Abstract
The macrophyte structure can influence the development of periphytic algal community in a shallow ecosystem. We investigated the interrelationship between periphytic algal community on artificial substrate and macrophyte species richness during four seasons in a tropical shallow reservoir. The periphyton was evaluated by biomass (as AFDM), algal abundance, species composition, and species richness. Limnological variables and macrophyte coverage were determined at sites with different macrophytes richness in littoral zone. Glass slides were used as the substrate and colonization time was 30 days. Periphytic algal structure was significantly influenced by seasonality and macrophyte richness, as well as the interaction of two factors. Periphytic algal density on natural and artificial substrates was negatively correlated with macrophyte richness and coverage. High biomass, algal biovolume and dominance of Zygnemaphyceae occurred when there was an increase of Utricularia foliosa coverage (summer). Our results showed that the seasonality, and to a lesser extent macrophyte species richness, explained significant portion of the variability of periphyton biomass, algal abundance, and taxonomic composition on artificial substrate. The changes in macrophyte community may have direct consequences on the periphyton structure in a shallow tropical reservoir.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Periphyton plays an important role in the functioning of lakes and reservoirs shallow, contributing significantly to primary production and nutrient cycling (Vadeboncoeur & Steinman, 2002). Several abiotic factors can influence the periphytic algal community structure in spatial and temporal scale, such as the nutrients and light availability, temperature, substrate composition and grazing (Stevenson, 1997). In tropical lakes and reservoirs, several studies reported strong influence of seasonality on the periphytic algal structure, especially light and nutrients availability (Moschini-Carlos et al., 2000; Borduqui & Ferragut, 2012; Pellegrini & Ferragut, 2012). However, such studies are still few for researchers to identify a pattern of variation in the periphytic algal structure, especially when considering the influence of aquatic macrophytes on the changes in the community.
The relationship between periphyton and macrophyte that plays a key role for the functioning of shallow ecosystems is widely recognized worldwide (Scheffer et al., 1993; Liboriussen & Jeppesen, 2006), as well as strong influence of macrophytes on the periphyton structure (Lalonde & Downing, 1991; Cattaneo et al., 1998). The relationship between periphyton and macrophytes is complex because these plants can also modify directly or indirectly environmental conditions for periphyton. The macrophytes participate in nutrient cycling processes through senescence, decomposition and excretion, as well as transferring sediment nutrients to the water (Carignan & Kalff, 1980). These biological processes can make available nutrients for periphyton (Burkholder, 1996). However, macrophytes may also negatively influence the periphyton through the competition for resources, production of allelopathic substances and reduced light availability due to shading (Erhard & Gross, 2006; Meerhoff et al., 2007). Another important aspect is that the macrophytes can determine the habitat structural complexity, which has influence on the periphytic algal biomass and species composition (Thomaz & Cunha, 2010; Ferreiro et al., 2014).
Previous studies have shown that the macrophytes architecture may influence positively or negatively the development of periphyton, as well as the variability of algal biomass, diversity, and species richness (Cattaneo et al., 1998; Jones et al., 2000; Laugaste & Reunanen, 2005; Santos et al., 2013). In addition, the macrophytes architecture can determine the amount of surface area available for colonization and modify the light and nutrients availability to the periphyton (Sand-Jensen & Borum, 1991). Thus, the spatial patterns of the periphytic algal structure may be explained by changes in the macrophyte community structure (abundance, biomass, growth forms). Another important aspect is that the composition and stability of the macrophyte community also depends on abiotic and hydrological characteristics of lakes (Thomaz et al., 2009). Thus, the macrophytes and periphyton community structure can change according to environmental changes of the lakes. Considering the interrelationship between periphyton and macrophyte, the structural characteristics of the macrophyte community, as richness and coverage, may influence the changes in the periphytic algal structure. Moreover, the macrophytes richness has direct and indirect effects on algal production hence on the nutrient retention in the wetland ecosystem (Engelhardt & Ritchie, 2001). All aspects mentioned above showed that macrophytes can strongly influence the spatial and temporal variability of periphytic algal community, especially in tropical lakes and reservoirs where high macrophytes abundance and diversity has been frequently reported by researchers (e.g., Pott & Pott, 2003; Chambers et al., 2008). Therefore, it is reasonable to assume that macrophyte richness may be a determinant of biomass, abundance, and species richness of periphytic algal.
Considering the effects of macrophytes community structure, such as architecture, coverage and species richness on the periphyton (Cattaneo et al., 1998; Engelhardt & Ritchie, 2001), we investigated the interrelationship between periphytic algal community and macrophyte species richness and coverage. We placed artificial substrates for periphyton growth at sites with differing macrophyte richness in four seasons in a tropical shallow reservoir (Ninféias Reservoir, Brazil). Our general hypothesis was that the macrophyte richness and seasonality are sources of variability in the periphyton biomass, algal abundance and taxonomic structure, but the seasonality is a strong driver of this variation.
