Abstract
We analyzed the diatom assemblages inhabiting the epipelic biofilm of a Pampean stream, characterized by their high basal nutrient levels, when exposed to a continuous surplus of inorganic nutrients. An in situ experience was conducted, increasing concentrations of N and P in water 3-fold from the basal concentration. Nutrient enrichment was achieved by the use of fertilizer bags distributed along the reach. The period of exposure was of 14 months. The effects of nutrient enrichment were analyzed following a BACIPS ANOVA design. The changes in nutrient concentration were associated with a significant increase in diatom density and a decrease in species richness and diversity. The additional nutrient load also caused the change in the diatom taxa proportion, favoring motile forms, Nitzschia species mainly. The fertilization in La Choza, caused a mild to moderate effect, indeed not immediate, on the diatom assemblage. These delayed responses of moderate intensity could be related with intrinsic characteristics of diatom assemblages pre-adapted to nutrient-rich environments. The rising urbanization and agricultural activity in the Pampean plain, may seriously impair the biodiversity of its rivers if the entrance of nutrients to these ecosystems is not mitigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased pressure by human beings is mainly expressed as the loss of natural habitats and its conversion to land dedicated to agriculture and farming. These changes in land affect biodiversity and functioning of both terrestrial and aquatic ecosystems (Sala et al., 2000; Bauer et al., 2002). In the case of the Pampa region, an extended agricultural area in Argentina (East South America), land use changes are combined with climate change predictions of higher rainfall and altered runoff patterns (Kitoh et al., 2011). Altogether, the region is facing scenarios of higher amounts of nutrients drained from the land and reaching the running waters. The predictions are that streams and rivers will have higher water discharge as well as greater nutrient concentrations, anticipating modifications on the river biodiversity as well as in the food web structure (Hart & Robinson, 1990; Liess & Hillebrand, 2004; Feijoó et al., 2014). This would results in significant alterations on ecosystem functioning (Biggs, 1996; Worm et al., 2002; Acuña et al., 2011). The nutrients excess may also affect the quality of water resources, with accompanying economic implications (Dodds et al., 2009; Stevenson & Sabater, 2010).
Several studies have recognized the link between rising nutrient concentrations and benthic algal biomass increase (Dodds et al., 2002; Dodds, 2006; Smith & Schindler, 2009; Gudmundsdottir et al., 2011; Sabater et al., 2011). Some of these studies were based on field manipulations in nutrient concentrations to assess benthic algal growth (Bothwell, 1989; Walton et al., 1995; Rier & Stevenson, 2006; Stevenson et al., 2006), while others consisted of large scale surveys investigating the relationships between nutrient enrichment and algal biomass (Welch et al., 1992; Dodds et al., 1997; Chetelat et al., 1999). Overall, there is a well-established evidence that nitrogen and phosphorus availability are tightly related to benthic algal biomass increase and community composition changes (Borchardt, 1996; Stevenson, 1996; Wyatt et al., 2010). Diatom assemblages respond to human impacts by changing the proportion of tolerant species to eutrophication, the proportion of nitrogen-heterotroph species, and the proportion of motile forms (Fore & Grafe, 2002).These effects have been proved in several field manipulation studies that have reported the increase of motile forms (e.g., taxa of Nitzschia and Navicula) and the variation in growth forms (Pringle, 1990; Kelly, 2003; Bellinger et al., 2006, Veraart et al., 2008; Wyatt et al., 2010; Gudmundsdottir et al., 2013).
Lowland streams in temperate areas are generally nutrient-poor (Kjeldsen et al., 1998; Vilbaste & Truu, 2003), but those in the Pampa are characterized by high basal nutrient concentrations (Giorgi, 1998; Feijoó et al., 1999) and show very high growth of primary producers such as algae and macrophytes (Acuña et al., 2011). The pampean streams run through depositional areas, dominated by volcanic sediments rich in phosphates and silicates (Martínez & Osterrieth, 1998; Giorgi et al., 2005). These are streams usually with stream bed made up of fine sediments (silt and clay), which are covered by epipelic biofilms rich in diatom taxa (Gómez, 1998; Gómez & Licursi, 2001). Neither nutrients (mainly N and P) nor light are usually limiting primary production in pampean streams (Giorgi, 1998; Feijoó et al., 1999; Acuña et al., 2011). Beyond the natural condition of nutrient-rich systems, these systems are submitted to rising nutrient concentrations because of the implementation of intensive agricultural practices. Understanding the effects of additional nutrient inputs to these nutrient-saturated systems (Armendariz et al., 2012; Artigas et al., 2013; Cochero et al., 2013; Ocón et al., 2013) is essential to foresee the potential implications associated to global changes for this region.
The Pampean plain contains one of the highest demographic and industrial concentrations in South America, as well as one of the largest agriculture and livestock production in the world. Such a heavy exploitation of the land by humans comes along with an intense use of agrochemicals. According to FAO (2013), the use of nitrogen-based fertilizers in Argentina doubled in the last 10 years, while the use of phosphorus-based fertilizers almost tripled during that period. These additional nutrients can cause an imbalance in the relationship nitrogen-phosphorus relationship (N:P), as well as new environmental conditions that would favor some species in detriment of others.
