Abstract
Life cycle and microdistribution patterns of Cordulegaster heros, a charismatic species for nature conservation, are poorly known. Life history characteristics and multiscale habitat preferences of the larvae were followed for one year in monthly intervals by systematic samplings in eight headwaters, which resulted in data on 2562 individuals. We hypothesized that meso- and microhabitat complexity play an important role in forming the population structure and microdistribution of the species. Based on the distribution of the consecutive larval instars, duration of later stages and time of molt and emergence, the larval development of C. heros in the Mecsek Mountains lasts for at least three, but with a maximum of four years. All three levels of the multi-habitat structure [habitat (sites), and meso- (riffle/pool sequence) and microhabitats (biotic and different particle-sized abiotic types)] have significant effects on the spatial distribution of the larvae. Densities and population structures vary among the sites, but mesohabitat type and microhabitat diversity (heterogeneity within a pool or riffle) govern the microdistribution. C. heros prefers pools with small or medium microhabitat heterogeneity and higher proportion of small particle-sized substrates, especially in younger stages. Older larvae are less sensitive for these effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The habitat of species, which may vary with space and time, is represented by different levels of scale, complexity, and heterogeneity. This is also true for the Odonates (Corbet, 1999). Streams can be defined as hierarchically organized systems incorporating stream segment, reach, pool/riffle sequence, and microhabitat subsystems. Habitats at all levels reside within the watershed environment, yet each segment, reach, or pool/riffle system plays a particular structural and functional role (physically and biologically) in the stream system and exists in a particular location in the watershed (Frissel et al., 1986). Mesohabitat sequences (pools and riffles) are key habitat elements of river ecosystems, as these areas can be identified as distinct habitats in most streams primarily on the basis of flow, depth, slope, and their specific physical characteristics (Pace et al., 2011). Habitat heterogeneity, derived from the variation of riffles (low water depth and high velocity) and pools (high water depth and low velocity), has an important role in structuring aquatic assemblages (Pace et al., 2011; Schmera & Erős, 2011). Microhabitat subsystems are defined as patches within pool/riffle systems with relatively homogenous substrate type, water depth, and velocity (Frissel et al., 1986). Substrate is the primary refuge of benthic residents, and plays the role of the principal habitat. The distribution pattern of benthic individuals and species occurrence highly depend on substrate size, consequently on microhabitat structure (Beisel et al., 1998; Marczak et al., 2006).
Cordulegaster heros Theischinger, 1979 is endemic to Central and Southeastern Europe. It has a relatively restricted area: besides Hungary, it occurs only in Austria, Czech Republic, Slovakia, Italy, and on the Balkans (Bedjanic & Salamun, 2003; Blaškovič et al., 2003; Staufer & Holuša, 2010; van Tol, 2013). C. heros is an IUCN Red-listed (near threatened, Kalkman et al., 2010) and a Natura 2000 species of community importance listed in annex II and IV (EC, 1992; Dévai, 2014), as well as a strictly protected dragonfly in Hungary (VM, 2012).
One of the longest larval developments is found in the family Cordulegastridae, where larvae have a sedentary lifestyle and inhabit relatively cool habitats (Corbet, 1999). These dragonflies typically occur in lotic waters, generally in small streams and brooks, where larvae live as shallow burrowers in the sediment (Ferreras-Romero & Corbet, 1999). Our knowledge on the larval development and habitat preference (including and focusing on meso- and microhabitats) is relatively poor and episodic. Marczak et al. (2006) studied the larvae of Cordulegaster dorsalis (Hagen in Selys, 1858) and revealed a size-mediated microhabitat preference in pools, with earlier instars preferring finer sediment with a higher organic fraction. This was also confirmed for C. heros by Lang et al. (2001) who detailed the life history characteristics describing a longitudinal distribution and microhabitat preferences of C. heros larvae (and C. bidentata Selys 1843, as well) in an Austrian stream system. They also noted abiotic parameters, which may have been responsible for the observed pattern. According to their findings, C. heros prefers second-order stream sections with lower calcium content and conductivity, and a higher proportion of coarse sediment compared to C. bidentata.
Life cycle characteristics, numbers of the larval stages, duration of the larval developments, and ecological requirements of a species may vary among different ecoregions, climatic conditions, altitudes, or habitat types (Corbet, 2002). However, only distributional data for C. heros exist. No ecological and/or ethological information on other populations of C. heros are known, including from the Hungarian populations.
Our basic aims were to explore the multiscale habitat preferences of C. heros. We hypothesized that the spatiotemporal distribution is determined by the meso- and microhabitat composition and structure of the streams. For this purpose, we explored and described the life cycle characteristics and spatial distribution of C. heros and tested the effects of seemingly important abiotic habitat characteristics at different spatial scales in the streams of the Mecsek Mountains.