Materials and methods
Study area
The research was conducted in Ninfeias Reservoir, which is an artificial reservoir designed for landscaping purposes inside Parque Estadual das Fontes do Ipiranga (23°39′15.60″S, 46°37′22.83″W) in the city of São Paulo, State of São Paulo, Brazil. This reservoir is a shallow mesotrophic and polymictic ecosystem with a 5433 m2 surface area, 7170 m3 volume. The mean depth is 1.32 m, maximum depth 3.6 m, and a mean theoretical residence time 7 days (Bicudo et al., 2002). The studied area is characterized by two climatic periods along the year: one is dry with lower air temperatures (20–25°C in 2010) during autumn and winter (March–August) and the other is rainy with higher temperatures (20–32°C in 2010) during spring and summer (September–February) (http://www.estacao.iag.usp.br/boletim.php).
Design sampling
Periphyton and water samples were collected at sites without macrophytes and with 1, 2, 3, and 4 macrophyte species during the autumn (May 2010), winter (July 2010) spring (October 2010) and summer (January 2011). Sampling was carried out in triplicate, totaling 60 sampling sites in the littoral zone.
The sites with different numbers of macrophyte species (0, 1, 2, 3, and 4 species) were identified in the reservoir bathymetric map (minimum distance of 10 m), considering all the species present in the water column. Subsequently, the sampling sites were chosen randomly. Based on number of macrophytes species (macrophytes species richness), habitat structure were designated as: Mf, macrophytes-free sites; Ny, Nymphaea spp. monospecific sites; 2M, sites with two macrophytes species (Nymphaea spp. and Utricularia foliosa L.); 3M, sites with three macrophytes species (Nymphaea spp., U. foliosa or Utricularia gibba L. and Panicum repens L.); 4M, sites with four macrophytes species [Nymphaea spp., U. foliosa, Eleocharis acutangula (Roxb.) Schult, P. repens and/or Eichhornia azurea (Sw.) Kunth]. Regarding growth forms: Nymphaea spp. has rooted floating leaves, U. foliosa, U. gibba and E. azurea is free-floating, E. acutangula and P. repens are emergent species. Nymphaea spp. is the most abundant species in the reservoir.
A square PVC (1 m2) was fixed at each sampling site to identify the place and also to hold a transparent acrylic support (26 × 10 cm) containing 10 glass slides (26 × 76 mm), which were positioned vertically to minimize the effect of the deposition of particulate matter (Fig. 1a–c). The acrylic support was fixed horizontally to 25-cm depth. The exposure time of the glass slides was 30 days. The experimental apparatus were placed in the middle of macrophyte stand to avoid border effects. Within apparatus, we also sampled the periphyton on petiole of Nymphaea that was carefully removed by scraping and water jets. The petiole size for sampling was standardized (30 cm) and criteria for selection were random.
Photos and drawing showing the experiment apparatus a floating PVC frame used for fixing the acrylic support and mark the sampling site, b position of the transparent acrylic support in the PVC structure, c transparent acrylic support for glass slides for colonization of periphyton, d square PVC frame used to estimate the macrophyte cover percentage at each sampling site. These photographs were taken during the autumn sampling
Analyzed variables
The air temperature and rainfall values were obtained by the Meteorological Station of the Instituto Astronômico e Geofísico da Universidade de São Paulo (estacao.iag.usp.br). Data from the 30 days prior to the sampling day were considered.
Water samples were collected to determine the physical and chemical variables in the middle of the macrophyte stand at the sampling sites. On the sampling day, water samples were filtered through Whatman GF/F membrane filters for dissolved nutrients analyses. Total nutrients analyses were carried out in the unfiltered samples within 30 days after the collection date. The following abiotic variables were analyzed: transparency (Secchi disk), light (LiCor LI-250A), temperature, electric conductivity (Digimed), dissolved oxygen (Golterman et al., 1978), alkalinity (Golterman & Clymo, 1971), pH (pHmeter Digimed), dissolved inorganic carbon, nitrite (N-NO2) and nitrate (N-NO3) (Mackeret et al., 1978), ammonium (N-NH4) (Solorzano, 1969), orthophosphate (P-PO4) and total dissolved phosphorous (TDP) (Strickland & Parsons, 1960), total nitrogen (TN) and total phosphorus (TP) (Valderrama, 1981), orthosilicate (Golterman et al., 1978), and particulate organic matter (POM) (APHA et al., 2005). We standardized the effect of time on the measurement of light between the sampling sites [(light at 30 cm/light subsurface) × 100].