Diatom assemblages are sensitive to nutrient conditions, and quickly adapt their communities’ composition and their relative abundance to the new conditions. This ability makes them attractive to understand and predict the effects of increased nutrients in the biological structure of the river ecosystem (van Dam et al., 1994; Kelly & Whitton, 1995; Kelly et al., 1998; Winter & Duthie, 2000; Kelly, 2003; Lobo et al., 2004; Böhm et al., 2013).
We aimed to understand the changes produced in the epipelic diatom community after a long-term (14 months) nutrient addition that tripled the basal nutrient concentration. Our hypothesis was that the experimental enrichment (with N and P) in La Choza stream would lead to an increase in diatoms density, as well as changes in diatom community structure. Such changes would enhance the proportion of tolerant taxa, and would favor the mobile growth forms (such as Nitzschia and Navicula species) able to avoid the stress within the benthic mat (Johnson et al., 1997). However, given the high nutrient concentration in the river, we also hypothesize that the response of the diatom communities would not be immediate after nutrient enrichment.
Study area
La Choza is a pampean stream located in the lowland prairies of Buenos Aires province (Argentina) (Fig. 1). The stream is characterized by a channel 4–10 m wide (Colautti et al., 2009), which mean depth is 60 cm. Its basin surface area is 48 km2 and drains deep loess deposits, mainly covered by temperate grassland grazed by cattle, and one quarter of the land is being devoted to agriculture (Artigas et al., 2013). The climate is temperate continental, resulting in wet springs and autumns. The average annual rainfall is 600–1200 mm, and the average monthly temperatures range from 10°C (June) to 24°C (January) (Ereño, 2002).
The experimental nutrient enrichment was conducted in a third order section (34o41′56′′S, 59o 04′16′′ W) of the stream, naturally devoid of riparian trees (Fig. 1). The stream bed is composed of carbonate calcium precipitates and deposits of silt and clay (Rodrigues Capítulo et al., 2010).
Materials and methods
Experimental manipulations
The experimental manipulation consisted in the artificial enrichment of a 100 m reach (named as the enriched, E reach), in comparison with an upstream 100 m reach, that served as control (hereafter named as the control, C reach). Both reaches were geomorphologically and hydrologically comparable (Artigas et al., 2013). The C reach was located 5.3 km upstream to ensure that the samples taken in both reaches were independent. Both reaches (C and E) were sampled monthly for 6 months prior to the nutrients addition (June–November 2007), and for 14 months (December 2007 to February 2009) since the enrichment onset. N and P concentrations were increased 3 times in the E reach with respect to the C reach. Mesh bags with 750 g of commercial fertilizer (Nitrofoska®, Basf, Amberes, Belgium; 12% P as PO4 3− and 10% N as NO3 −) and 250 g of urea were used to produce the desired enrichment in the E reach of the La Choza Stream. Twelve in-water bags were distributed along the treatment reach, and nutrient concentrations were adjusted twice a week in order to follow the natural variations of water flow and nutrient concentrations in the stream (Artigas et al., 2013).
Physical–chemical parameters
Dissolved oxygen (mg l−1), pH, temperature (°C), and conductivity (μS cm−1), were measured using a Horiba-U10 portable Water Quality Checker (Horiba, Co., Japan).Water samples for nutrient analysis were collected in triplicate in both reaches on each sampling occasion. Samples were filtered through pre-combusted glass fiber filters (Whatman GF/F, Whatman International) and analyzed for ammonium (NH4 +, μg N l−1), nitrate (NO3 −, μg N l−1), and soluble reactive phosphorus (SRP, μg P l−1) concentrations according to standard methods (APHA, 1998). Total dissolved inorganic nitrogen (total DIN, μg N l−1) was calculated as the sum of nitrate, nitrite, and ammonia.
Diatom assemblages
The epipelic biofilm samples were collected monthly during the experimental period (6 and 14, before and after enrichment, respectively), by pipetting 10 aliquots of streambed subsamples from each stream reach. Each aliquot was 4 ml in volume and corresponded to 1 cm2 of the superficial sediment layer (Gómez & Licursi, 2001). The samples for diatom identifications were first cleaned with hydrogen peroxide (H2O2) at 60°C in stove during 12 h, then washed thoroughly by centrifugation with distilled water, and 100 μl of sample were mounted on microscope circular slides (Ø 22 mm, Menzel-Glazer®) using Naphrax®. Four hundred valves from each sample were identified using an Olympus BX51 microscope with interference, phase-contrast and Nomarski–differential-interference-contrast optics at 1000× magnification. The monographs of Krammer (1992, 2000), Krammer & Lange-Bertalot (1986, 1988, 1991a, b), Rumrich et al. (2000) and Patrick & Reimer (1966, 1975) were used for the diatom taxa identification. The quantification of the total abundance of diatoms was performed at 400× magnification using a Sedgwick–Rafter chamber (APHA, 1998).
Species diversity was calculated using the Shannon and Wiener index according to Ludwig & Reynolds (1988).The diatoms were classified by their growth form according to Molloy (1992), and by their metabolic characteristics with respect to the nitrogen metabolism according to van Dam et al. (1994).
