Abstract
Recent alpine stream studies have aimed to predict biotic responses to changing environmental conditions, hydrological regime and climate, but there is little information on macroinvertebrate drift shifts in this context and most research was largely restricted to snow-free period. We compared seasonal drift–benthos patterns in proglacial streams of Val Roseg (Switzerland) between 2008/2009 and 1997/1998. Samples were collected monthly (including winter) in a glacial lake outlet stream, a proglacial stream, and their post-confluence stream (main channel) in both periods. Measured physical and chemical parameters indicated the largest glacial meltwater influence in the proglacial stream and its lower inflow in 2008/2009 compared to 1997/1998. Drift largely coincided with benthic structure at each site, although drift–benthos patterns were at times asynchronous, depending on site and flow conditions. Our results indicate that the initial stages of hydrological “freeze-melt” events and the period of snow cover are most favorable for macroinvertebrates in glacial streams. Drift density was significantly lower in 2008/2009 than in 1997/1998. In 2008/2009, we observed lower chironomid, but higher ephemeropteran and plecopteran drift densities. The drift–benthos changes between periods likely reflect behavioral and life-history responses of proglacial stream macroinvertebrates to seasonal shifts in environmental conditions in response to glacial retreat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alpine streams, recognized as patchy and pulsing ecosystems whose structural and functional attributes are susceptible to multiscale spatio-temporal changes, often have been model systems for studying habitat heterogeneity, hydrological connectivity, and potential responses of flora and fauna to climate change (Milner & Petts, 1994; Brown et al., 2007; Jacobsen et al., 2014). The overall heterogeneity and complexity of alpine streams are predisposed by the (a) relative contributions of different water sources (glacial meltwater, snowmelt/rainfall, groundwater), (b) degree of glacial influence, (c) surface energy balance and climate variability at higher altitudes, (d) catchment morphology and underlying geology, (e) distribution, type, and influence of tributaries, and (f) seasonal expansion and contraction (i.e., freeze-melt cycles) of surface waters within the floodplain channel network (Milner & Petts, 1994; Smith et al., 2001; Khamis et al., 2014 and references therein).
Whereas most glacier-fed (proglacial; kryal) streams have high flows caused by snow/glacial meltwater outbursts in spring and summer, and much lower flows with more dominant groundwater contribution in winter (i.e., during periods of freezing, snow cover, and reduced surface flows) (e.g., Malard et al., 1999; Robinson et al., 2004; Milner et al., 2009), proglacial lake outlets appear more environmentally benign than proglacial streams (Hieber et al., 2002). Reduced harshness in lake outlets is usually evident through (a) higher temperatures and faster warming rates, (b) lower seasonal and diel flow variations, (c) higher channel stability associated with lower sediment scouring, and (d) a distinct, lake-influenced community structure and dynamics (Milner & Petts, 1994; Hieber et al., 2002). Thus, although sharing similar general characteristics, glacial streams can differ greatly in their origin and nature, even at small spatio-temporal scales (Brown et al., 2003). In alpine regions worldwide, changing environmental conditions at larger temporal scales (e.g., temperature-induced precipitation shift from snow to rain) have caused rapid glacier retreat (Dyurgerov & Meier, 2000; IAHS (ICSI) – UNEP – UNESCO, 2005). Glacial retreat has resulted in inter-annual, seasonal, and diurnal changes in thermal and flow regimes, thereby affecting macroinvertebrate distributions and biodiversity as well as life histories (Milner et al., 2008, 2009; Jacobsen et al., 2012, 2014; Blaen et al., 2014).
Drift is an important re-distribution and colonization mechanism of stream-dwelling organisms, driven by a variety of abiotic (flow, temperature, water quality) and biotic (behavior, life history, benthic density, predation, competition) processes (reviews by Waters, 1972; Müller, 1974; Brittain & Eikeland, 1988). Any member of the benthic fauna may at some time “enter drift”, although most drift studies have focused on macroinvertebrates, biota widely known in their sensitivity to changes in environmental and biotic properties (Allan, 1995; Castella et al., 2001; Milner et al., 2001). Accordingly, shifts in macroinvertebrate drift should reflect spatio-temporal changes in stream habitat and benthic community characteristics.