The periphyton on artificial and natural substrate was removed by scraping and washing with distilled water. Periphyton on artificial substrate samples were filtered through Whatman GF/F glass-fiber filter for determining ash free dry mass (AFDM, APHA et al., 2005). For the periphytic algae quantitative analysis on artificial substrate and natural, the samples were fixed with acetic Lugol. The algal counting was made in an inverted microscope Zeiss Observer following Utermohl (1958) method. Counting limit was established through the species rarefying curve and until reaching 100 individuals of the most common species (Ferragut et al., 2013). Only for periphyton on artificial substrate, the algal biovolume was obtained from Fonseca et al. (2014) or calculated according to Hillebrand et al. (1999). Algal species with relative biovolume higher than or equal to 10% of the total sample were considered as descriptors and species with relative biovolume greater than or equal to 50% were considered as dominant. Species richness was determined by number of species per sample.
Total macrophytes coverage and each individual species at sampling sites were performed using a square PVC frame of 1 m2 containing 100 small squares made with nylon thread (Fig. 1d), following methods in Thomaz et al. (2004). This method was chosen because it is a non-destructive sampling method. A single observer performed the count of macrophytes to standardize the quantification. The macrophytes species richness and coverage were related to the changes in the periphytic algal community.
Statistical analyses
Principal component analysis (PCA) was performed to reduce the dimensionality of abiotic data. This multivariate descriptive analysis was performed from the covariance matrix and the data transformed by log(x + 1).
The Pearson correlation coefficient (r) and coefficient of determination of linear regression (R 2) was used to measure the degree of linear relationship between macrophyte richness and coverage with periphytic algal density on artificial and natural substrate (r > 0.5, P < 0.05). Considering the difference of water depth between sampling sites, we used Pearson correlation analysis (r) to determine if periphyton AFDM, algal density, biovolume and species richness varied with water depth.
The influence of seasonality and macrophyte richness and interaction of these factors on the structural characteristics of periphyton community (AFDM, total density, total biovolume, and species richness) was analyzed using a permutational multivariate analysis of variance (two-way PERMANOVA). This test was also performed to examine the influence of seasonality and macrophyte richness on algal class biovolume. This analysis was performed using log-transformed data, Bray–Curtis similarity and 4999 permutations in the statistical program Past 3.01 (Hammer et al., 2001).
The relationships between algal community and environmental factors were analyzed using a canonical correspondence analysis (CCA). The main matrix was composed of 38 species whose relative biovolume was above 2% of the total density in each sample and the secondary matrix was composed of five abiotic variables, which were selected based on the PCA. The analysis was done using log-transformed data. Multivariate analysis (PCA, CCA) were done using PC-ORD 5.15 for Windows (McCune & Mefford, 2011).
Results
Climate and limnological variables
The highest temperature values were recorded in summer (23.9°C) and the lower values in winter (17.1°C) during the sampling period. The cumulative monthly rainfall was higher in autumn and summer (179.5 and 201.1 mm, respectively) than in winter and spring (89.7 and 69.1 mm, respectively).
Table 1 summarizes the temperature, water transparency, light availability and water-column depth sites with differences in macrophyte richness during the seasons.
The PCA of limnological variables in sites with different numbers of macrophytes species explained 78% of the total variance in the first two axes (Fig. 2). The scores from the spring and summer data were ordered on the negative side of axis 1 and the autumn and winter data on the positive side, separating the dry period (autumn and winter) of the rainy period (spring and summer). On the positive side, sampling units of spring and summer were correlated with high values of light, TDP and HCO3 (r = 0.6–0.7), but summer was more associated with high temperatures and high chlorophyll-a and TDP and spring with pH and HCO3. On the opposite side, sampling units of autumn and winter were highly correlated with high nitrogen concentration (r = ≥0.9).
Principle component analysis (PCA) of limnological variables at sites with different numbers of macrophytes species during the seasons. Mf macrophyte-free sites, Ny Nymphaea spp. sites, 2M, 3M, 4M sites with 2–4 macrophyte species. Chloa phytoplankton chlorophyll-a, CO 2 CO2 free, HCO 3 bicarbonate, Cond conductivity, Depth depth, DO dissolved oxygen, pH pH, NH 4 ammonium, NO 2 nitrite, NO 3 nitrate, TN total nitrogen, TDP total dissolved phosphorus, TP total phosphorus, PM particulate matter, Si orthosilicate, Rad subaquatic radiation, Temp temperature, Transp transparency (Secchi disk)
Macrophytes
Nymphaea spp. was generally the most abundant species, except at the 2M sites in autumn and winter when the contribution of U. foliosa was slightly higher (Fig. 3). The highest Nymphaea coverage was found in spring (52–89%). On average, total macrophyte coverage was lower in autumn and winter (data range 13–62%, mean 40%) than in the spring and summer (data range 81–94%, mean 88%). The correlation between the macrophytes coverage and richness was positive (Spearman: r = 0.68, P < 0.001, n = 48).
