above. Many fish did not display natural behavior (i.e., hid under cover or moved away) after entering the experimental pool. The holding positions of nine charr and 10 salmon were determined.
Two-way or one-way analysis of variance (ANOVA) was used to test the differences in foraging microhabitat metrics between charr and salmon. Species (charr/salmon) and study reach (upstream/downstream) were used as independent variables.
Swimming stamina trial
Swimming stamina trials were conducted in the field during July 2012. A small water cascade was constructed using sandbags to induce rapid flow in the upper end of study reach #1. Then, a stamina tunnel (cylindrical transparent tube, 65-cm long with an 8-cm internal diameter) was installed with an experimental fish. Both ends of the stamina tunnel were closed with 1-cm mesh caps. Swimming endurance time (SET), which was the time when the fish could no longer swim despite prodding from the downstream end, was recorded. Velocity at the tunnel outlet was 66.4 ± 1.6 cm s−1 (mean ± SD). Water temperature during the experiment was 14.4–16.1°C. A total of 30 charr and 30 salmon were tested alternately. After the swimming stamina trial, the fish were anesthetized with phenoxyethanol, fork length was measured to the nearest 1 mm, and body weight was measured to the nearest 0.1 g. Condition factor [(body weight)·(fork length)−3·104] was calculated for each fish. Digital videos of the swimming stamina trials are available in Supplementary Material (Videos B and C).
One-way ANOVA and analysis of covariance (ANCOVA) were used to test for differences in the SET between charr and salmon. Natural logarithmic transformation was used for the SET. Either fork length or condition factor was used as the covariate in the ANCOVA. The adjusted means of the model were tested again after excluding the interaction term when the interaction between the covariate and independent variable (species) was not significant (P > 0.05) (Sokal & Rohlf, 2011).
All statistical analyses were performed using IBM SPSS Statistics (Version 20, IBM Corp., Armonk, NY, USA).
Results
Altitudinal distributions
Charr density (per 70 m of reach) was higher in the upstream reaches, whereas salmon density was higher in the downstream reaches, resulting in changes in the proportion of charr from 5 to 95% in the upstream direction (Fig. 3). The proportion of charr rebounded somewhat in the most downstream reach near the river mouth.
The abiotic environment available for fish changed along the altitudinal gradient. Both water temperature and velocity decreased towards the upstream reaches (Fig. 4). The environmental gradient in the upstream area was characterized by narrow, shallow, steep, and low-water discharge habitats, resulting in changes in the width from 6.2 to 2.2 m, depth from 25 to 15 cm and gradient from 0.6 to 11% in the upstream direction. Significant correlations were detected between the proportion of charr, the charr/salmon densities, and the abiotic environmental variables (Table 1); however, the correlation coefficients for temperature relations were not higher than those for gradient and water velocity. Very small differences in temperature were observed between reaches #3 and #7 (∆ = 0.2°C), even though salmon and charr were most abundant in reaches #3 and #7, respectively (Fig. 3).
Residuals of the altitude-proportion of charr relationship were also negatively correlated with residuals of the altitude-velocity relationship (Table 1), indicating that water velocity was consistently linked to the proportion of charr even after taking account of altitudinal trends.
The mean fork lengths of charr and salmon varied among study reaches (charr, 85–134 mm; salmon, 86–137 mm). However, neither charr nor salmon fork length changed along the altitudinal gradient (all P > 0.05). The charr and salmon condition factors decreased in the upstream direction (Fig. 4). Significant correlations were observed between the charr and salmon condition factors and the abiotic environmental variables (Table 1).
Foraging microhabitats
The underwater observations revealed that charr favored slower water velocity and were positioned closer to the bottom than masu salmon, regardless of whether they were in an upstream or downstream reach (Fig. 5, Table 2). Microhabitat temperature did not differ between charr and salmon found within the same reaches (Fig. 5, Table 2). Charr favored slower water velocity and tended to be found closer to the bottom under the allopatric conditions, although a significant difference was only detected for water velocity (Fig. 6, Table 2).
Swimming stamina trial
Swimming endurance time (SET) against a fixed water velocity was significantly longer for masu salmon than for charr (Table 3). SET increased with fork length but was longer for salmon than that for charr at a given fork length (Fig. 7a). ANCOVA did not identify a significant difference in regression slopes, but identified significant differences for adjusted means (i.e., intercept) (Table 3). In contrast, the regression slopes and intercepts of SET on condition factor did not differ between charr and salmon (Fig. 7b, Table 3), indicating that the difference in SET between the species could be explained by the difference in condition factor.