To date, most drift studies have examined abiotic and biotic factors affecting spatio-temporal drift patterns across different stream types (e.g., Cellot, 1989; Pringle & Ramírez, 1998; Sertić Perić et al., 2011), including those in alpine landscapes (Ilg et al., 2001; Robinson et al., 2002, 2004; Hieber et al., 2003). Alpine drift studies have focused mostly on the influence of habitat heterogeneity and seasonal variability of hydrological connectivity on macroinvertebrate benthos–drift relationships at larger spatial scales within a single or among different catchments (but see Waringer, 1992 and Saltveit et al., 2001 for finer spatial scale drift surveys within a single alpine channel). Because of logistic sampling problems in winter (Brittain & Milner, 2001), these studies also were largely restricted to the snow-free season and little is known regarding the winter ecology of macroinvertebrates (drift, benthos) within alpine proglacial streams (Schütz et al., 2001). By detecting the drift and benthos patterns within glacial headwaters during periods of extent snow cover, we would be able to assess and compare the responses of glacier-fed stream habitats to hydrological changes caused by climate change, such as shifts from snow—towards rain-dominated precipitation regime and glacial retreat, respectively.
To our knowledge, the present study is the first to examine macroinvertebrate benthos–drift relationships in proglacial streams over an annual cycle, during winter in particular. The main objectives of the study were to compare (a) year-round abiotic (physical and chemical) properties of different proglacial streams; (b) biotic characteristics (density, taxonomic richness of benthos and drift) between proglacial sites over an annual cycle; and (c) physical and chemical variables, and drift in a proglacial stream at two time periods (1997/1998 and 2008/2009) between which substantial glacier retreat occurred (ca. 480 m in distance). We aimed to gain insight into potential future outcomes of environmental change within glacier-fed streams following glacial retreat, especially regarding macroinvertebrate response patterns in drift. Study streams consisted of a proglacial stream, proglacial lake outlet, and the confluence of the two streams, thus spanning a large range in environmental conditions exhibited by glacial streams in general.
Materials and methods
Study system and site description
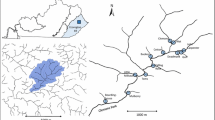
The study was conducted in the upper Val Roseg catchment (49.5 km2; 9°53′57″E, 46°29′28″N; 1760–4049 m a.s.l.) in the Bernina Massif, Switzerland (Fig. 1a). Detailed descriptions of the Val Roseg area and floodplain channel types are given in Tockner et al. (1997). The Roseg catchment arises from two first-order glacial melt streams: (1) a proglacial (kryal) stream originating from Tschierva glacier, and (2) an outlet stream from proglacial Roseg Lake (area = 0.36 m2; depth = ca. 7 m; altitude = 2,159 m) below the Roseg glacier. The lake was formed naturally about 50 years ago behind the medial moraine of the two glaciers that dammed the Roseg river below the Roseg glacier. The two glaciers (Roseg and Tschierva) have decreased in size rapidly with climate change (on average 40 m per year during the period 1997–2010) (Swiss Glacier Monitoring Network).
Map of Switzerland (a) with enlargements of the Val Roseg catchment (b) and floodplain channel network in the upper catchment (c). Arrow alongside the channel indicates the flow direction. Study sites are marked with black circles; L1 and L2 are lake outlet sites, P is a proglacial site, and M is a main channel site just below the confluence of the lake outlet and proglacial stream
Below the confluence of the proglacial stream (length ca. 900 m) and lake outlet (length ca. 950 m), the Roseg river forms a single-thread channel (upper main channel) incised in glacial till (length ca. 600 m). Downstream, it broadens within a braided glacial floodplain (length ca. 2,750 m) followed by a canyon-constrained reach (length ca. 7,050 m), and ending with the river mouth at Pontresina (Fig. 1b). Mean annual discharge of the Roseg river is 2.79 m3/s (1955–2009) (Swiss National Hydrological and Geological Survey). Despite distinct inter-annual variation in discharge, the general flow regime exhibits typical maxima in summer during glacial ablation and minima in winter (Fig. 2).
The glacial floodplain is covered mainly by glaciers and bare rock (75%), an additional 18% by grassland and 3.6% by woody vegetation. Subnival and low-alpine zones have patchy grasslands mainly consisting of pioneer plants (lichens, mosses), curved sedge (Carex curvula All.), and mat-grass (Nardus stricta L.). Green alder [Alnus viridis (Chaix) DC] and willow (Salix spp.) shrubs as well as early successional stages of larch (Larix decidua Mill.) occur on a low-alpine plateau along the upper main channel incised in glacial till. Treeline in the Val Roseg (2100–2300 m a.s.l.) is restricted mainly to lower-valley side slopes, and is dominated by larch, stone pine (Pinus cembra L.), and mugo pine (Pinus mugo Turra).