Periphyton on artificial substrate
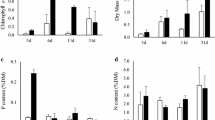
The periphyton structural attributes differed among the sites with different numbers of macrophyte and during the seasons (Fig. 4a–d). Periphyton biomass (AFDM) showed the highest values in macrophyte-free sites, except in the spring when the values were low at all sites (Fig. 4a). Algal density was higher in the sites with lower macrophyte richness in autumn and winter, while the density in spring was reduced at all sites (Fig. 4b). In summer, the algal density was, on average, higher than in the spring and the coefficient of variation was the lowest among seasons (CV 13%). Algal species richness also showed the lowest values in the sites with the higher macrophyte species richness (including 2M in winter), except in the summer when algal richness was increased in all sites (Fig. 4d). Biomass (AFDM), algal density, and species richness presented, on average, the lowest values in spring. Species richness showed lower values (2–4 times) in spring compared to the other seasons (Fig. 4d). Periphyton AFDM, algal density, biovolume, and species richness were negatively correlated with macrophyte richness species during autumn and winter (Spearman correlation coefficient: r = −0.4 to −0.5, P ≤ 0.05). This result was also observed during spring and summer, but the values were low and not significant. Pearson correlation between periphyton attributes (AFDM, algal density, biovolume, and species richness) and water depth at each sampling site was weak and non-significant.
Periphyton AFDM, total algal density, biovolume and species richness (white bar, n = 3, ±SD), and macrophyte cover (gray area, n = 3) at sites with differences in macrophyte richness during the seasons (Mf: macrophyte-free sites, Ny: Nymphaea spp. sites, 2M, 3M, 4M: sites with 2–4 macrophyte species)
Bacillariophyceae, Chlorophyceae, Cyanophyceae, and Zygnemaphyceae were the most representative classes in the periphyton during the study period (Fig. 5). The relative biovolume of algal classes varied greatly among sites in autumn and spring, while Zygnemaphyceae was dominant in summer. In contrast, in winter the contribution of the different classes changed with increasing macrophyte richness.
Two-way PERMANOVA showed that periphyton structure was significantly influenced by seasonality, macrophyte richness, and by the interaction of two factors (Table 2). Periphyton attributes variation was explained by seasonality and macrophytes richness, but the seasonality had greater weight in the variability of the attributes. Algal classes did not show great qualitative changes, but the class biovolume was significantly different between seasons and sites, and showed significant interaction between the factors (Table 3).
Species descriptors changed with seasonality and macrophyte richness (Fig. 6). These species represented on average 44, 58, 55, and 69% of the periphyton algal biovolume in the autumn, winter, spring and summer, respectively. In autumn Netrium digitus (Ehrenberg) Itzighson & Rothe (53%) was the most important species in Mf site, Cosmarium margaritatum (Lundell) Roy & Bisset in Ny and 3M sites and Oscillatoria sp. in 2M and 4M sites. In winter, Netrium digitus was the most abundant species in Uf and 2M sites, Cosmarium botrytis and Ochromonas danica in Ny sites, Pleurotaenium simplicissimum Grönblad in 3M sites and Spirogyra sp. in 4M sites. In the spring, P. simplicissimum was dominant (77–55%), except at sites without macrophytes and 2M. In the summer N. digitus was dominant in almost all sites (67–51%), except in 4M sites (39%).
Considering that 158 periphytic algae species were quantified, we used only those species with relative density greater than 2% of the total density (38 species) in the sample for CCA (Fig. 7; Table 4). The eigenvalues for axis 1 (λ = 0.221) and 2 (λ = 0.178) explained 24.4% of the total data variability. The high species-environment correlation for axis 1 (r = 0.856) and 2 (r = 0.735) indicated a strong relationship between species distribution and environmental variables. The canonical coefficients showed that the TN concentration was the most important variable in the ordination axis 1 and light in axis 2. The first axis of the ordination represented seasonality, showing that the sites of the spring and summer were associated with higher values of light and TDP (r > 0.7). Contrarily, the sites during winter and autumn were correlated to high TN, nitrate and ammonium concentrations (r = −0.5). Three species showed stronger correlations with the macrophytes sites during autumn and winter (r > 0.5: Peridinium umbunatum Stein, Chromulina elegans Doflein, Chromulina pygmaea Nygaard) while others three characterize the sites during spring and summer [r > 0.5: Stauroneis phoenicenteron (Nitzch.) Ehrenberg, Ulnaria cf. acus (Kützing) M.Aboal, Cosmarium pseudoconnatum var. pseudoconnatum Nordstedt].