Materials and methods
Study sites
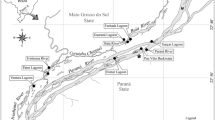
The recordings of the larval distribution were conducted in monthly intervals from June 2011 to May 2012 at eight headwaters in the Mecsek Mountains, SW Hungary (Fig. 1, for list of sites see figure legend). This area has some of the highest densities of this red-listed species in Hungary (Boda et al., 2011). Most of the sampling sites were located along second-order stream sections except the, KOR and BAR, which were first-order headwaters. All streams can be characterized as perennial submountain streams, though during extremely dry summers (like in 2012), some of them dried out completely for more than a month. Odonata fauna from these sites consisted of two species: we observed the absolute dominance of C. heros, with Calopteryx virgo (Linnaeus, 1758) being the only co-occurring species.
Study sites in Mecsek Mountains, SW Hungary. Baranya stream (BAR) 46°09′36″N, 18°16′28″E, 290 m elevation above sea level (a.s.1.); Hidasi stream (HID) 46°11′41″N, 18°19′03″E, 312 m a.s.1.; Hosszúhetényi stream (HOS) 46°10′31″N, 18°20′55″E, 306 m a.s.1.; Körtvélyesi stream (KOR) 46°08′56″N, 18°14′43″E, 290 m a.s.1.; Mázai stream (MAZ) 46°15′29″N, 18°23′33″E, 210 m a.s.1.; Ól stream (OLV) 46°15′49″N, 18°22′02″E, 216 m a.s.1.; Petőczi stream (PET) 46°07′27″N, 18°02′49″E, 212 m a.s.1.; Vár stream (VAR) 46°13′40″N, 18°19′19″E, 281 m a.s.1
Sampling procedure
In every month, at each site, along a 200 m long section, 10 randomly chosen riffles and 10 randomly chosen pools (different in each occasion) were sampled within an area of two square meters. Each point was sampled for 3 min using a hand net according to the ‘kick and sweep’ method. Consecutive samplings were conducted in an upstream direction from the lowest point of the site. In the case of each sampled riffle and pool, the composition of the microhabitats (based on the AQEM types following Hering et al. (2004), see also supplementary Table S1), water depth, water width (maximal width of the water surface at each sampling unit), and velocity were measured.
For all captured individuals, head width (maximum distance between the lateral margins of the compound eyes), total body length (maximum distance between the mouthparts and the end of the cerci measured along the dorsal surface), length of wing sheaths (maximum length of forewing-pad, if presents), length of the labium (maximum length of the prementum), diameter of the mentum, and length of the metafemur (maximum length parallel to the dorsal margin) were measured to the nearest 0.01 mm using a digital caliper. Larval stages were separated using an isometrically (head width) and an allometrically (length of wing sheath) growing body dimension. Firstly, we determined provisional thresholds for each larval stage based on the head width, which is the most frequently used body dimension in separation of larval stages (Corbet, 2002). The final, penultimate, antepenultimate, and preceding instars were designated as F, F-1, F-2, and F-3. All larvae were assigned to one of these clearly identifiable instars or designated as ‘early instars’ (E). Then in case of the older stages (from F-3 to F), the classification was refined on the basis of the relationship between the head width and the length of wing sheath using the full dataset and the monthly separated datasets.
We also determined the time of molt of the larvae based on the presence of a thick coating of allochthonous particles on the body surface and the characteristics (hardness and color) of larval integument. We distinguished three categories ‘very clean,’ ‘clean,’ and ‘dirty.’ The ‘very clean’ larvae have a light yellow, soft, and flexible integument and the body does not have a thick coating. These larvae were likely molted within the last 24 h (Corbet, 2002). Such larvae were distinguishable from larvae with a dark and hard integument bearing moderate (‘clean’) or large (‘dirty’) amounts of allochthonous particles on the body surface. The sex of captured larvae was also determined. We could not determine the sex for small larvae (n = 1523) because to their young age. We followed a common practice described by Ferreras-Romero & Corbet (1999) for measuring morphological characteristics, designating larval stages, and determining sex and time of molt. After identification, measuring, and counting, the larvae were returned to their original mesohabitat spot.