The four sampling sites in 2008/2009 were situated in the subnival and low-alpine zones (Fig. 1c). Site names refer to the sampled stream reach: L1 and L2 are the lake outlet sites (upper/broader and lower/incised reach, respectively), P is the proglacial reach, and M is the main channel (incised reach). Site L1 was located directly at the outlet (i.e., ca. 5 m below) of the glacial Lake Roseg. Sites L2 and P were located at the distal end of the lake outlet and proglacial reach, ca. 20 m before their confluence, respectively. Site M was the post-confluence site situated within the incised main channel (Fig. 1c). In 1997, sites P and M were 0.7 and 1.2 km below the Tschierva glacier terminus, respectively (Burgherr & Ward, 2001); whereas in 2007 these distances were 1.2 km for site P and 1.7 km for site M. Sites labeled P and M in this paper correspond to codes M-10 and M-1 used in Tockner et al. (1997) and Malard et al. (1999), and M3 and M4 in Burgherr & Ward (2001), respectively.
Drift, benthic macroinvertebrates, benthic organic matter
As one of the objectives of the present study was to compare 2008/2009 with 1997/1998 drift data, we adopted the methodology of the earlier drift studies (Robinson et al., 2002, 2004). Briefly, monthly drift and benthic samples were collected during two consecutive field days (a) between April 1997 and April 1998 (with the exception of November 1997, and February and March 1998) and (b) between October 2008 and August 2009 (with the exception of April and July 2009) (Fig. 2). The lake outlet sites were not sampled in 1997/1998. Sampling also was not possible at site L1 in December, January, and March 2009 because of high avalanche risk.
Triplicate samples of drifting macroinvertebrates and associated organic matter were collected using a steel frame mounted with a drift sampler. Drift samplers were cylindrical plastic tubes (aperture 0.01 m2) fitted with a 1-m long nylon net (100-µm mesh). Because an earlier study showed no distinct diel drift periodicity (Robinson et al., 2002), drift was sampled near mid-day at each site on each sampling date in this study. Sampling duration lasted between 10 and 30 min, depending on net clogging by glacial flour. We used a MiniAir2 velocity meter (Schiltknecht messtechnik AG, Gossau, Switzerland) to measure the flow velocity at the aperture of each sampler to calculate the volume of water filtered by each net. Velocity measures were taken at the beginning and end of each sample and averaged to account for clogging hydraulic effects. To compare with drift samples, triplicate benthic macroinvertebrate samples were collected in riffle/run habitats at each site using a Hess sampler (0.042 m2, 100-µm mesh).
In the field, drift and benthic samples were preserved with 4% formalin (1997/1998) or 70% ethanol (2008/2009). In the laboratory, macroinvertebrates were handpicked from each sample, identified to the lowest possible taxonomic level (genus in most cases) and counted using a stereomicroscope. After removing the organisms, sample residuals were dried at 60°C, weighed, combusted at 550°C for 4 h, and reweighed to estimate the transported (TOM) and benthic organic matter (BOM) as ash-free dry mass (AFDM).
Physical and chemical characteristics of the sample sites
On each sampling date at each site, water temperature (T) and specific conductance (Cond) were measured with a field portable meter (WTW model LF325, Weilheim, Germany at 20°C; accuracy: ±0.5% of value), and a 1-l water sample was collected for chemical analysis. The water samples were stored in polyethylene bottles, refrigerated in the dark cool place (i.e., in a cool box at 4 ± 2°C), and processed within 24 h of collection. From the water samples, nitrate–N (NO3 −-N), nitrite-N (NO2 −-N), ammonium-N (NH4 +-N), particulate nitrogen (PN), phosphate-P (PO4 3−-P), particulate phosphorus (PP), dissolved organic carbon (DOC), particulate organic carbon (POC), and total inorganic carbon (TIC) were determined in the laboratory following the methods of Tockner et al. (1997). Discharge data were obtained from the Swiss National Hydrological and Geological Survey at the Roseg gaging station located 7.1 km downstream of the floodplain (Fig. 1b). Because the lower valley is constrained and has no major tributaries, this station accurately reflects hydrologic variability in the upstream study sites, and has been used for long-term Val Roseg flow data (Finn et al., 2010).
Data analysis
Measures selected as the most indicative for physical and chemical characterization of the study streams were summarized as means, standard deviations (SD), and coefficients of variation (CV). In addition, all measured parameters were analyzed by principal component analysis (PCA) to illustrate the spatial and temporal habitat changes during 2008/2009. Physical and chemical variables were log(x + 1) transformed before analysis to ensure equal variance and prevent the PCA from being driven by variables with extreme variation.