CCA ordination of periphytic algal biovolume and five environmental variables at sites with differences in macrophyte richness during the seasons. Species correlation with axes 1 and 2, and the respective codes are given in Table 4. Score abbreviation: first letters refers to seasons (A autumn, W winter, Sp spring, S summer) and second letters indicates site categories (Mf = macrophyte free-sites, Ny = Nymphaea sites; 2–4M = sites with 2-4 macrophytes species). Vector: NH 4 ammonium, N-NO 3 nitrate, TN total nitrogen, TDP total dissolved phosphorus and %Rad subaquatic radiation (light)
Periphytic algal density on artificial and natural substrate
Simple linear regressions showed that periphytic algal density on artificial and natural substrates decreases significantly with increasing macrophytes species richness and coverage (Fig. 8). In both substrates, algal density were negatively correlated with macrophyte coverage and species richness (Pearson correlation coefficient: r = 0.4–0.6, P < 0.05).
Discussion
The shallow reservoir of this study is characterized by an extensive littoral zone with a high abundance of aquatic macrophytes, especially Nymphaea spp. and Utricularia foliosa, which vary in abundance over the year (Bicudo et al., 2002). In this scenario, we found that changes periphytic algal community structure on artificial substrate were related to macrophyte richness and seasonality. Although changes in community structure due to seasonality have been more significant and evident (as in CCA), permutational multivariate analysis of variance indicated that variation of macrophyte richness was also a variability source. The findings appears to confirm our initial hypothesis that changes in the periphyton biomass and algal abundance, richness and species descriptors would be more strongly associated with seasonality. Previous studies reported that the changes in macrophyte community can be an important source variation to phytoplankton and periphyton structure in this reservoir (Fonseca & Bicudo, 2011; Fermino et al., 2011; Pellegrini & Ferragut, 2012). Seasonality is a factor that commonly determines the variation of periphyton biomass, species composition and productivity in tropical reservoirs and lakes, especially nutrients concentration and water temperature (e.g., Moschini-Carlos et al., 2000; Cetto et al., 2004; O’Reilly, 2006; Borduqui & Ferragut, 2012). Our results were similar to those of other researchers who have found that the macrophyte community structure was important for periphyton structure in temperate lakes, but was less important than other environmental variables, especially the light and nutrients availability (Lalonde & Downing, 1991; Vadeboncoeur et al., 2006).
Periphyton algal biovolume and biomass (as AFDM) on artificial substrate had the highest values during the summer at all sampling sites (with and without macrophytes). Although the limnological conditions were quite similar in spring and summer according to PCA, mainly light and P availability, the macrophyte community structure was different between seasons. During the spring, there was absolute dominance of Nymphaea (on average 65%), which is a rooted macrophyte with leaves large and broad. In summer, Nymphaea was replaced by Utricularia foliosa (on average 36 and 31%, respectively). Considering the descriptors species in spring, we found the higher relative biovolume Pleurotaenium simplicissimum (Mf and 2M sites) and Stauroneis phoenicenteron (Ny, 3M, and 4M sites), which have adaptive strategies to compete under conditions of low light and nutrients availability, such as adherence form and mobility, respectively (Cohn & Weitzell Jr., 1996; Domozych et al., 2007). Unlike, periphyton had high algal biomass and total biovolume and dominance of Zygnemaphyceae at all sampling sites during the summer, especially Netrium digitus and Cosmarium margaritatum. These species are k-strategists and has a low ratio surface/volume usually being associated with protected and less disturbed ecosystems. The high abundance of desmids is commonly associated with the presence of macrophyte stands in tropical and temperate lakes and reservoirs (e.g., Cattaneo et al., 1998; Coesel, 2001; Felisberto & Rodrigues, 2005). We found the highest organic matter content (AFDM) and desmids biovolume in periphyton during periods of high U. foliosa coverage. This free-floating macrophyte has been frequently characterized by high periphyton biomass and abundance of Zygnemaphyceae, especially desmids (Peroutka et al., 2008; Santos et al., 2013). The architecture of U. foliosa appears to favor the accumulation of biomass due to high surface area availability for colonization and also to allow good penetration of light (Santos et al., 2013). In this sense, macrophyte architecture presents a strong effect on the periphyton biomass and taxonomic composition of algal assemblages (Cattaneo et al., 1998; Jones et al., 2000).