Discussion
We confirmed altitudinal niche partitioning between white-spotted charr and masu salmon in accordance with the abiotic environmental gradient. The proportion of charr increased with decreasing water temperature and decreasing water velocity among reaches. The underwater observations identified significant differences between charr and salmon in water velocity of their preferred microhabitat but not in microhabitat temperature within reaches. Therefore, water velocity significantly affected the distributions of charr and salmon within and among reaches. The proportions of charr and charr density were more closely related with gradient and water velocity than with water temperature. Our results support the gradient-induced habitat availability hypothesis (Maruyama, 1977, 2005; Yamamoto, 1991), rather than the temperature hypothesis (Imanishi, 1951).
Mean water current velocity decreased in the upstream high-gradient reaches. This result is not intuitive but such a pattern has been observed in other rivers (Rahel & Hubert, 1991; Growns & Davis, 1994; Torgersen et al., 2006). A high gradient induces many water cascades, which generate turbulence and lower-velocity habitats in headwaters (Yamamoto, 1991). In addition, lower discharge decreases water velocity in headwaters. Although the water current velocity initially increases downstream, it decreases subsequently in downstream lowland areas. Our results show that both water velocity and the proportion of charr rebounded somewhat in the most downstream reach near the river mouth. A similar pattern (i.e., upstream: masu salmon, downstream: white-spotted charr) was also noted by Sato (1958), who suggested that the distribution pattern was explained by the bottom-dwelling lentic propensity of white-spotted charr. Maruyama (2005) reported that white-spotted charr occurred in a low-gradient river mouth owing to the presence of lush shrub and reed shelters.
The finding that white-spotted charr prefer lower water velocity and swim closer to the bottom than masu salmon has been reported previously (Sato, 1963; Furukawa, 1978; Furukawa-Tanaka, 1988; Nakano, 1995; Yamamoto et al., 2001; Miyasaka et al., 2003) and was confirmed in the present study. Nakano (1995) suggested that the different use of foraging microhabitat is species-specific rather than a result of inter-specific competition, which is consistent with our finding that the species-specific propensity of foraging microhabitat did not change between sympatric and allopatric conditions. Moreover, adult female charr select lower water velocity areas as spawning sites compared with female masu salmon (Maruyama, 1981; Nakamura, 1999); therefore, a difference in spawning activity between the two species may be another factor affecting the altitudinal distributions of offspring (parr). Arctic charr (Salvelinus alpinus) also prefer lower water velocity habitats compared with those of Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) (Heggenes & Saltveit, 2007). Therefore, charrs (Salvelinus spp.) and salmon (Oncorhynchus spp./Salmo spp.) have distinct fundamental niches with respect to water current velocity.
Water current velocity can affect growth and survival of salmonids (Parker & Barnes, 2014), and the effect of water current velocity on swimming performance may differ by temperature (Ojanguren & Brañta, 2000). Thus, further study is necessary to elucidate the influence of water velocity via a temperature change on behavior and the demographics of white-spotted charr and masu salmon.
Masu salmon were superior to white-spotted charr with respect to swimming stamina. The swimming fatigue shown by charr is consistent with their habitat preference for lower water velocity areas. Dolly Varden also have inferior swimming speed and stamina to masu salmon (Kyoshi, 1997). Thus, inferior swimming ability of charrs may be genus-specific. Interestingly, the difference in swimming endurance time (SET) between charr and salmon was explained by the difference in condition factor alone when condition factor was used as a covariate. Different condition factors are reflected by different body shapes; i.e., salmon have deeper bodies than charr. A deeper body is often associated with adaptation to increased water velocity (Pakkasmaa & Piironen, 2000; Seiler & Keeley, 2007). We speculate that the longitudinal gradient in condition factor observed in the present study may be adaptive phenotypic plasticity to the longitudinal gradient in water velocity. However, condition factor is a measure of growth condition; therefore, a higher condition factor toward downstream areas may simply be owing to the temperature-related growth condition (Morita et al., 2011).