Because drift data were not normally distributed and could not be normalized by common transformations, non-parametric tests were employed (Statistica 9.1, StatSoft, Inc., 2010). Monthly drift and benthic macroinvertebrate (densities, richness, and most frequent taxa) differences were analyzed using Friedman’s repeated measures ANOVA. Kendall’s coefficient of concordance (τ) was calculated to estimate the relationship between rankings of the repeated measures among sites. Kendall’s τ ranges from 0 to 1 in which values near zero represent lack of agreement, while values near 1 represent high synchrony in the rankings of repeated measures among sites. We used the Kruskal–Wallis (K–W) test to analyze drift and benthic macroinvertebrate density and amplitude differences among sites. If differences were significant, a non-parametric Tukey-type multiple comparison test for unequal numbers of data was employed (Zar, 1984).
Friedman’s ANOVA was used to compare inter-month drift densities between sites and months common to the two sampling periods (1997/1998 and 2008/2009). Kendall’s τ was used to estimate drift pattern synchrony between 1997/1998 and 2008/2009. A non-parametric Wilcoxon matched pairs test was used to test for inter-period drift differences within individual months and for inter-period differences in physical and chemical parameters at a site. A P value of 0.05 was taken to indicate statistical significance in all tests.
Results
Physical and chemical characteristics in 2008/2009
The physical and chemical measures reflected the difference in water sources (i.e., glacial water influence) between sites (Table 1; Fig. 3). Compared to the proglacial and main channel sites, lake outlet sites showed lower variability (CVs) in measured physical and chemical parameters during 2008/2009 (Table 1). Water temperatures were on average 1°C lower in the proglacial (P) and main channel (M) compared to the lake outlet sites (L1, L2). In contrast, mean conductivity, NO3-N, and PP were higher in the proglacial and main channel than in the lake outlet sites. Extremely high CVs for PP and DOC at sites P and M were mostly a result of high values during high precipitation in November and spring runoff in June. For example, June PP values at sites P and M were 60-fold greater, while November PP values at site M (1.83 ± 0.39 g P/L) were an order of magnitude higher than respective annual means.
Ordination of a PCA based on monthly measurements (n = 8 for sites L2, P and M; n = 5 for site L1) of 14 physical and chemical variables at each site during the study period. a Ordination diagram of the 14 normalized physical and chemical variables in the PCA. Codes are given in “Materials and methods”; b site position on the PCA (PCA-1 × PCA-2) based on measured physical and chemical variables. Error bars illustrate temporal changes in measured physical and chemical variables at each study site. August data were omitted because of missing measurements of POC, PP, and PN
TOM was low at all sites during the 2008/2009 period. With the exception of June, when TOM peaked at sites P (125.5 g/m3) and M (70.5 g/m3), average TOM ranged from 0.06 g/m3 (site P in October) to 3.1 g/m3 (site M in November). The highest amounts of BOM were recorded in May 2009 at most sites (L1, 9.5 ± 4.3 g/m2; M, 5.0 ± 5.4 g/m2; P, 4.7 ± 4.4 g/m2). An exception was the lower lake outlet site (L2) that had the lowest BOM content during the entire study period (Table 1). Minimal amounts of BOM were present in June and August 2009, averaging for all sites in that period 0.44 ± 0.26 g/m2.
PCA illustrated the environmental differences between sites according to physical and chemical parameters (Fig. 3). The first two axes of the PCA explained 52% of the total variability between sites. PCA aligned sites L1, L2, M, and P along a gradient of decreasing temperature and increasing conductivity, NO3-N, and TIC. The PCA of measured physical and chemical parameters also revealed a higher temporal (i.e., seasonal) variability at sites M and P compared to the lake outlet sites (L1, L2).
Benthic community patterns in 2008/2009
There were significant temporal differences in macroinvertebrate benthic density and with all sites showing a highly synchronous pattern (Friedman’s ANOVA, χ 2 (n = 4, df = 5) = 13.49, P < 0.05, τ = 0.67) (Fig. 4). During the 2008/2009 period, benthic density was significantly lower at the lake outlet sites than in the main and proglacial sites (K–W test, H (3, n = 96) = 18.67, P < 0.001). Kendall’s coefficients corroborated these findings, showing a strong concordance between L1 and L2 (τ = 0.92), and P and M (τ = 0.94). Highest benthic densities were recorded in May at sites L1, P, and M, mostly due to increased numbers of Chironomidae (mostly Diamesinae and Orthocladinae), Plecoptera (juvenile Plecoptera; Nemouridae), and Ephemeroptera (Baetidae) (Fig. 5). At site L2, the highest benthic density was recorded in November, when high numbers of Baetidae (>1900 ind./m2), Heptageniidae (>900 ind./m2), Leuctridae (>180 ind./m2), Perlodidae (>90 ind./m2), and Nemouridae (>80 ind./m2) were collected.