Data analysis
Only those species present in at least 2% of the total sample dataset and with more than 2% of relative abundance in at least 1 sample were included for the statistical analysis. The effects of nutrient enrichment on the physico-chemical data and diatom variables were analyzed following a BACIPS ANOVA design between the control and enriched reaches (Stewart-Oaten & Bence, 1986). The proportion of variance (often termed eta squared, η 2) explained by the BA factor plus the CE × BA interaction was calculated as an estimate of the magnitude of the effect of nutrient enrichment (Artigas et al., 2013).
Cochran’s test (Cochran, 1941) was used to test for homogeneity of variance on the transformed data, and then a 3-factor ANOVA was performed: (1) Periods: before, after (BA); (2) Reach: control, enriched (CE); and (3) Time: sampling times, nested within periods [TIME (BA)].
Impacts can cause different patterns in the resulting tables of these analyses. The interaction between the factors Period and Reach (BA × CE) will be significant if there are immediate or ‘press’ effects, i.e., if the variables in the impacted site are shifted to a new average condition in the after period. Alternatively, if there are gradual or ‘pulse’ effects, the interaction between the factors Reach and Time [CE × TIME (BA)] will be significant, with no significant interaction in BA × CE. Both interactions will be significant if there is not only a substantial difference between the sites and periods, but also a large variation within each period in each site (Cochero et al., 2013).
Differences between diatom assemblages in C and E reach before and after nutrient addition were analyzed by a multidimensional scaling (MDS) ordination analysis, using the Bray–Curtis similarity measure; species abundance data were ln (x + 1) transformed (Warwick & Clarke, 1993; McCune & Grace, 2002).
Results
Physical and chemical variables
The physical and chemical parameters (Table 1) differed between reaches and periods. The differences were significant (CE × TIME [BA], P < 0.05) between C and E reaches in conductivity, pH, dissolved oxygen, SRP, and DIN concentrations (Table 1). During the pre-enrichment period, the conductivity values were slightly higher in the E than in the C reach, and these differences were enhanced after fertilization (P < 0.001).The temperature variations followed the typical seasonal ranges (summer: 32.8–16.2°C, autumn: 14.9–8.3°C, winter: 10–4.6°C and spring: 26.1–14.3°C), but the pattern did not differ between reaches. While the dissolved oxygen concentration averaged 9.8 mg l−1 in both reaches during pre-enrichment, it significantly (P = 0.003) decreased in the E reach (average 8.6 mg l−1; Table 1) during enrichment. The pH values during the enrichment were higher in the C than in E reach (P = 0.001).
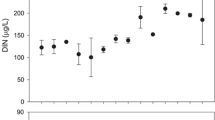
Nutrient addition increased the average SRP concentrations by about 5-fold in the E reach (Fig. 3) when compared to the C reach (CE × BA, P = 0.027), and the DIN concentration by about 2-fold when compared to the C reach (Table 1). However, the DIN, had an average concentration of 0.65 mg l−1 before the fertilization period in the C reach, and of 1.38 mg l−1 in the E reach. During the fertilized period the average concentration of DIN in both reaches decreased. Although the effect of fertilization was significant [CE × TIME (BA), P < 0.001], this was mainly due to the first 2 months after fertilization; following that time the DIN remained similar in both reaches. The DIN:SRP relationship changed from 3.2 to 4.6 in the C reach and from 5.6 to 1.9 in the E reach (Table 1), indicating an imbalance due to denitrification processes (Cochero et al., 2013) in La Choza stream.
Diatom assemblage
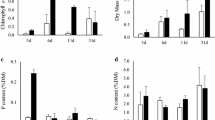
A total of 200 diatom species were identified during the study, and 64 of them were used for the statistical analyses (Table 2). The most frequent and abundant species throughout the study were Denticula kuetzingii, Nitzschia frustulum, Hippodonta hungarica, Nitzschia amphibia, N. palea, Ulnaria ulna, Gomphonema parvulum, Melosira varians, Nitzschia inconspicua, Achnanthidium minutissimum, Tryblionella kuetzingii, Caloneis bacillum, and Nitzschia supralitorea. Species richness and diversity were significantly lower (P < 0.001) in both reaches, particularly in the E reach at the end of the experiment (Figs. 2, 3; Table 3). However, cell density increased significantly, reaching a maximum of 82 × 106 cell cm−2 in the E reach at the end of the experiment, while the C reach attained 17 × 106 cell cm−2 (Fig. 4; Table 3). A clear separation in species densities in E reach during enrichment period was observed in the MDS ordination (Fig. 5). The stress value obtained was 0.1; according to Clarke & Warwick (1994) this value corresponds to a good ordination with no real prospect of misleading interpretation.
Enrichment promoted the growth of Nitzschia palea, N. inconspicua, and N. frustulum significantly (Table 2). Meanwhile Denticula kuetzingii and Hippodonta hungarica were significantly favored by enrichment in the early stages of fertilization but declined at the end of the experiment and were replaced by N. frustulum mainly. The BACIPS ANOVA results evidenced a significant immediate effect in the Nitzschia palea density in the E reach, while the effect were gradual for the rest of species mentioned above (Table 2).