Statistics
We used a goodness-of-fit-test to determine the sex ratio of C. heros larvae. Density distribution histograms and scatter plots were used to separate larval instars. To validate the classification of individuals to larval stages, we used discriminant analysis (LDA) with all measured body dimensions except body length, as variables. To test the differences of the abundances among the watercourses, one-way analysis of variances (ANOVA) was used. Grouping of the sites was made based on the monthly density of larval instars using hierarchical cluster analysis (Bray–Curtis index, UPGMA). To examine the differences of abiotic variables between riffles and pools, principal component analysis (PCA) and permutational multivariate analysis of variance (PERMANOVA, Bray–Curtis index, number of permutations: 9999) were used. PCA and PERMANOVA were made in the case of all the watercourses and also were based on the site groups from the cluster analysis. The differences between the numbers of collected individuals in mesohabitats were determined using independent samples t tests or Welch-tests (depending on the result of the Levene’s test). We tested the influence of the three habitat levels, the meso- and microhabitat composition (by calculating a Simpson diversity index for each riffle and pool), and hydrological characteristics on the abundance (also for grouped habitats and for each larval instar) using general linear models (GLMs) with calculation of Sum of Squares type III. Prior to those analyses, according to the autocorrelation tests, no strong (R 2 > 0.75), significant correlations were found among the variables, so all the factors and co-variables were included in the full models. Later, velocity was excluded because of its non-significant effect. Prior to analyses which included the abiotic dataset, the width, depth, and velocity variables were standardized to a range of 0–100 to allow for comparison with microhabitat data. To test and visualize the mesohabitat preferences of different larval stages, we fitted `species response curves (SRC)’ to the first axis of the samples PCA scatter plot using generalized addictive models (GAMs, df = 2, final model selection was based on Akaike information criterion (AIC)).
Density distribution histograms, univariate tests, and GLMs were conducted in R statistical environment ver. 3.12.0 (R Development Core team, 2014). PCA biplots, GAMs, and SRC figure were made using CANOCO for Windows Version 5.0 (Ter Braak & Smilauer, 2012). For hierarchical cluster analysis, discriminant analysis, and multivariate tests, we used PAST software version 3.01 (Hammer et al., 2001).
Results
Life cycle, larval development, and phenology
A total of 2562 C. heros larvae were captured during the study. Numbers of total captured individuals per sites were between 41 and 1152, and the monthly total catches were between 93 and 536 (Table 1). Average numbers of individuals per square meter were between 0.17 and 4.8 (for more details see supplementary Table S2).
Based on the relative frequency distributions of head width and length of wing sheath measurements, and the relationship between these body dimensions (Fig. 2 and supplementary Fig. S1), we were able to identify the instars F–F-3 and E. All larvae were assigned to one of these categories. Based on the result of LDA, 95.1% of larvae were correctly classified; uncertain classifications occurred only between F-2, F-3, and E stages (supplementary Table S3). The largest body length was almost 43 mm with 9.4 mm head width, whereas for the smallest captured larvae, these parameters were only 3 and 1.2 mm. Descriptive data on the morphological features of the different instars were summarized in supplementary Table S4.
Scatter plot and relative frequency distribution histograms of the head width and length of wing sheath measurements resulted in 4 + E recognizable larval instars (male and female larvae, and early instars (E) whose sex could not yet be identified) of Cordulegaster heros (n = 2562). (Instars are marked as E–F, for more information, see the text)
The sex ratio of F and F-1 larvae did not differ significantly from 1:1 (χ2 = 0.5035, df = 1, P = 0.4779). The proportion of the early instars (E) was the highest from July to October, and the final instar (F) reached the highest proportion between December and April (Table 1). Their proportion decreased in May and June, right after the start of emergence (Boda et al., 2015).
Based on the hardness and color of the integument and the dirt coverage of the larval body, larvae entered the final instar (F) exclusively between May and August. Molting to the penultimate instars (F-1, F-2, and F-3) and within early stages (E) also occurred predominantly in the same period (summer), with a few exceptions in other periods (Table 2).
Effects of the habitat structure on the spatial distribution
Habitat characteristics at all spatial scale (from microhabitats to habitat level) had significant effects in forming the spatial distribution of C. heros (Table 3). The model explained only 25.3% of the total variance, but all three levels significantly contributed to the development of number of individuals, though in various degrees. Habitat-level differences (between sites) explained the most variation (46.7%), mesohabitat structure (riffle-pool) explained 22.4%, and habitat-mesohabitat interaction explained 27.4% variation. The effect of microhabitat diversity explained the lowest variation (1.7%), but also proved to be significant (Table 3).