Average proportion of the most frequent benthic/drift taxa at each study site during the study period. Asterisks indicate months when sampling was not possible at site L1. Note scaling of axes. Chi Chironomidae, Cop Copepoda, Eph Ephemeroptera, Ple Plecoptera, Other other taxa. Note high proportion of Copepoda at site L1
During winter, benthic density did not differ between sites and the pattern was asynchronous (χ 2 (n = 3, df = 2) = 2.00, P > 0.05, τ = 0.33). The main channel site (M) then had the highest density (Fig. 4), mostly composed of Diamesinae, Orthocladinae, Baetidae, Heptageniidae, juvenile Chloroperlidae, and Nemouridae (Fig. 5). These taxa comprised 78–95% of the benthic community abundance at site M (Table 2—Online Resource 1). The lowest benthic densities were observed in February at sites L1 and L2 (<100 ind./m2), and in August at sites P (<140 ind./m2) and M (<40 ind./m2).
Benthic taxon richness was highest in the main channel (M) and lowest in the lake outlet sites (L1, L2); the latter showed a high degree of concordance (τ = 0.86) in richness during the study period. All sites had a similar seasonal pattern with significantly lower taxon richness during the high-discharge season relative to the rest of the year (χ 2 (n = 4, df = 5) = 14.71, P = 0.01, τ = 0.74). Site M displayed relatively stable taxon richness during the study period (CV = 46%), although having highest values in December and May and lowest richness in August (Fig. 4). Other sites displayed similar and lower taxon richness, although higher inter-month variability (CV = 58–61%). Taxon richness at site P was similar to the lake outlet during autumn until mid-winter (τ = 0.97), but it became more similar to site M (τ = 0.99) after February (Fig. 4).
Drift patterns in 2008/2009
Although significant temporal differences were not confirmed (χ 2 (n = 4, df = 5) = 4.14, P > 0.05, τ = 0.21), at all sites, lowest drift densities were observed in winter (January and February) and high values in autumn (October, November) and spring (March, May, and June) (Fig. 4). The autumn drift increase was accompanied by increased numbers of drifting Chironomidae (i.e., Diamesinae) in the proglacial (18% of the drift) and main (33%) channel sites, and Ephemeroptera in the lake outlet (22%) (Table 2—Online Resource 1). The spring increase reflected greater drift densities of Chironomidae (Diamesinae; 49% of the drift) and Plecoptera (mostly Nemouridae; 30%), especially in the proglacial and main channel sites (Fig. 5).
Analysis of drift at individual sites separated site L1 from the other sites (H (3, n = 96) = 30.57, P < 0.0001) by having the lowest drift densities during the study period (Figs. 4, 5). Other sites had higher drift densities, with a conspicuous drift peak at site P in March and smaller drift peaks at sites P and M in June (Fig. 4). Drift peaks did not always overlap with benthic peaks. For example, a drift increase opposite to the respective benthic pattern was recorded in February and March at site L2 (Fig. 5). Similar patterns also were observed at site P in October and June and at site M, where drift densities were opposite to those of benthic densities (Fig. 5).
Drift composition largely coincided with the benthic structure at each site (Table 2—Online Resource 1). An exception was site L1, where harpacticoid copepods appeared in the drift with a major peak in February (Fig. 5). Drift taxon richness showed no between-site synchronicity or a conspicuous seasonal pattern (χ 2 (n = 4, df = 5) = 5.00, P > 0.05, τ = 0.25), as was the case for benthic richness (Fig. 4). At site L2, for most dates (except January, February, and March), drift richness had two-fold higher values than benthic richness (Fig. 4). This difference was mostly associated with taxa that were in the drift but not in the benthos at a site: Leuctridae (comprised 7% of the total drift at L2), Copepoda (3%), Hydrachnidia (1%), Simuliidae (1%), Tanypodinae and Tanytarsini (0.3% each), and Empididae (0.2%) (Table 2—Online Resource 1). At sites P and M, drift richness in June was almost four-fold higher than respective benthic richness (Fig. 4), mostly due to drift-present but benthos-absent taxa belonging to Limoniidae (comprised 0.2% of the June drift at site P; 1.2% at site M), Baetidae (2% at P; 4.5% at M), and Leuctridae (0.05% at P; 0.4% at M). In August, at the lake outlet sites (L1, L2), drifting Hydrachnidia (6% of the August lake outlet drift), Copepoda (3%), and Simuliidae (3%) led to two-fold higher drift taxon richness in comparison to respective benthic richness (Fig. 4).