Enrichment caused a low impact on species richness (η 2 < 0.20), but caused moderate impact on its abundance and diversity (0.20 > η 2 ≤ 0.8) (Fig. 6). Regarding the species densities, the effect of the fertilization was greater on Nitzschia frustulum, N. inconspicua, Denticula kuetzingii, and Hippodonta hungarica (0.20 > η 2 ≤ 0.8), whereas for Nitzschia palea the effect was lower (η 2 < 0.20).
Proportion of variance explained by nutrient enrichment on diatom diversity, abundance, and richness and selected species from BACIPs analysis, the values represent the η 2 values (NIFR Nitzschia frustulum, DKUE Denticula kuetzingii, NINC Nitzschia inconspicua, HHUN Hippodonta hungarica, NPAL Nitzschia palea)
The proportion of Nitzschia species increased significantly (P < 0.05) in the enriched reach as a consequence of nutrient addition (Fig. 7), while different species (e.g., Denticula kuetzingii and Hippodonta hungarica) were dominant in the Control reach. The proportion of Navicula species did not show significant differences between reaches (Fig. 7; Table 2).
The nutrient enrichment determined changes in the proportion of growth forms of the diatom community. The postrate motile forms (e.g., Nitzschia species) were significantly (P < 0.05) more abundant in the E reach in the post enrichment period (Table 2; Fig. 8), while the adnate forms (e.g., Denticula species) prevailed in the C reach (P < 0.05).
Discussion
Increase in nutrient loadings of aquatic ecosystems has been predicted by different scenarios of global change (Hulme & Sheard, 1999). Our study shows that nutrient enrichment in a Pampean stream, already characterized by high basal nutrient levels (Feijoó et al., 1999; Artigas et al., 2013), caused an increase in total diatom density and a decrease in species richness and diversity. The additional nutrient load also caused the change in the diatom taxa proportion, favoring motile forms, Nitzschia species mainly.
Several studies have already reported that diatom density may increase as a consequence of nutrient addition in streams. Usually, these studies have been performed in systems with lower basal nutrient status (Kelly, 2003; Bellinger et al., 2006; Gudmundsdottir et al., 2013), where light can be the mainly modulating factor (Veraart et al., 2008) to nutrient enrichment. In La Choza stream the significant increase in diatom density was associated with the increase of nutrient concentration, as well as with the full light availability.
The most remarkable effect caused by nutrient enrichment on the diatom community in La Choza was the change in the diatom species proportion within the diatom community. In all of the cases the response was not immediate, and most of the changes were obvious only at the end of the enrichment. Such a delay in the response to nutrient enrichment was also observed in a forested stream, where light was limiting (Veraart et al., 2008) and where changes coupled to short windows of opportunity (i.e., higher light availability coupled with nutrient enrichment).
Diatom species showed differential responses to enrichment. Most of the taxa that decreased in response of the nutrient addition (Caloneis bacillum, Nitzschia amphibia, Hippodonta hungarica, Denticula kuetzingii, Achnanthidium minutissimum, and Ulnaria ulna) are considered as mesotrophic, and have a recognized sensitivity to increased conductivity, organic matter and nutrient concentration (van Dam et al., 1994; Gómez & Licursi, 2001; Licursi et al., 2010). However, those species that increased their densities as consequence of the experimental enrichment were more tolerant to higher nutrient concentrations; this was the case of Nitzschia frustulum, N. inconspicua, and N. palea. These are common and abundant diatom species in Pampean river systems affected by agricultural and urban land uses, as consequence of high organic matter and nutrients amounts (Gómez & Licursi, 2001; Licursi & Gómez, 2002; Sierra et al., 2013).These species have also been reported to be able of using organic nitrogen (Hellebust & Lewin, 1977; van Dam et al., 1994; Tuchman et al., 2006). In La Choza stream these taxa could be favored by greater availability of organic matter (from increased production of autochthonous organic matter) and by the imbalance of the N:P en E reach. An increase in the relative abundance of Nitzschia species has been associated to high sediment and phosphorus loads (Kelly, 2003; Licursi & Gómez, 2009), and was reported by Pan et al. (2012) as indicator of both water quality and habitat degradation.
Cell motility can influence the ecological success of certain species (Hudon & Legendre, 1987). Motility may allow it to actively seek limiting resources, such as light or nutrients, and, thus, reduce the intensity of density-dependent effects (Mc Cormick & Stevenson, 1991). Motile diatom species have a competitive advantage over their attached relatives as they can actively expose themselves to optimal light conditions and avoid oxidative damage under supersaturating irradiance condition (Cohn & Weitzell, 1996; Cartaxana & Serôdio, 2008; Barnett et al., 2015). Another advantage of motility is that the cells can move to nutrient-richer microhabitats when nutrients are limiting growth (Hoagland et al., 1982; Hoagland, 1983; Svensson et al., 2014). According to Passy (2007) motile forms are comparatively free of both resource limitation and disturbance stress. Accordingly, high abundance of motile species has been reported associated to physical disturbance by siltation in benthic habitat of rivers (Bahls et al., 1992; Fore & Grafe, 2002; Licursi & Gómez, 2009; Pan et al., 2012), and also in nutrient-rich habitats (Kelly, 2003; Vilbaste & Truu, 2003; Passy, 2007; Gottschalk & Kahlert, 2012; Pan et al., 2012). In addition, in La Choza stream, nutrient enrichment favored the shift of adnate growth form (e.g., Denticula species) to motile growth forms (Nitzschia species, mainly). Bellinger et al. (2006) reported increase in the proportion of motile forms in an experimental enrichment in an African stream. Changes in predominant growth forms were also reported by Gudmundsdottir et al. (2013), in a study of nitrogen enrichment of sub-Artic streams, but that produced the decrease of motile diatoms (e.g., Nitzschia and Navicula) and the increase of attached diatoms by mucilage pads. Whether this difference is due to the use of only nitrogen in the nutrient enrichment, while in the rest of discussed studies enrichment both nitrogen and phosphorus were used, remains to be tested.