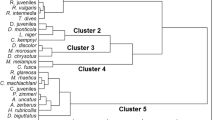
Among habitats (sites), the highest total number of individuals (1152) during 12 months occurred at BAR. Lowest numbers of individuals were found at HID, VAR, HOS (41, 45, 69). KOR and OLV had medium numbers (349, 536), and PET and MAZ produced lower numbers (169, 201) (Fig. 3A). Significant differences were found in the monthly numbers of individuals per square meter among the streams (ANOVA, F = 30.519, df = 7, P < 0.001). Incorporating the monthly larval instar density distributions, the eight streams could be classified to four ‘site groups’ with highly different population structure (Figs. 3B, 4). Group I contained the first order streams (BAR, KOR) where the total numbers of individuals were relatively high and the proportions of the younger instars (F-3 and E) were also the highest (68%, 37%, respectively). For Group II streams (MAZ, OLV), the very high (82%, 74%) proportion of F-2 and F-3 instars was the most remarkable feature. In Group III (HID, VAR), the common feature was the very low total numbers of individuals. For these streams, the proportion of the larval instars could not be seen as a reliable predictor. In Group IV (HOS, PET), the very high proportion (72%, 75%) of the final instars (F, F-1) was the common feature (Fig. 4).
Habitat-level distribution of C. heros in the Mecsek Mountains. A Density distribution of C. heros in eight sampling sites. Box interquartile range with median line, whisker: standard error of mean; n = 2562). B Grouping of eight sites based on population structures (densities of larval stages) of C. heros (Bray–Curtis, UPGMA). (For abbreviations of site names, see capture of Fig. 1; roman numbers (I–IV) indicate habitat (stream) groups)
Larval development of C. heros in previously grouped headwaters. The arrows and numbers indicate the time of the inferred development steps between larval instars (for more details see the text). Data of group III were not shown because of the very low numbers of individuals. Light gray ‘very clean’ individuals; dark gray ‘clean’ individuals; black ‘dirty’ individuals. (For abbreviations of site names, see capture of Fig. 1)
The different population structures among the groups of streams might have an effect on the response of the population to the meso- and microhabitat structure; therefore, we also paid attention on the different site groups in the following analyses.
During mesohabitat-level analyses, we first tested whether the main characteristics (width, depth, and velocity) and microhabitat composition of the two mesohabitats in different site groups (I–IV) were similar. Based on these variables, all pool and riffle samples were plotted and analyzed using PCA biplots (Fig. 6A, supplementary Fig. S2). In the case of the total dataset (all streams together, Fig. 6A), the riffles and pools were separated along the first component axis. The main variables distinguishing between mesohabitat types were microhabitat composition, velocity, and water depth along the first PCA axis. Width of the mesohabitats varied widely within the habitat types and explained along the second component axis. Pools were deeper and their characteristic microhabitat types were biotic (FPOM, CPOM, and somewhat the xylal) and abiotic with small particle size (argyllal and psammal). Accordingly, riffles were more shallow mesohabitats with dominance of larger particle stony microhabitats (macro-, meso-, and microlithal, and akal). Additional PCA analyses of the formerly defined four groups of streams with different population structure (supplementary Fig. S2) led almost to the same results. The only slight contradiction could be seen in the case of Group I, where riffles rather than pools were characterized more by psammal microhabitat, whereas the opposite occurred in the other groups. Significant differences between pools and riffles were revealed based on abiotic factors in cases of each stream group using One-way PERMANOVA tests (Group I: F = 187.3, P = 0.0001; Group II: F = 364.1, P = 0.0001; Group III: F = 255.7, P = 0.0001; Group IV: F = 268.2, P = 0.0001).
Significant differences were found in the number of individuals between riffles and pools at each sampling sites (BAR: t = 5.983, df = 22, P < 0.000; HID: t = 3.962, df = 13.542, P = 0.002; HOS: t = 5.053, df = 12.519, P < 0.000; KOR: t = 3.788, df = 12.083, P = 0.003; MAZ: t = 4.623, df = 11.665, P = 0.001; OLV: t = 4.264, df = 11.338, P = 0.001; PET: t = 2.553, df = 19.676, P = 0.019; VAR: t = 2.440, df = 22, P = 0.023). Numbers of larvae were significantly higher in pools than in riffles (Fig. 5).
Density distribution of C. heros larvae in pools and riffles in each sampling site shows differences between the two mesohabitat types. (For abbreviations of site names, see capture of Fig. 1, box interquartile range with median line; whisker standard error of mean; dot outliers; asterisk extreme outliers; n = 2562)
Based on the full dataset (all sites), the GLM model showed that all the parameters (width, depth, mesohabitat type, and the microhabitat diversity) had significant effects on the spatial distribution (numbers of individuals per square meter) of C. heros (supplementary Table S5). The mesohabitat type showed the strongest effects with numbers of individuals in pools being twice as high as in riffles. Microhabitat composition (given as Simpson diversity of microhabitat types) also seemed to be important, but its effect on the numbers of individuals was lower; if the diversity was increasing, the numbers of individuals were slightly decreasing. Changes in other parameters (width and depth) were also significant with minimal effects on the numbers of individuals.