Environmental factors other than water velocity should not be ruled out to comprehensively understand the altitudinal distributions of white-spotted charr and masu salmon. First, altitudinal gradients in stream productivity may affect charr and salmon distributions. Higher gradients usually increase the proportion of pools and decrease the proportion of riffles. Because most aquatic insect production occurs in riffles, food availability decreases toward upstream areas (Yamamoto, 1991). Finstad et al. (2011) demonstrated that the optimal water temperature ranges for growth and feeding of Arctic charr and brown trout are almost identical. However, Arctic charr exhibit double the growth efficiency throughout the temperature range; therefore, Arctic charr would outcompete brown trout in cold low-productivity habitats (Finstad et al., 2011). A similar situation may occur during altitudinal niche partitioning between white-spotted charr and masu salmon (Yamamoto, 1991).
Higher gradient channels usually have abundant shelters for charr, such as rock interstices (Maruyama, 1977). Behavioral observations suggest that white-spotted charr require shelter in contrast to masu salmon (Sato, 1958; Maruyama, 1977; Furukawa, 1978). The common Japanese name for S. leucomaenis is “IWANA,” which means fish living in a rock hole (Kitahara et al., 2001), indicating that charr prefer to enter rock interstices. Therefore, higher gradient streams are suitable for charr because of the abundance of shelter and an increase in the amount of lower water velocity habitat. Although this study used coarse-grained analysis for habitat availability, Inoue & Nunokawa (2002) successfully related habitat structure and masu salmon density using a fine-scale habitat availability analysis (i.e., 0.5 × 0.5 m grids). Such a fine-scale patchiness analysis with shelter availability would help demonstrate the habitat suitability of charr in the upstream high-gradient reaches.
In conclusion, our results suggest that gradient- or velocity-related habitat feature was a major determinant of the observed altitudinal distribution pattern of masu salmon and white-spotted charr during the summer. Habitat heterogeneity related to velocity would play an important role for the coexistence of white-spotted charr and masu salmon. However, the proportions of charr and salmon often change with season, and the dominant species in a given reach can reverse between summer and winter (Morita et al., 2011; Sahashi & Morita, 2014). Altitudinal distributions during winter should be investigated in a future study. Ecosystem heterogeneity through space and time would guarantee the coexistence of species (Miyashita et al., 2014).
References
Bain, M. B., J. T. Finn & H. E. Booke, 1985. Quantifying stream substrate for habitat analysis studies. North American Journal of Fisheries Management 5: 499–500.
Fausch, K. D., S. Nakano & K. Ishigaki, 1994. Distribution of two congeneric charrs in streams of Hokkaido Island, Japan: considering multiple factors across scales. Oecologia 100: 1–12.
Finstad, A. G., T. Forseth, B. Jonsson, E. Bellier, T. Hesthagen, A. J. Jensen, D. O. Hessen & A. Foldvik, 2011. Competitive exclusion along climate gradients: energy efficiency influences the distribution of two salmonid fishes. Global Change Biology 17: 1703–1711.
Furukawa, T., 1978. Habitat segregation and space use of Japanese charr and red-spot masu salmon. Anima 62: 17–23. (in Japanese).
Furukawa-Tanaka, T., 1988. The ecology of salmonid fishes in Japanese mountain streams III. Interactive food segregation between red-spot masu salmon, Salmo (Parasalmo) masou macrostomus, and Japanese charr, Salvelinus leucomaenis, in relation to their social structure. Doctoral thesis, Kyoto University. (in Japanese with English summary).
Growns, I. O. & J. A. Davis, 1994. Longitudinal changes in near-bed flows and macroinvertebrate communities in a Western Australian stream. Journal of the North American Benthological Society 13: 417–438.
Heggenes, J. & S. J. Saltveit, 2007. Summer stream habitat partitioning by sympatric Arctic charr, Atlantic salmon and brown trout in two sub-arctic rivers. Journal of Fish Biology 71: 1069–1081.
Imanishi, K., 1951. White-spotted charr and masu salmon. Forestry Commentary Series 35: 275–301. (in Japanese).
Inoue, M. & M. Nunokawa, 2002. Effects of longitudinal variations in stream habitat structure on fish abundance: an analysis based on subunit-scale habitat classification. Freshwater Biology 47: 1594–1607.
Kato, K., 1977. Natural hybrids of salmonid fishes from the Nippara River, Tokyo. Japanese Journal of Ichthyology 23: 225–232. (in Japanese with English summary).
Kimoto, K., M. Kagehira, K. Azechi & K. Nagasawa, 2015. Longitudinal changes in fish assemblage in a mountain stream, northeastern Kyushu, southern Japan. Japanese Journal of Ichthyology 62: 1–12. (in Japanese with English summary).