Changes in diatoms were not the only ones caused in the biota of La Choza. Complementary studies related with Functional Feeding Groups (FFG) of invertebrates (Ocón et al., 2013) during the experimental nutrient addition in La Choza stream, revealed a significant increase in filtering-collectors and a significant decrease in gathering-collectors in the Enriched reach, while predators and grazers did not show statistically significant changes. Their results also indicate that after the enrichment the diatoms became the least abundant basal food web resource of all the FFGs. The authors found that in the guts of Pomacea canaliculata (main scraper in La Choza stream), at the treatment site, the proportion of diatoms had become significantly lower and mainly vascular plants were dominant.
Conclusion
Nutrient addition in La Choza stream was associated with changes in density, richness, diversity, growth forms, and the tolerance of species to eutrophication. The additional fertilization in La Choza for 14 months, produced in a system with high basal levels of nutrients and high irradiance, caused a mild to moderate effect, indeed not immediate, on the diatom assemblage. These delayed responses of moderate intensity could be related with intrinsic characteristics of diatom assemblages pre-adapted to nutrient-rich environments. Our results indicate that the rising urbanization and agricultural activity in the Pampean plain, may seriously impair the biodiversity of its rivers if the entrance of nutrients to these ecosystems is not mitigated.
References
Acuña, V., C. Vilches & A. Giorgi, 2011. As productive and slow as a stream can be the metabolism of a Pampean stream. Journal of the North American Benthological Society 30: 71–83.
American Public Health Association (APHA), 1998. Standard Methods for Examination of Water and Wastewater, 20th ed. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington DC.
Armendariz, L., C. Ocón & A. Rodrigues Capítulo, 2012. Potential responses of oligochaetes (Annelida, Clitellata) to global changes: experimental fertilization in a lowland stream of Argentina (South America). Limnologica 42(2): 118–126.
Artigas, J., E. García-Berthou, D. E. Bauer, M. I. Castro, J. Cochero, D. Colautti, A. Cortelezzi, J. Donato, A. Elosegi, C. Feijoó, A. Giorgi, N. Gómez, L. Leggeri, I. Muñoz, A. Rodrigues-Capitulo, A. M. Romaní & S. Sabater, 2013. Global pressures, specific responses: the effects of nutrient enrichment in streams. Environmental Research Letters 8: 014002.
Bahls, L. L., R. Bukantis & S. Tralles, 1992. Benchmark Biology of Montana Reference Streams. Montana Department of Health and Environmental Sciences; WaterQuality Bureau, Helena.
Barnett, A., V. Méléder, L. Blommaert, B. Lepetit, W. Vyverman, K. Sabbe, C. Dupuy & J. Lavaud, 2015. Growth form defines physiological photoprotective capacity in intertidal benthic diatoms. The ISME Journal, Nature Publishing Group 2015(9): 32–45.
Bauer, D. E., J. Donadelli, N. Gómez, M. Licursi, C. Ocón, A. C. Paggi, A. Rodrigues Capítulo & M. Tangorra, 2002. Ecological status of the Pampean plain streams and rivers (Argentina). Verhandlungen des Internationalen Verein Limnologie 28: 259–262.
Bellinger, B. J., C. Cocquyt & C. M. O’Reilly, 2006. Benthic diatoms as indicators of eutrophication in tropical streams. Hydrobiologia 573: 75–87.
Biggs, B. J. F., 1996. Patterns in benthic algae of streams. In Stevenson, R. J., M. L. Bothwell, & R.L. Lowe (eds), Algal Ecology Freshwater Benthic Ecosystems, Academic Press. San Diego: 31–56, 753.
Böhm, J. S., M. Schuch, A. Düpont & E. A. Lobo, 2013. Response of epilithic diatom communities to downstream nutrient increases in Castelhano Stream, Venâncio Aires City, RS, Brazil. Journal of Environmental Protection 4: 20–26.
Borchardt, M. A., 1996. Nutrients. In Stevenson, R. J., M. L. Bothwell, & R. L. Lowe (eds), Algal Ecology Freshwater Benthic Ecosystems, Academic Press. San Diego: 183–227, 753.
Bothwell, M. L., 1989. Phosphorus—Limited growth dynamics of lotic periphytic diatom communities: areal biomass and cellular growth rate responses. Canadian Journal of Fisheries and Aquatic Sciences 46: 1293–1301.
Cartaxana, P. & J. Serôdio, 2008. Inhibiting diatom motility: a new tool for the study of the photophysiology of intertidal microphytobenthic biofilms. Limnology and Oceanography 6: 466–476.