We assumed that the effects of the habitat structure on the numbers of individuals might be different among site groups with different population sizes and structures (Group I–IV), and each parameter could affect different larval instars in various ways. Therefore, we ran the model for each site group (supplementary Table S5) and also for each larval instar separately (supplementary Table S6).
Mesohabitat type (pools or riffles) was the most important factor in all site groups, especially in cases of Group I and II streams (supplementary Table S5). This effect is highly significant in site groups II–IV, and marginally significant (P = 0.056) in Group I. The microhabitat composition showed significant effects in cases of Group I and IV streams.
The type of mesohabitat had significant effects on the number of individuals per square meter for all larval instars (supplementary Table S6), but its importance continuously decreased from the early instars to the final instar. This effect was also confirmed by the GAM model (Fig. 6B and supplementary Table S7). Smaller larvae clearly prefer the pools more than larger larvae. The microhabitat composition has slightly modifying but significant effects on the early instars, though the significant effects were minor for F and F-1 larvae. Water depth and width also showed significant but minimal effects on the numbers of individuals for all stages.
Biplot of principal component analysis (PCA) of all sampled riffles and pools of eight streams based on microhabitat composition and hydrological characteristics (panel A, eigenvalues were given on the axes as %, gray dots—riffles, and black dots—pools) and results of the GAM model fitting larval density to the first axis of the sample PCA scatter plot separating pools and riffles (panel B). The distinct separation of mesohabitat types based on microhabitat composition and water depth is visible along the first PCA axis (panel A); early stage larvae (E) show strong preference for pools, which also exists but strongly and continuously decreases in cases of older (F-3–F) stages (panel B)
Because the microhabitat composition significantly affected the numbers of individuals, especially in the cases of early instars, we plotted and analyzed numbers of individuals (total and all stages separately) against the proportion of each microhabitats (supplementary Fig. S4) in order to reveal microhabitat preferences of the various body sized larvae. There was no difference among the microhabitat preferences of the different life stages, and the trends were the same throughout the larval periods. In the cases of most of the microhabitat types, numbers of individuals were decreasing with increasing proportion of the given microhabitat type in the pools and riffles. The exceptions were the two microhabitats with the smallest particle sizes (psammal and argyllal), where the preferences moved to the middle of the proportion scale. The high numbers of individuals were linked to higher, but not the highest, proportions of these types of microhabitats in a parabolic shaped distribution.
In summary, the preference of the species to the higher proportion of the small particle-sized mineral substrates is higher in all larval stages than to the other types (supplementary Fig. S3). In the pools, the proportion of the biotic and fine particle-sized mineral substrate types (FPOM, CPOM, argyllal, and psammal) was higher (Fig. 6A and supplementary Fig. S2) than in riffles. The species shows clear preference for pools in all larval stages. All these effects are the strongest for the earlier larval stages (E), and the effects decrease during larval development (Fig. 6B, supplementary Table S6 and S7).
Discussion
Life cycle, larval development, and phenology
An accurate study of the life cycle of dragonflies requires the analysis of larval instars, which account for the longest period of their life history (Hawking & New, 1996). Biometry of certain morphological features, therefore, allows interpretation of their life cycle (Goretti et al., 2001). For Cordulegastridae, larval development may last for 2–5 years, with 14–15 larval stages being described (Corbet, 2002). From these larval stages, only the last few stages can clearly be separated based on morphological features. The time that individuals spend in earlier stages is short because many molting events occur within a short period.
Lang et al. (2001), the only known publication about C. heros life cycle and ecology, used the same method to separate 5 + E larval instars based on the measurements of 688 individuals originating from a stream system in upper Austria (300–400 m elevation a.s.l.). In the Mecsek Mountains, we found 4 + E larval instars based on data of 2562 individuals. Decreasing the annual and monthly average temperature (e.g., Corbet, 1999), together with increasing latitude (Valle, 1938; Ander, 1950 and Kormondy, 1959 cited in Corbet, 1983), increases the numbers of the stadia and the length of the development time, while emergence and molting is shifted to later. The same effect can be seen in the case of increasing altitude (Ferreras-Romero & Corbet, 1999). Our sampling sites are located at slightly lower latitude (2° to the south of the Austrian sites), but in a sub-mediterranean climate with presumably much higher temperature (mean annual temperature +2°C, number of the sunshine hours per year is +350) and about 100 m lower altitude (210–310 m a.s.l.) than the Austrian sites. The warmer climate may explain the lower number of larval instars and the shorter development period. Faster larval development of the Hungarian population compared with the Austrian populations might explain the overlapping head widths among stages.