1997/1998–2008/2009 comparisons
No significant inter-period differences were found in physical and chemical measures at the two sites (P, M) common in both periods (Wilcoxon matched pairs test, P > 0.05) (Table 1). A detailed physical and chemical assessment of the study sites in 1997/1998 is given in Tockner et al. (1997), Burgherr & Ward (2001), and Robinson et al. (2002), whereas here we present data for 2008/2009 and 1997/1998 relevant to this study (Table 1). Mean temperature, conductivity, NO3-N, DOC, and TOM were generally higher, and BOM lower, in 2008/2009 than in 1997/1998. Similar to 2008/2009, PP values showed high variability in 1997/1998. In autumn 1997/1998, a pattern of variable discharge with several peaks similar to the one in 2008/2009 was observed (Fig. 2). However, there was a trend toward an earlier date of spring runoff in 2008/2009 compared to 1997/1998 (Fig. 2). Larger discharge peaks (>8 m3/s) occurred in June/July in 1997/1998, while such peaks occurred already during late May in 2008/2009.
There were no significant inter-month (i.e., seasonal) differences in the total and most frequent taxa (Chironomidae, Ephermeroptera, Plecoptera) drift densities at sites M + P in both periods (Fig. 6). At both sites, Ephemeroptera and Plecoptera showed a high (τ > 0.70), and Chironomidae a low, degree (τ < 0.30) of inter-period synchrony in drift densities, and total drift density showed an intermediate inter-period synchrony (0.50 > τ < 0.60).
Total drift densities (mean + SE) (a) and drift densities of the most frequent drift taxa (b–d) at sites P and M on sampling dates in months common to the two sampling periods (1997/1998 and 2008/2009). Note scaling of axes. Friedman’s ANOVA results (χ 2) and Kendall’s concordance coefficients (τ) indicate inter-month and inter-period differences in drift density, respectively. χ 2 statistics indicate the significance of inter-month variation; τ quantifies the degree of synchrony between 1997/1998 and 2008/2009 drift density rankings (τ = 1 indicates complete synchrony/concordance; τ = 0 indicates no synchrony/concordance)
Significant inter-period differences were found in total, Chironomidae and Ephemeroptera drift densities within individual months (Wilcoxon matched pairs test, P < 0.05), whereas Plecoptera showed no significant differences between study periods (P > 0.05) (Fig. 6). In general, the inter-period pattern in total drift density greatly overlapped with Chironomidae drift, showing a significant decrease in drift in 2008/2009 compared to 1997/1998. Such a pattern was most obvious at site M where each month displayed a significant between period difference (0.001 > P ≤ 0.052). Ephemeroptera drift densities at site M differed significantly between study periods only in May (P < 0.05) and October (P < 0.01). At site P, October values in total and Chironomidae drift densities, as well as the Ephemeroptera drift in May, showed a significant increase in 2008/2009 compared to 1997/1998 (P < 0.05). In contrast, total and Chironomidae drift at site P in May (P = 0.052) and June (P < 0.05) 2008/2009 displayed a significant decrease relative to that in 1997/1998 (Fig. 6).
Discussion
Temporal shifts in habitat characteristics
Physical and chemical characteristics of the alpine floodplains and channel networks are greatly influenced by the degree of glacial influence, flow patterns, and system freeze melt cycles (i.e., expansion–contraction dynamics) (Malard et al., 2000; Füreder et al., 2001; Brown et al., 2007; Cauvy-Fraunié et al., 2014). It is expected that glacier recession initially results in a higher release of melt water and thus increased flows (Stahl et al., 2008). In the longer term, stream flow decreases because of glacier volume reduction (i.e., changes in long-term glacier storage effects) (Barnett et al., 2005; Milner et al., 2009). The annual discharge fingerprints shown in Fig. 2 suggest such a long-term trend, but its credibility is doubtful as our sampling campaigns were a decade apart. However, the historical (1955–2007) Val Roseg flow records reported by Finn et al. (2010) show a significant decrease in winter (low-flow season) and annual minimum flows, and an increased number of annual short-term flow reversals in the Val Roseg. The long-term storage effect is almost entirely climate-dependent and corresponds to glacier volume variations balanced between glacier mass accumulation and ablation (i.e., glacier mass balance). It affects inter-annual runoff variability by increasing it during periods of glacier growth and retreat, whereas minimizing variation at moderate percentages (ca. 40%) of glacier cover (see references in Jansson et al., 2003). Accordingly, the upper Val Roseg, with 41.7% of glacier cover (Uehlinger et al., 2003), still seems to display an increased runoff variability, most likely due to the ongoing glacier retreat.