Clarke, K. R. & R. M. Warwick, 1994. Change in Marine Communities: an Approach to Statistical Analysis and Interpretation. Natural Environment Research Council.
Chetelat, J., F. R. Pick & A. Morin, 1999. Periphyton biomass and community composition in rivers of different nutrient status. Canadian Journal of Fisheries and Aquatic Sciences 56(4): 560–569.
Cochero, J., A. M. Romaní & N. Gómez, 2013. Delayed response of microbial epipelic biofilm to nutrient addition in a pampean stream. Aquatic Microbial Ecology 69: 145–155.
Cochran, W. G., 1941. The distribution of the largest of a set of estimated variances as a fraction of their total. Annals of Eugenics 11: 47–52.
Cohn, S. A. & R. E. Weitzell Jr, 1996. Ecological considerations of diatom cell motility. I. Characterization of motility and adhesion in four diatom species. Journal of Phycology 32: 928–939.
Colautti, D. C., M. E. Maroñas, E. D. Sendra, L. C. Protogino, F. Brancolini & D. Campanella, 2009. Ictiofauna del arroyo La Choza, cuenca del Río de la Reconquista (Buenos Aires, Argentina). Biología Acuática 26: 55–62.
Dodds, W. K., 2006. Eutrophication and trophic state in rivers and streams. Limnology and Oceanography 51(1): 671–680.
Dodds, W. K., V. H. Smith & B. Zander, 1997. Developing nutrient targets to control benthic chlorophyll levels in streams: a case study of the Clark Fork River. Water Research 31: 1738–1750.
Dodds, W. K., V. H. Smith & K. Lohman, 2002. Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Canadian Journal of Fisheries and Aquatic Sciences 59: 865–874.
Dodds, W. K., W. W. Bouska, J. L. Eitzmann, T. J. Pilger, K. L. Pitts, A. J. Riley, J. T. Schloesser & D. J. Thornbrugh, 2009. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environmental Science & Technology 43: 12–19.
Ereño, C. 2002. Climatología de la cuenca. En: Borthagaray, JM (Comp). El Río de la Plata como territorio. FADU-UBA Ed. Infinito-Furban. Buenos Aires. 51–75.
FAO, 2013. Food and Agriculture Organization of the United Nations. FAOSTAT. http://faostat.fao.org.
Feijoó, C. S., A. Giorgi, M. E. García & F. Momo, 1999. Temporal and spatial variability in streams of a Pampean basin. Hydrobiologia 394: 41–52.
Feijoó, C., L. Leggieri, C. Ocón, I. Muñoz, A. Rodrigues Capítulo, A. Giorgi, D. Colautti, N. Ferreiro, M. Licursi, N. Gómez & S. Sabater, 2014. Stoichiometric homeostasis in the food web of a chronically nutrient-rich stream. Society of Freshwater Sciences 33(3): 820–831.
Fore, L. S. & C. Grafe, 2002. Using diatoms to assess the biological condition of large rivers in Idaho (USA). Freshwater Biology 47: 2015–2037.
Giorgi, A., 1998. Factores reguladores del fitobentos de arroyos de llanura. PhD thesis No. 711, Universidad Nacional de La Plata, La Plata.
Giorgi, A., C. Feijoó & G. Tell, 2005. Primary producers in a Pampean stream: temporal variation and structuring role. Biodiversity and Conservation 14: 1699–1718.
Gómez, N., 1998. Use of epipelic diatom for evaluation of water quality in the Matanza-Riachuelo (Argentina), a pampean plain river. Water Research 32: 2029–2034.
Gómez, N. & M. Licursi, 2001. The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquatic Ecology 35(2): 173–181.
Gottschalk, S. & M. Kahlert, 2012. Shifts in taxonomical and guild composition of littoral diatom assemblages along environmental gradients. Hydrobiologia 694: 41–56.
Gudmundsdottir, R., J. S. Olafsson, S. Palsson, G. M. Gislason & B. Moss, 2011. How will increased temperature and nutrient enrichmentaffect primary producers in sub-Arctic streams? Freshwater Bio logy 56: 2045–2058.
Gudmundsdottir, R., S. Palsson, E. R. Hannesdottir, J. S. Olafsson, G. M. Gislason & B. Moss, 2013. Diatoms as indicators: the influences of experimental nitrogen enrichment on diatom assemblages in sub-Arctic streams. Ecological Indicators 32: 74–81.
Hart, D. D. & C. T. Robinson, 1990. Resource limitation on a stream community: phosphorus enrichment effects on periphyton and grazers. Ecology 71: 1494–1502.
Hellebust, J. A. & J. Lewin, 1977. Heterotrophic nutrition. In Werner, D. (ed), The Biology of Diatoms. University of California Press, Berkeley Press, Berkeley and New York: 169–197, 498.
Hoagland, K. D., 1983. Short term standing crop and diversity of periphytic diatoms in a eutrophic reservoir. Journal of Phycology 19: 30–38.
Hoagland, K. D., S. C. Roemer & J. R. Rosowski, 1982. Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae). American Journal of Botany 69(2): 188–213.
Hudon, C. & P. Legendre, 1987. The ecological implications of growth forms in epibenthic diatoms. Journal of Phycology 23: 434–441.
Hulme, M. & N. Sheard, 1999. Climate change scenarios for Argentina. Climatic Research Unit, Norwich.