Based on our one-year-long study, we cannot accurately determine the length and describe the whole process of larval development, but we were able to reveal some important aspects of larval development. The larvae reach emergent size in a shorter time than in Austria. Also the timing of molt between consecutive larval stages is shorter in the Hungarian population (Fig. 4). Our results suggest the following (numbers also refer to the numbered arrows on Fig. 4, indicating the processes). (1) Lang et al. (2001) described that the proportion of the final instars decreased (due to the emergence) in June and July, whereas in Hungary, the start of the emergence period occurred earlier (Fig. 4, Group IV., and see also Boda et al., 2015). (2) The newly hatched individuals (E) appeared in very high numbers in July and August, and the early instars were quite short, with most of the early larvae reaching F-3 by November (Fig. 4, Group I and II.). (3) Nevertheless, a remarkable number of young larvae spent the winter in these early stages, their integument became dirty and they enter the F-3 in spring (Fig. 4, Group I and II.). (4) The instar distribution in Group II indicates that some of the early larvae, most probably those which hatched very early, may reach the F-2 stage in the same year. Entering from F-3 to F-2 seems to be almost continuous during the year and larvae spent at least several months (approximately half a year) in F-2 stage. (5) Stepping from F-2 to F-1 and from F-1 to F stages occurred exclusively between May and August, in parallel with the emergence period. The larvae spent a whole year in both of these last two stages. (6) We found larvae in remarkably lower numbers of each stage during winter months. The reason for this is not a decrease in the numbers of individuals, but most probably the burrowing life style and decreased activity of the larvae during winter. Capture efficiency of Cordulegaster species can be lower during winter than in other periods of the year (Buchwald, 1988; Böcker, 1993; Heidemann & Seidenbusch, 1993; Lang et al., 2001).
Based on the points above, the larval development of C. heros in the Mecsek Mountains lasts for at least three, but maximum 4 years depending on the biotic and abiotic characteristics of the habitat and the weather conditions of the years. Corbet (1983) mentioned that the length of the larval development of C. heros is 4–5 years, but no other information can be found. For the better known and closely related C. boltonii, the literature mentions different periods for larval development depending on geographical location: 2–3 years in Southern Spain and Southern France (Schütte, 1997; Ferreras-Romero & Corbet, 1999), 4–5 years in the Pyrenees, in the Alps, in former Yugoslavian mountains, and in Southern Germany (Robert, 1958 and Kiauta, 1964 cited in Donath, 1987; Ferreras-Romero & Corbet, 1999), but it can be more than 5 years in the United Kingdom (Corbet et al., 1960).
Effects of the habitat structure on the spatial distribution
Our analyses revealed all three levels of the multi-habitat structure have significant effects on the spatial distribution of the C. heros larvae (Table 3). Various densities and different population structures could be seen among the sites (Fig. 3A, B), although the meso- and microhabitat structure in the site groups were not highly different (supplementary Fig. S2). Differently structured populations might respond differently to the same environment (Dixon & Baker, 1988; Suhling, 1999). In Groups I and II, the numbers of the younger larvae (Group I: E and F-3; Group II: F-3 and F-2) were high, whereas larger larvae (F-1, F) were found only occasionally. Species may have needed to re-colonize this section following a strong disturbance (e.g., drought). Because of the long larval development period, not enough time had passed for re-establishing the typical population structure. In Group III, the total numbers of individuals were low and the species had low density populations at these sites. This may be due to the streams running in very hard bedrock at these sites, hydropetric substrate (bedrock outcrop) being common there and also the amount of other substrates is lower than at the other streams. Accordingly, these two sites are not optimal for this shallow burrower species. In Group IV, we found the opposite situation; the dominance of the later stages could be seen. Due to the lack of adequate prior data, we can offer only hypothetical explanations: (1) Contrary to the others, in these streams small size fishes might be the top predators, which prey on younger instars, but are not able to eat bigger larvae. (2) Further unknown environmental effects might also play roles in the formation of this population structure. For example, C. heros never occupied these streams in the past or after a longer dry period, the population went locally extinct. Three years ago, some female adults recolonized this section and placed their eggs here, but in the next two years there were no further ovipositions by females from elsewhere. (3) Cannibalism is not rare among dragonflies (e.g., Van Buskirk, 1989), and insufficient nutrient supply may lead the large larvae to eat the small juveniles.