The physical and chemical (mostly thermal) settings of Val Roseg headwaters in 2008/2009 indicated that the glacial influence was greatest in the proglacial reach below Tschierva glacier, lowest in the outlet stream of the proglacial lake, and moderate in the channel below the confluence of the proglacial stream and lake outlet. The same pattern was observed in 1997/1998 (Burgherr & Ward, 2000; Robinson et al., 2004), although some inter-period shifts in stream ionic and organic matter concentrations were found that suggested a reduction of glacial influence in 2008/2009 (Table 1). For example, we measured conductivity and NO3 −-N increases in 2008/2009, which reflects increases in groundwater and/or snowmelt inputs (Tockner et al., 1997; Füreder et al., 2001; Roy et al., 2011). Because comparison of 1997/1998–2008/2009 data might not give a true reflection of temporal trends (no measures in the intervening time period), the observed shifts in physical and chemical variables in Roseg streams may not be solely due to long-term climate-induced thermal and hydrological shifts (cf. Malard et al., 1999; Milner et al., 2009). These shifts could result from natural year-to-year variability from one site to another, but our results match previous predictions on the potential hydrological and habitat shifts in glacier-fed streams under global change (Brown et al., 2007; Milner et al., 2009).
Lake outlet effect on macroinvertebrate benthos and drift
Due to the high similarity of benthic and drift assemblages between sites P and M, and sites L1 and L2, we infer that abiotic similarities between sites were important determinants of macroinvertebrate community structure and drift patterns. The high similarity in physical and chemical variables between the proglacial and main channel (Table 1; Fig. 3) further confirmed that the main channel is fed primarily by glacial meltwater, although the glacial influence is mitigated by the lake outlet (Tockner et al., 1997; Hieber et al., 2002). On the other hand, the two lake outlet sites—L1, laid down within the upper, broad channel close to the lake and L2, situated within the lower, incised lake outlet reach—were faunal clearly different, although their high physical and chemical similarities suggest that they would have matching benthos and drift trends. Thus, it seems that spatial proximity of the sites does not necessarily imply their complete uniformity and, apparently, habitat characteristics of alpine glacial streams are mainly controlled by a glacier presence, its vicinity and seasonality, and at a lower hierarchical level by the presence of a proglacial lake, and channel topography and morphology (Füreder et al., 2002; Hieber et al., 2002).
Having the highest water temperatures and lowest seasonal variability in measured abiotic parameters (Fig. 3), our lake outlet sites were relatively stable and environmentally more benign in comparison to their non-outlet (proglacial, main channel) counterparts. However, they had significantly lower benthic macroinvertebrate richness and density. This result was in contrast to expectations that taxon richness and abundances are generally lower in harsh (kryal, high elevation) than in more benign (glacial lake outlet; mid/low elevation) alpine streams (Milner & Petts, 1994; Burgherr & Ward, 2000; Jacobsen & Dangles, 2012 and references therein). The lake outlet result was driven by very low abundances or absence of filter feeders (Table 2—Online Resource 1) that are usually highly abundant in non-glacial lake outlets (Richardson & Mackay, 1991). Our findings suggest that the glacial lake affected benthic macroinvertebrate structure mainly by ameliorating physical and chemical conditions, but not by increasing suspended organic matter content typical of other outlet streams that favor high filter feeder abundances (Maiolini et al., 2006). Contrary to that found in non-glacial lake outlets, glacial alpine lakes may act as sinks rather than sources of organic matter to outlet streams (cf. Hieber et al., 2002; Maiolini et al., 2006). Besides the effects of organic matter inputs, drift–benthos relationships in alpine lake outlets also could be affected by rapid lake surface thermal fluctuations induced by climatological forcing; lake origin, morphology, and stratification patterns; and stochastic weather events (e.g., high-velocity winds, high-precipitation events) (Oesch et al., 2005; Lisi et al., 2013).
Winter role in macroinvertebrate community structure and drift
Our study is one of the few year-round ecological studies of alpine glacier-fed streams that included regular sampling during winter (also see Schütz et al., 2001). Our records of low benthic densities in August and high densities in May (sites P and L1) and November (site L2) are consistent with earlier findings by Burgherr & Ward (2000). These data indicate that the initial phases of expansion (i.e., melting in spring) and contraction (i.e., freezing in late autumn/early winter) in alpine glacial streams are more favorable for benthic macroinvertebrates than the summer peak flow period (Burgherr & Ward, 2001; Robinson et al., 2001). Consistent with earlier Val Roseg studies, we suggest that winter might be a favorable period for macroinvertebrates in glacial streams. We believe that the winter effect was probably influenced by fine-scale patchiness of the snow cover at our sites. The main channel was snow-free (open) during the winter period (December–March; personal observation), thus it was more sunlight exposed and possibly provided greater food supply (periphyton) for macroinvertebrates (cf. Schütz et al., 2001) as reflected in the high densities and richness in winter. In contrast, sites that were snow covered had low densities until the channels became open. A similar “snow-cover-effect” was observed by Schütz et al. (2001), where a snow-covered site had up to six-fold lower macroinvertebrate densities and taxon richness than a snow-free site on the same stream. Another source for the high winter densities in the main channel could be drift from the upstream proglacial and lake outlet streams, which was usually higher in the proglacial stream and lake outlet sites than in the main channel.