Johnson, R. E., N. C. Tuchman & C. G. Peterson, 1997. Changes in the vertical microdistribution of diatoms within a developing periphyton mat. Journal of the North American Benthological Society 16: 503–519.
Kelly, M. G., 2003. Short term dynamics of diatoms in an upland stream and implications for monitoring eutrophication. Environmental Pollution 125: 117–122.
Kelly, M. G. & B. A. Whitton, 1995. Trophic diatom index—a new index for monitoring Eutrophication in Rivers. Journal of Applied Phycology 7: 433–444.
Kelly, M. G., A. Cazaubon, E. Coring, A. Dell’Uomo, L. Ector, B. Goldsmith, H. Guasch, J. Hürlimann, A. Jarlman, B. Kawecka, J. Kwandrans, R. Laugaste, E. A. Lindstrøm, M. Litao, P. Marvan, J. Padisák, E. Pipp, J. Prygiel, E. Rott, S. Sabater, H. van Dam & J. Vizinet, 1998. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. Journal of Applied Phycology 10: 215–224.
Kitoh, A., S. Kusunoki & T. Nakaegawa, 2011. Climate change projections over South America in the late 21st century with the 20 and 60 km mesh Meteorological Research Institute atmospheric general circulation model (MRI-AGCM). Journal of Geophysical Research 116(D6): 14920.
Kjeldsen, K., T. M. Iversen, J. Thorup & T. Winding, 1998. Benthic algal biomass in an unshaded first-order lowland stream: distribution and regulation. Hydrobiologia 377: 107–122.
Krammer, K., 1992. Pinnularia: eine Monographie der europäischen Taxa. Biblioteca Diatomologica 26: 1–353.
Krammer, K., 2000. The genus Pinnularia. In Lange-Bertalot, H. (ed.), Diatoms of Europe, Vol. 1. ARG GantnerVerlag, Ruggell.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae teil 1: naviculaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Vol. 2. GustavFischerVerlag, Stuttgart.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae: teil 2: bacillariaceae, epthemiaceae, surirellaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Vol. 2. Gustav Fischer Verlag, Stuttgar.
Krammer, K. & H. Lange-Bertalot, 1991a. Bacillariophyceae teil 3: centrales, fragilariaceae, eunotiaceae. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Vol. 2. Gustav Fischer Verlag, Stuttgart.
Krammer, K. & H. Lange-Bertalot, 1991b. Bacillariophyceae Teil 4: achnanthaceae, literaturverzeichnis. In Ettl, H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Vol. 2. Gustav Fischer Verlag, Stuttgart.
Licursi, M. & N. Gómez, 2002. Benthic diatom and some environmental condition in three lowland streams of Pampean Plain. Annales de Limnologie 38(2): 109–118.
Licursi, M. & N. Gómez, 2009. Effects of dredging on benthic diatom assemblages in a lowland stream. Journal of Environmental Management 90(2): 973–982.
Licursi, M., N. Gómez & J. Donadelli, 2010. Ecological optima and tolerances of coastal benthic diatoms in a freshwater mixohaline zone from the Río de la Plata estuary. Marine Ecology Progress Series 418: 105–117.
Liess, A. & H. Hillebrand, 2004. Invited review: direct and indirect effects in herbivore –periphyton interactions. Archiv fur Hydrobiologie 159(4): 433–453.
Lobo, E. A., V. L. M. Callegaro, G. Hermany, N. Gomez & L. Ector, 2004. Review of the use of microalgae in South America for monitoring rivers, with special reference to diatoms. Vie et Milieu 53(2/3): 35–45.
Ludwig, J. A. & J. F. Reynolds, 1988. Statistical Ecology. Wiley, New York. 337.
Martínez, D. E. & M. Osterrieth, 1998. Geoquímica de la Sílice Disuelta en el Acuífero Pampeano en la Vertiente Sudoriental de Tandilia. Serie Correlación Geológica 13: 241–250.
Mc Cormick, P. V. & R. J. Stevenson, 1991. Mechanisms of benthic algal succession in lotic environments. Ecology 72: 1835–1848.
McCune, B. & J. B. Grace, 2002. Analysis of Ecological Communities. MjM Software Design, Gleneden Beach: 300.
Molloy, J. M., 1992. Diatom communities along stream longitudinal gradients. Freshwater Biology 28: 59–69.
Ocón, C., M. V. Lopez-van Oosterom, M. I. Muñoz & A. Rodrigues-Capítulo, 2013. Macroinvertebrate trophic responses to nutrient addition in a temperate stream in South America. Fundamental and Applied Limnology 182(1): 17–30.
Pan, Y., R. M. Hughes, A. T. Herlihy & P. R. Kaufmann, 2012. Non-wadeable river bioassessment: spatial variation of benthic diatom assemblages in Pacific Northwest rivers, USA. Hydrobiologia 684: 241–260.
Passy, S. I., 2007. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquatic Botany 86: 171–178.
Patrick, R. & C. W. Reimer, 1966. The Diatoms of the United States, Exclusive of Alaska and Hawaii. Monograph Academy of Natural Sciences of Philadelphia, Philadelphia: 131–152.