At the mesohabitat scale, C. heros populations in the Mecsek Mountains prefer pools with small or medium microhabitat heterogeneity and higher proportions of small particle-sized substrate. This is especially true in younger stages, whereas older larvae are less sensitive for these effects. Our findings are mainly consistent with those of Lang et al. (2001) who found that the small larvae clearly preferred larger patches of fine sediments with lower heterogeneity, whereas larger larvae were also frequent in more heterogeneous patches, for example, sandy patches surrounded by coarse gravel particles, cobbles, and stones. However, our results refine the former knowledge about the habitat preference of C. heros, as we showed that there is no distinct differences and opposition in microhabitat preference of different larval stages. Only the strength of the preference differs. Younger larvae need a high proportion of small particle-sized substrate material for burrowing, which can be found in pools with higher water depth and lower velocity. Larger larvae can use patches covered with coarser substrate material, but the very high heterogeneity of the substrate seems to be disadvantageous for all stages.
References
Bedjanic, M. & A. Salamun, 2003. Large golden-ringed dragonfly Cordulegaster heros Theischinger 1979, new for the fauna of Italy (Odonata: Cordulegastridae). Natura Sloveniae 5(2): 19–29.
Beisel, J. N., P. Usseglio-Polatera, S. Thomas & J. C. Moreteau, 1998. Stream community structure in relation to spatial variation: The influence of mesohabitat characteristics. Hydrobiologia 389: 73–88.
Blaškovič, T., E. Bulánková & J. Šíbl, 2003. First record of Cordulegaster heros ssp. heros Theischinger, 1979 (Cordulegastridae, Odonata) from Slovakia. Biologia, Bratislava 58(2): 293–294.
Boda, R., Gy Rozner, A. Czirok, I. Szivák & Z. Csabai, 2011. New data on the distribution of Cordulegaster heros Theischinger, 1979 in Mecsek Mountains and its surroundings. Acta Biologica Debrecina Supplementum Oecologica Hungarica 26: 21–28.
Boda, R., Cs Bereczki, A. Ortmann–Ajkai, P. Mauchart, B. Pernecker & Z. Csabai, 2015. Emergence behaviour of the red listed Balkan Goldenring (Cordulegaster heros Theischinger, 1979) in Hungarian upstreams: Vegetation structure affects the last steps of the larvae. Journal of Insect Conservation. doi:10.1007/s10841-015-9776-3.
Böcker, L., 1993. Größenspezifische Verteilung der Larven von Cordulegaster boltonii (Donovan) und C. bidentatus (Selys) über den Bachlauf—Untersuchungen an allo- und sympatrischen Bächen im Gießener Raum. Libellula 12: 225–247.
Buchwald, R., 1988. Die Gestreifte Quelljungfer Cordulegaster bidentatus (Odonata) in Südwestdeutschland. Carolinea 46: 49–64.
Corbet, P. S., 1983. A Biology of Dragonflies. E.W. Classey Ltd, Faringdon.
Corbet, P. S., 1999. Dragonflies: Behaviour and Ecology of Odonata. Harley Books, Colchester.
Corbet, P. S., 2002. Stadia and growth ratios of Odonata: a review. International Journal of Odonatology 5(1): 45–73.
Corbet, P. S., C. Longfield & N. W. Moore, 1960. Dragonflies. Collins, London.
Dixon, S. R. & R. L. Baker, 1988. Effects on size on predation risk, behavioural response on fish, and cost of reduced feeding in larval Ischnura verticalis (Coenagrionidae: Odonata). Oecologia 76: 200–205.
Dévai, Gy, 2014. Ritka hegyiszitakötő—Cordulegaster heros Theischinger, 1979 [Balkan Goldenring—Cordulegaster heros Theischinger, 1979]. pp. 181–184. In Haraszthy, L. (ed.), Natura 2000 fajok és élőhelyek Magyarországon [Natura 2000 species and habitats in Hungary]. Pro Vértes Természetvédelmi Közalapítvány, Csákvár. (in Hungarian).
Donath, H., 1987. Untersuchungen in einer Larvenkolonie von Cordulegaster boltonii (Donovan) in der Niederlausitz. Libellula 6: 105–116.
EC 1992. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora.
Ferreras-Romero, M. & P. S. Corbet, 1999. The life cycle of Cordulegaster boltonii (Donovan, 1807) (Odonata: Cordulegastridae) in the Sierra Morena Mountains (southern Spain). Hydrobiologia 405: 39–48.
Frissel, C. A., W. J. Liss, C. E. Warren & M. D. Hurley, 1986. A hierarchical framework for stream habitat classification: viewing streams in a watershed context. Environmental Management 10(2): 199–214.
Goretti, E., D. Ceccagnoli, La P. Gianandrea & M. V. Di Giovanni, 2001. Larval development of Aeshna cyanea (Müller, 1764) (Odonata: Aeshnidae) in Central Italy. Hydrobiologia 457: 149–154.
Hammer, O., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaentologica Electronica 4(1): 9.