Kitahara, Y., J. Kubota, M. Taniwaki, M. Tokugawa, O. Hayashi, T. Maeda, S. Matsui & M. Watanabe, 2001. Japanese Language Unabridged Dictionary, 2nd ed. Shogakukan lnc, Tokyo.
Kyoshi, H., 1997. On the results of swimming endurance survey in fish. Civil Engineering Research Institute for Cold Region Monthly Report 534: 36–40. (in Japanese).
Maruyama, T., 1977. How distributions of Japanese charr and masu salmon are determined? The Freshwater Fishes 3: 113–117. (in Japanese).
Maruyama, T., 1981. Comparative ecology on the fluvial forms of Salmo (Oncorhynchus) masou masou (Brevoort) and Salvelinus leucomaenis (Pallas) (Pisces, Salmonidae). I. Structure of spawning redds and spawning sites in Kamidani. River Yura. Japanese Journal of Ecology 31: 269–284. (in Japanese with English summary).
Maruyama, T., 2005. 4.2 Freshwater environment. In Taniuchi, T., T. Nakabo, H. Somiya, A. Taniguchi, I. Aoki, A. Hino, S. Watanabe, H. Abe, T. Fujii & T. Akimichi (eds), Scientific Encyclopedia of the Fish. Asakura Publishing Co., Ltd, Tokyo: 224. (in Japanese).
Miyasaka, H., S. Nakano & T. Frukawa-Tanaka, 2003. Food habit divergence between white-spotted charr and masu salmon in Japanese mountain streams: circumstantial evidence for competition. Limnology 4: 1–10.
Miyashita, T., T. Amano & T. Yamakita, 2014. Effects of ecosystem diversity on species richness and ecosystem functioning and services: a general conceptualization. In Nakano, S., T. Yahara & T. Nakashizuka (eds), Integrative Observations and Assessments. Springer, Tokyo: 29.
Miyazawa, S., M. Okamoto & S. Kondo, 2010. Blending of animal colour patterns by hybridization. Nature Communications 1: 66.
Morita, K. & T. Suzuki, 1999. Shifts of food habit and jaw position of white-spotted charr after damming. Journal of Fish Biology 55: 1156–1162.
Morita, K. & Y. Yamamoto, 2002. Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conservation Biology 16: 1318–1323.
Morita, K., S. H. Morita & T. Nagasawa, 2011. Seasonal changes in stream salmonid population densities in two tributaries of a boreal river in northern Japan. Ichthyological Research 58: 134–142.
Nakamura, T., 1999. Comparison of physical characteristics of the spawning redds between the fluvial Japanese charr Salvelinus leucomaenis and the masu salmon Oncorhynchus masou masou in the headwaters of the Kinu River, central Japan. Nippon Suisan Gakkaishi 75: 802–809. (in Japanese with English summary).
Nakano, S., 1995. Competitive interactions for foraging microhabitats in a size-structured interspecific dominance hierarchy of two sympatric stream salmonids in a natural habitat. Canadian Journal of Zoology 73: 1845–1854.
Ogitani, M., K. Sekiné & K. Tojo, 2011. Habitat segregation and genetic relationship of two heptageniid mayflies, Epeorus latifolium and Epeorus l-nigrus, in the Shinano-gawa River basin. Limnology 12: 117–125.
Ojanguren, A. F. & F. Brañta, 2000. Thermal dependence of swimming endurance in juvenile brown trout. Journal of Fish Biology 56: 1342–1347.
Pakkasmaa, S. & J. Piironen, 2000. Water velocity shapes juvenile salmonids. Evolutionary Ecology 14: 721–730.
Parker, T. M. & M. E. Barnes, 2014. Rearing velocity impacts on landlocked fall Chinook salmon (Oncorhynchus tshawytscha) growth, condition, and survival. Open Journal of Animal Sciences 4: 244–252.
Rahel, F. J. & W. A. Hubert, 1991. Fish assemblages and habitat gradients in a Rocky Mountain-Great Plains stream: biotic zonation and additive patterns of community change. Transactions of the American Fisheries Society 120: 319–332.
Sahashi, G. & K. Morita, 2014. Fall-winter collection of two salmonid species: seasonal changes in population densities in four tributaries of the Kushiro river system. Ichthyological Research 61: 189–192.