Unlike the other attributes, the periphytic algal density on artificial substrate was significantly reduced when there was increase of macrophyte coverage, particularly above 60%. However, the sites with the highest number of macrophytes species also showed the highest macrophytes coverage. Thus, the two features of macrophyte community in each sampling sites had influence on the periphyton, as observed by the algal density. This environmental condition resulted in periphytic algal growth reduction on artificial and natural substrate (Nymphaea spp.). Certainly, the increased macrophyte coverage and richness was unfavorable for the periphytic algal growth due to increased competition for resources, and mainly due to shading. Studies showed that high periphyton biomass can cause decline of macrophytes (Roberts et al., 2003), but the reverse is also true (McCormick et al., 1998). The macrophyte stands can cause significant shading on algal communities (Sand-Jensen & Borum, 1991; McCormick et al., 1998). Meerhoff et al. (2007) reported that shading is an important factor in controlling periphyton algal biomass in the littoral zone of shallow lakes/reservoirs. As expected in shallow lakes (Scheffer et al., 1993), high macrophyte coverage occurred in the greater water transparency period (clear phase), but our results suggest that this phase is unfavorable for the periphyton algal growth on artificial and natural substrate in the reservoir.
There is a consensus that the physical and chemical characteristics of the substrate can influence the periphyton primary production and species composition, especially the natural substrates (Vadeboncoeur et al., 2006). In this study, the use of artificial substrate eliminated the effect of substrate microtopography and nutrients release on periphyton. However, we found a negative relationship between the macrophytes richness and coverage and periphytic algal density on artificial and natural substrate (Nymphaea). This same tendency was observed with other periphyton attributes on Nymphaea (BGP, unpublished data). Also, Camargo & Ferragut (2014) reported that periphytic algal biomass and density on Eleocharis acutangula were negatively correlated with macrophyte coverage. Consequently, these results suggest that the high macrophytes richness and coverage may not favor periphytic algal growth on either artificial or natural substrates.
Considering that development of periphyton can be influenced by the position of substrate in the ecosystem (Lowe & Pan, 1996), would be expected some influence of water-column depth on periphyton in the present study. Although the position of the substrates position in the water column have been standardized (25 cm below the surface), the distance between substrate and sediment varied greatly between sampling sites. However, we found a nonlinear significant relationship between the periphyton attributes (biovolume, density, biomass and species richness) on artificial substrate and water depth of the sampling sites. This result suggests that other environmental factors acted more strongly on variation of periphyton attributes in this reservoir, where the macrophytes abundance is very high almost all year (on average 67%). According to Lalonde & Downing (1991), the relationship between epiphyton biomass and water depth not only varied with lake trophy but also did not rule out the influence of macrophyte architecture on the variation of biomass. Unlike the clear and direct relationship of epipelon biomass with water-column depth (Vadeboncoeur et al., 2014), the periphyton developed in the upper layer of water column in middle of dense macrophyte stands appears to be less sensitive to the effects of depth, mainly regarding the effects of seasonality and macrophyte community structure. Nevertheless, this relationship needs to be investigated in shallow ecosystems with high abundance of aquatic macrophytes.
Our results showed that the seasonality, and to a lesser extent macrophyte species richness, explained significant portion of the variability of periphytic biomass, algal abundance and taxonomic composition on artificial substrate. The macrophyte structure (architecture, species richness, coverage) can be a strong driver of periphytic algal structure, especially growth and descriptors species composition. As observed in temperate lakes and tropical wetland ecosystem (Lalonde & Downing, 1991; Cattaneo et al., 1998; Engelhardt & Ritchie, 2001), we found that changes in macrophyte community may have direct consequences on the periphyton structure in the tropical shallow reservoirs/lakes. The complexity of littoral habitats is a major difficulty for identifying drivers of factors periphyton community structure, but our results showed evidence of the richness and the macrophyte cover can have a negative influence on the community, at least in lakes and reservoirs shallow with dense stands of macrophytes.
References
APHA, AWWA & WEF, 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington, DC.
Bicudo, D. C., M. C. Forti & C. E. M. Bicudo, 2002. Parque Estadual das Fontes do Ipiranga (PEFI): unidade de conservação que resiste à urbanização de São Paulo. Secretaria do Meio Ambiente do Estado de São Paulo, São Paulo.
Borduqui, M. & C. Ferragut, 2012. Factors determining periphytic algae succession in a tropical hypereutrophic reservoir. Hydrobiologia 683: 109–122.
Burkholder, J. M., 1996. Interactions of benthic algae with their substrata. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego: 253–297.
Camargo, V. M. & C. Ferragut, 2014. Estrutura da comunidade de algas perifíticas em Eleocharis acutangula (Roxb.) Schult (Cyperaceae) em reservatório tropical raso, São Paulo, SP, Brasil. Hoehnea 41(1): 31–40.
Carignan, R. & J. Kalff, 1980. Phosphorus sources for aquatic weeds: water or sediments? Science 207: 987–989.