Hawking, J. H. & T. R. New, 1996. The development of dragonfly larvae (Odonata: Anisoptera) from two streams in north-eastern Victoria, Australia. Hydrobiologia 317: 13–30.
Heidemann, H. & R. Seidenbursch, 1993. Die Libellenlarven Deutschlands und Frankreichs: Handbuch für Exuviensammler. Erna Bauer, Keltern.
Hering, D., O. Moog, L. Sandin & P. F. M. Verdonschot, 2004. Overview and application of the AQEM assessment system. Hydrobiologia 516: 1–20.
Kalkman, V. J., J.-P. Boudot, R. Bernard, K.-J. Conze, G. De Knijf, E. Dyatlova, S. Ferreira, M. Jović, J. Ott, E. Riservato & G. Sahlén, 2010. European Red List of Dragonflies. IUCN & Publications Office of the European Union, Luxembourg.
Lang, C., H. Müller & J. A. Waringer, 2001. Larval habitats and longitudinal distribution patterns of Cordulegaster heros Theischinger and C. bidentata Sélys in an Austrian forest stream (Anisoptera: Cordulegastridae). Odonatologica 30(4): 395–409.
Marczak, B. L., S. J. Richardson & C. M. Classen, 2006. Life history phenology and sediment size association of the dragonfly Cordulegaster dorsalis (Odonata: Cordulegastridae) in an ephemeral habitat in southwestern British Columbia. Canadian Field Naturalist 120(3): 347–350.
Pace, G., P. Andreani, M. Barile, A. Buffagni & S. Erba, 2011. Macroinvertebrate assemblages at mesohabitat scale in small sized volcanic siliceous streams of Central Italy (Mediterranean Ecoregion). Ecological Indicators 11: 688–696.
R Development Core Team, 2014. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.rproject.org.
Schmera, D. & T. Erős, 2011. The role of sampling effort, taxonomical resolution and abundance weight in multivariate comparison of stream dwelling caddisfly assemblages collected from riffle and pool habitats. Ecological Indicators 11: 230–239.
Schütte, C., 1997. Egg development and early instars in Cordulegaster boltonii immaculifrons Selys: a field study (Anisoptera: Cordulegastridae). Odonatologica 26: 83–87.
Suhling, F., 1999. Effects on fish on the microdistribution of different larval size groups of Onychogomphus uncatus (Odonata: Gomphidae). Archiv für Hydrobiologie. 144(2): 229–244.
Staufer, M. & O. Holuša, 2010. First record of Cordulegaster heros ssp. heros Theischinger in the Czech Republic and notes on Cordulegaster species occurrence in the southern part of Moravia (Odonata: Cordulegastridae). Libellula 29(3/4): 197–204.
Ter Braak, C. J. F. & P. Smilauer, 2012. CANOCO Reference Manual and User’s guide: Software for Ordination (ver. 5.0). Biometris, Wageningen and Ceské Budejovice.
VM 2012. 100/2012. (IX. 28.) VM rendelete a védett és a fokozottan védett növény- és állatfajokról, a fokozottan védett barlangok köréről, valamint az Európai Közösségben természetvédelmi szempontból jelentős növény- és állatfajok közzétételéről szóló 13/2001. (V. 9.) KöM rendelet és a növényvédelmi tevékenységről szóló 43/2010. (IV. 23.) FVM rendelet módosításáról [Decree of the Ministry of Environmental Protection about the protected and strictly protected plant and animal species, the strictly protected caves, and publishing the plant and animal species of nature conservation importance in the European Commission]. Magyar Közlöny 2012(128): 20903-21019. (in Hungarian)
van Buskirk, J., 1989. Density-dependent cannibalism in larval dragonflies. Ecology 70(5): 1442–1449.
van Tol, J., 2013. Fauna Europaea: Odonata. Fauna Europaea version 2.6.2, http://www.faunaeur.org (last visited: 10.02.2015).
Acknowledgements
Authors’ thanks are due to the students (Bernadett Reitzi, Gréta Bognár and Dániel Németh) of University of Pécs for their extensive help during field works. We thank to Thomas G. Horvath (University of Koblenz-Landau) for providing language improvements. Thanks also for the valuable and constructive comments of the associate editor and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Checo Colón-Gaud
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boda, R., Bereczki, C., Pernecker, B. et al. Life history and multiscale habitat preferences of the red-listed Balkan Goldenring, Cordulegaster heros Theischinger, 1979 (Insecta, Odonata), in South-Hungarian headwaters: does the species have mesohabitat-mediated microdistribution?. Hydrobiologia 760, 121–132 (2015). https://doi.org/10.1007/s10750-015-2317-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2317-y