Sato, T., 1958. Studies on Gogi charr 12 The Gogi in the Saijochoyuki, Hiba-gun. Journal of the Hiba Society of Natural History 46: 2–6. (in Japanese).
Sato, T., 1963. Studies on so-called Gogi (Salvelinus pluvius (Hilgendorf)) in Hiroshima Prefecture. In Hiroshima Board of Education (ed.), Research Reports of Cultural Assets in Hiroshima Prefecture III (Vol. Natural monument). The Hiroshima Board of Education, Hiroshima: 3–30. (in Japanese).
Sato, T., K. Watanabe, M. Arizono, S. Mori, M. Nagoshi & Y. Harada, 2008. Intergeneric hybridization between sympatric Kirikuchi char and red-spotted masu salmon in a small Japanese mountain stream. North American Journal of Fisheries Management 28: 547–556.
Seiler, S. M. & E. R. Keeley, 2007. Morphological and swimming stamina differences between Yellowstone cutthroat trout (Oncorhynchus clarkii bouvieri), rainbow trout (Oncorhynchus mykiss), and their hybrids. Canadian Journal of Fisheries and Aquatic Sciences 64: 127–135.
Sokal, R. R. & F. J. Rohlf, 2011. Biometry, 4th ed. W. H. Freeman and Company, New York.
Taniguchi, Y., F. J. Rahel, D. C. Novinger & K. G. Gerow, 1998. Temperature mediation of competitive interactions among three fish species that replace each other along longitudinal stream gradients. Canadian Journal of Fisheries and Aquatic Sciences 55: 1894–1901.
Taniguchi, Y. & S. Nakano, 2000. Condition-specific competition: implications for the altitudinal distribution of stream fishes. Ecology 81: 2027–2039.
Tokeshi, M., 1999. Species Coexistence: Ecological and Evolutionary Perspectives. Blackwell Science Ltd., Oxford.
Torgersen, C. E., C. V. Baxter, W. L. Hiram & B. A. Mclntosh, 2006. Landscape influences on longitudinal patterns of river fishes: spatially continuous analysis of fish-habitat relationships. American Fisheries Society Symposium 48: 473–492.
Tsuboi, J., T. Iwata, K. Morita, S. Endou, H. Oohama & K. Kaji, 2013. Strategies for the conservation and management of isolated salmonid populations: lessons from Japanese streams. Freshwater Biology 58: 908–917.
Yamamoto, S., 1991. Charr the ecology and fishing. Tsuribitosha, Tokyo. (in Japanese).
Yamamoto, S., I. Sanjyo & M. Kohara, 2001. Physical environment of the microhabitat of Japanese charr Salvelinus leucomaenis in mountain stream of the Chikuma system. Bulletin of Nagano Prefectural Fisheries Experimental Station 5: 1–8. (in Japanese).
Acknowledgments
We thank Kazumasa Ohkuma for the stamina tunnels, Shinsuke Endou and Taku Yoshiyama for help with fieldwork, and Toru Nagasawa for valuable discussions. This study was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant nos. 22780187 and 25450293).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Power, R. Knudsen, C. Adams, M. J. Hansen, J. B. Dempson, M. Jobling & M. Ferguson / Advances in Charr Ecology and Evolution
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2015_2571_MOESM2_ESM.mp4
Supplementary Video A. Digital video showing the foraging microhabitats of white-spotted charr and masu salmon parr during underwater observations in the Ohkamaya River. The white-spotted charr, Salvelinus leucomaenis, is in the photograph at left and the masu salmon, Oncorhynchus masou, is in the photograph at right. (MP4 6501 kb)
10750_2015_2571_MOESM3_ESM.mp4
Supplementary Video B. Digital video showing the masu salmon, Oncorhynchus masou, swimming stamina trial using a stamina tunnel. (MP4 2332 kb)
10750_2015_2571_MOESM4_ESM.mp4
Supplementary Video C. Digital video showing the white-spotted charr, Salvelinus leucomaenis, swimming stamina trial using a stamina tunnel. (MP4 1492 kb)
Rights and permissions
About this article
Cite this article
Morita, K., Sahashi, G. & Tsuboi, Ji. Altitudinal niche partitioning between white-spotted charr (Salvelinus leucomaenis) and masu salmon (Oncorhynchus masou) in a Japanese river. Hydrobiologia 783, 93–103 (2016). https://doi.org/10.1007/s10750-015-2571-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2571-z