Cattaneo, A., G. Galanti, S. Gentinetta & S. Romo, 1998. Epiphytic algae and macroinvertebrates on submerged and floating-leaved macrophytes in an Italian lake. Freshwater Biology 39: 725–740.
Cetto, J. M., J. A. Leandrini, S. A. Felisberto & L. Rodrigues, 2004. Comunidade de algas perifíticas no reservatório de Iraí, Estado do Paraná, Brasil. Acta Scientiarum, Biological Sciences 26: 1–7.
Chambers, P. A., P. Lacoul, K. J. Murphy & S. M. Thomaz, 2008. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595: 9–26.
Coesel, P. F. M., 2001. A method for quantifying conservation value in lentic freshwater habitats using desmids as indicator organisms. Biodiversity and Conservation 10: 177–187.
Cohn, S. A. & R. E. Weitzell Jr, 1996. Ecological considerations of diatom cell motility. I. Characterization of motility and adhesion in four diatom species. Journal of Phycology 32: 928–939.
Domozych, D. S., L. Elliott, S. N. Kiemle & M. R. Gretz, 2007. Pleurotaenium trabecula, a desmid of wetland biofilms: the extracellular matrix and adhesion mechanisms. Journal of Phycology 43: 1022–1038.
Engelhardt, K. A. M. & M. E. Ritchie, 2001. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 411: 687–689.
Erhard, D. & E. M. Gross, 2006. Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aquatic Botany 85: 203–211.
Felisberto, S. A. & L. Rodrigues, 2005. Abundance of periphytic desmids in two Brazilian reservoirs with distinct environmental conditions. Acta Limnologica Brasiliensia 17: 433–443.
Fermino, F. S., C. E. M. Bicudo & D. C. Bicudo, 2011. Seasonal influence of nitrogen and phosphorus enrichment on the floristic composition of the algal periphytic community in a shallow tropical, mesotrophic reservoir (São Paulo, Brazil). Oecologia Australis 15(3): 476–493.
Ferragut, C., D. C. Bicudo & I. S. Vercellino, 2013. Amostragem e medidas de estrutura da comunidade perifítica. In Schwarzbold, A., A. Burliga & L. C. Torgan (eds), Ecologia do perifíton. Rima, São Carlos: 157–177.
Ferreiro, N., A. Giorgi & C. Feijoó, 2014. Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquatic Ecology 47: 389–401.
Fonseca, B. M. & C. M. Bicudo, 2011. Phytoplankton seasonal and vertical variations in a tropical shallow reservoir with abundant macrophytes (Ninféias Pond, Brazil). Hydrobiologia 665: 229–245.
Fonseca, B. M., C. Ferragut, A. Tucci, L. O. Crossetti, F. Ferrari, D. C. Bicudo & C. E. M. Bicudo, 2014. Biovolume de cianobactérias e algas de reservatórios tropicais do Brasil com diferentes estados tróficos. Hoehnea 41: 9–30.
Golterman, H. L. & R. S. Clymo, 1971. Methods for chemical analysis of freshwaters. In International Biological Program. Blackwell Scientific Publications, Oxford.
Golterman, H. L., R. S. Clymo & M. A. M. Ohmstad, 1978. Methods for Physical and Chemical Analysis of Freshwaters. Blackwell Scientific Publications, Oxford.
Hammer, O., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9.
Hillebrand, H., C. D. Durselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Jones, J. I., B. Moss, J. W. Eaton & J. O. Young, 2000. Do submerged aquatic plants influence periphyton community composition for the benefit of invertebrate mutualists? Freshwater Biology 43: 591–604.
Lalonde, S. & J. A. Downing, 1991. Epiphyton biomass is related to lake trophic status, depth, and macrophyte architecture. Canadian Journal of Fisheries and Aquatic Sciences 48: 2285–2291.
Laugaste, R. & M. Reunanen, 2005. The composition and density of epiphyton on some macrophyte species in the partly meromictic Lake Verevi. Hydrobiologia 547: 137–150.
Liboriussen, L. & E. Jeppesen, 2006. Structure, biomass, production and depth distribution of periphyton on artificial substratum in shallow lakes with contrasting nutrient concentrations. Freshwater Biology 51: 95–109.
Lowe, R. L. & Y. Pan, 1996. Benthic algal communities as biological monitors. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego, CA: 705–740.
Mackeret, F. J. H., J. Heron & J. F. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Titus Wilson and Son Ltda, Kendall.
McCormick, P. V., R. B. E. Shuford III, J. B. Backus & W. C. Kennedy, 1998. Spatial and seasonal patterns of periphyton biomass and productivity in the northern Everglades, Florida, USA. Hydrobiologia 362: 185–208.