Patrick, R. & C. W. Reimer, 1975. The Diatoms of the United States, Exclusive of Alaska and Hawaii. Monograph Academy of Natural Sciences of Philadelphia, Philadelphia: 132.
Pringle, C. M., 1990. Nutrient spatial heterogeneity—effects on community structure, physiognomy, and diversity of stream algae. Ecology 71: 905–920.
Rier, S. T. & R. J. Stevenson, 2006. Response of periphytic algae to gradients in nitrogen and phosphorus in streamside mesocosms. Hydrobiologia 561: 131–147.
Rodrigues Capítulo, A., N. Gómez, A. Giorgi & C. Feijoo, 2010. Global changes in pampean lowland stream: implications for biodiversity and functioning. Hydrobiologia 657: 53–70.
Rumrich, U., H. Lange-Bertalot & M. Rumrich, 2000. Iconographia Diatomologica—Annotated Diatom Micro-graphs: diatoms of the Andes, from Venezuela to Patagonia/Tierra delFuego, Vol. 9. GanterVerlag, Ruggell.
Sala, O. E., F. S. Chapin III, J. J. Armesto, E. Berlow, J. Blommfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774.
Sabater, S., J. Artigas, A. M. Romaní, A. Gaudes, I. Muñoz & G. Urrea, 2011. Long-term moderate nutrient inputs enhance autotrophy in a forested mediterranean stream. Freshwater Biology 56: 1266–1280.
Sierra, M. V., N. Gómez, A. V. Marano & M. Di Siervi, 2013. Caracterización funcional y estructural del biofilm epipélico en relación al aumento de la urbanización en un arroyo de la llanura pampeana (Argentina). Ecología Austral 23: 108–118.
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology and Evolution 24(4): 201–207.
Stevenson, R. J., 1996. An introduction to algal ecology in freshwater benthic habitats. In Stevenson, R. J., M. L. Bothwell, & R. L. Lowe (eds), Algal Ecology Freshwater Benthic Ecosystems, Academic Press. San Diego: 3–30, 753.
Stevenson, R. J. & S. Sabater, 2010. Understanding effects of global change on river ecosystems: science to support policy in a changing world. Hydrobiologia 657: 3–18.
Stevenson, R. J., S. T. Rier, C. M. Riseng, R. E. Schult & M. J. Wiley, 2006. Comparing effects of nutrients on algal biomass in streams in two regions with different disturbance regimes and with applications for developing nutrient criteria. Hydrobiologia 561: 149–165.
Svensson, F., J. Norberg & P. Snoeijs, 2014. Diatom cell size, coloniality and motility: trade-offs between temperature, salinity and nutrient supply with climate change. PLoS ONE 9(10): e109993. doi:10.1371/journal.pone.0109993.
Stewart-Oaten, A. & J. R. Bence, 2001. Temporal and spatial variation in environmental impact assessment. Ecological Monographs 71: 305–339.
Tuchman, N. C., M. A. Schollett, S. T. Rier & P. Geddes, 2006. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 561: 167–177.
van Dam, H., A. Mertens & J. Sinkeldam, 1994. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Netherlands Journal of Aquatic Ecology 28: 117–133.
Veraart, A. J., A. M. Romaní, M. Tornés & S. Sabater, 2008. Algal response to nutrient enrichment in forested oligotrophic stream. Journal of Phycology 44: 564–572.
Vilbaste, S. & J. Truu, 2003. Distribution of benthic diatoms in relation to environmental variables in lowland streams. Hydrobiologia 493: 81–93.
Walton, S. P., E. B. Welch & R. R. Horner, 1995. Stream periphyton response to grazing and changes in phosphorus concentration. Hydrobiologia 302: 31–46.
Warwick, R. M. & K. R. Clarke, 1993. Increased variability as a symptom of stress in marine communities. Journal of Experimental Marine Biology and Ecology 172: 215–226.
Welch, E. B., J. M. Quinn & C. W. Hickey, 1992. Periphyton biomass related to pointsource nutrient enrichment in seven New Zealand streams. Water Resources 26(5): 669–675.
Winter, J. G. & H. C. Duthie, 2000. Epilithic diatoms as indicators of stream total N and total P concentration. Journal of the North American Benthological Society 19: 32–49.
Worm, B., H. K. Lotze, H. Hillebrand & U. Sommer, 2002. Consumer versus resource control of species diversity and ecosystem functioning. Nature 417: 848–851.
Wyatt, K. H., R. J. Stevenson & M. R. Turestky, 2010. The importance of nutrient co-limitation in regulating algal community composition, productivity and algal-derived DOC in an oligotrophic marsh in interior Alaska. Freshwater Biology 55: 1845–1860.
Acknowledgments
This study was funded by the project GLOBRIO of the Banco Bilbao Vizcaya Argentaria (BBVA) Foundation. We would like to express our thanks to the anonymous reviewers for improvements in this manuscript. This is ILPLA Scientific Contribution No. 940.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Rights and permissions
About this article
Cite this article
Licursi, M., Gómez, N. & Sabater, S. Effects of nutrient enrichment on epipelic diatom assemblages in a nutrient-rich lowland stream, Pampa Region, Argentina. Hydrobiologia 766, 135–150 (2016). https://doi.org/10.1007/s10750-015-2450-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2450-7