McCune, B. & M. J. Mefford, 2011. PC-ORD: Multivariate Analysis of Ecological Data. Version 6.0. MjM Software, Gleneden Beach, OR.
Meerhoff, M., C. Iglesias, F. T. De Mello, J. M. Clemente, E. Jensen, T. L. Lauridsen & E. Jeppesen, 2007. Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshwater Biology 52: 1009–1021.
Moschini-Carlos, V., R. Henry & M. L. M. Pompêo, 2000. Seasonal variation of biomass and productivity of the periphytic community on artificial substrata in the Jurumirim Reservoir (São Paulo, Brazil). Hydrobiologia 434: 35–40.
O’Reilly, C. M., 2006. Seasonal dynamics of periphyton in a large tropical lake. Hydrobiologia 553: 293–301.
Pellegrini, B. G. & C. Ferragut, 2012. Variação sazonal e sucessional da comunidade de algas perifíticas em substrato natural em um reservatório mesotrófico tropical. Acta Botanica Brasilica 26: 807–818.
Peroutka, M., W. Adlassnig, M. Volgger, T. Lend, W. G. Url & I. K. Lichtscheid, 2008. Utricularia: a vegetarian carnivorous plant? Plant Ecology 199: 153–162.
Pott, V. J. & A. Pott, 2003. Dinâmica da vegetação aquática do Pantanal. In Thomaz, S. M. & L. M. Bini (eds), Ecologia e manejo de macrófitas aquáticas. Editora da Universidade Estadual de Maringá, Maringá: 145–162.
Roberts, E., J. Kroker, S. Körner & A. Nicklisch, 2003. The role of periphyton during the re-colonization of a shallow lake with submerged macrophytes. Hydrobiologia 506–509: 525–530.
Sand-Jensen, K. & J. Borum, 1991. Interactions among phytoplankton periphyton and macrophytes in temperate freshwaters and estuaries. Aquatic Botany 41: 137–175.
Santos, T. R., C. Ferragut & C. E. M. Bicudo, 2013. Does macrophyte architecture influence periphyton? Relationships among Utricularia foliosa, periphyton assemblage structure and its nutrient (C, N, P) status. Hydrobiologia 714: 71–83.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends Ecology Evolution 8: 275–279.
Solorzano, L., 1969. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceanography 14: 799–801.
Stevenson, R. J., 1997. Scale-dependent determinants and consequences of benthic algal heterogeneity. Journal of the North American Benthological Society 16: 248–262.
Strickland, J. D. H. & T. R. Parsons, 1960. A manual of seawater analysis. Bulletin Fisheries Research Board of Canada 125: 1–185.
Thomaz, S. M. & E. R. Cunha, 2010. The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages composition and biodiversity. Acta Limnologica Brasiliensia 22: 218–236.
Thomaz, S. M., T. A. Pagioro, L. M. Bini, M. C. Roberto & R. R. A. Rocha, 2004. Limnological characterization of the aquatic environments and the influence of hydrometric levels. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and Its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 75–102.
Thomaz, S. M., P. Carvalho, A. A. Padial & J. T. Kobayashi, 2009. Temporal and spatial patterns of aquatic macrophyte diversity in the Upper Paraná River floodplain. Brazilian Journal Biology 69: 617–625.
Utermohl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen der Internationalen Vereinigung fur Theoretische und Angewandte Limnologie 9: 1–38.
Vadeboncoeur, Y. & A. D. Steinman, 2002. Periphyton function in lake ecosystems. Scientific World Journal 2: 1449–1468.
Vadeboncoeur, Y., J. Kalff, K. Christoffersen & E. Jeppesen, 2006. Substratum as a driver of variation in periphyton chlorophyll and productivity in lakes. Journal of the North American Benthological Society 25(2): 379–392.
Vadeboncoeur, Y., S. P. Devlin, P. B. McIntyre & M. J. Vander Zanden, 2014. Is there light after depth? Distribution of periphyton chlorophyll and productivity in lake littoral zones. Freshwater Science 33(2): 524–536.
Valderrama, G. C., 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Marine Chemistry 10: 109–112.
Acknowledgments
MLS and BGP thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant No. 2011/14751-2 and 2009/52253-4, respectively) for a Master of Science fellowship, and CF thanks FAPESP for financial support (No. 2009/52253-4). The authors are also grateful to all the students and technicians involved in the laboratory and fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisak
Rights and permissions
About this article
Cite this article
de Souza, M.L., Pellegrini, B.G. & Ferragut, C. Periphytic algal community structure in relation to seasonal variation and macrophyte richness in a shallow tropical reservoir. Hydrobiologia 755, 183–196 (2015). https://doi.org/10.1007/s10750-015-2232-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2232-2