Spatio-temporal comparisons of benthos–drift patterns
In general, drift patterns coincided with benthic patterns at each site (see Fig. 4). We suggest that benthos–drift patterns reflected the annual dynamics of the glacial streams’ expansion and contraction periods. These results confirm earlier Val Roseg studies showing a strong interplay between hydrologic seasonality and in-stream distribution, dispersal, and colonization patterns of benthic fauna (Burgherr & Ward, 2001; Robinson et al., 2002, 2004). If the annual flow regime is predictable, organisms can adapt life-history events (egg hatching, larval development, adult emergence) with the hydrological pattern (Lytle & Poff, 2004). Organisms might avoid poor environmental and unstable conditions by developing at low temperatures under snow and ice cover (e.g., winter species of Plecoptera and Ephemeroptera) or avoid competition during more optimal conditions by timing larval development during summer high flows and overwintering as eggs or larvae in the hyporheic zone (e.g., summer species such as Diamesa spp.) (Schütz et al., 2001). During our study, the “summer species scenario” prevailed at the most glacier-influenced sites (P, M), while the “winter species scenario” was more pronounced at the lake outlet sites (L1, L2) (Table 2—Online Resource 1).
Exceptions to this summer/winter species scenario were (1) increased benthic densities of larval Diamesa spp. in late spring and summer at site L1; (2) asynchronous occurrence of larval mayfly taxa (mostly Baetis alpinus and Rhithrogena loyolaea) with peak densities in winter at sites L1 and M, in autumn at site L2, and in spring at site P; and (3) year-round occurrence of plecopterans (mostly Protonemoura spp. and Leuctra spp.) in glacier-influenced sites (P, M). These exceptions may be explained firstly by a glacial melt flow pulse that increased the glacial influence in the upper lake outlet (L1), thereby enhancing conditions for Diamesa spp. (e.g., Kownacka & Kownacki, 1975; Burgherr & Ward, 2001). Furthermore, the mayflies Baetis and Rhithrogena are univoltine species in these glacial streams and overwinter as larvae (Sartori & Landolt, 1999) and they are often found in stable alpine streams with water temperatures above 4°C (Ward, 1994; Milner et al., 2001). These taxa had winter larval peaks in the upper lake outlet (L1) and main channel (M), suggesting they experienced a temperature-induced developmental delay (cf. Brittain, 1990) or a density decrease with the reduced winter flows at sites L2 and P (cf. Brittain & Saltveit, 1989). Lastly, Plecoptera, generally described as cold stenotherms with high feeding plasticity, appear well adapted to colder (arctic, alpine) environments (Fuller & Stewart, 1977; Brittain, 1990). Their egg development and nymphal growth are less temperature dependent, and at low temperatures, they can enter nymphal diapause that lengthens the life cycle, increases adult body sizes and thus fecundity (Brittain, 1990). These life-history adaptations may explain the year-round appearance of Plecoptera at our sites, but especially in glacier-influenced sites P and M.
Our findings of the higher drift taxon richness at the lower lake outlet site (L2) than the respective benthic richness were surprising, as in most studies, even at kryal lake outlets, benthos taxon richness was always higher than drift richness (Hieber et al., 2003; Robinson et al., 2004). The reversed pattern observed at L2 was most obvious in autumn (see Fig. 4) and corresponded to flow peaks. We suggest the flow peaks likely caused streambed disturbance that dislodged benthic macroinvertebrates from upstream reaches (Borchardt, 1993). Most taxa in the drift but absent in benthic samples (Leuctridae, Baetidae, Limoniidae, Simuliidae, Hydrachnidia, and Copepoda) are closely affiliated to substrate surfaces through feeding activity and habitat use (e.g., Kowarc, 1992; Di Sabatino et al., 2000; Ilg & Castella, 2006), and thus susceptible to substrate movement during high flows. Lastly, the high copepod drift during winter at L1 likely originated from the lake outflow as documented in various studies (Sandlund, 1982; Hieber et al., 2003; Jones, 2010).
Possible future scenarios of environmental shifts in glacier-fed streams
A decade ago, sample sites were much closer to the glacier, had lower temperatures (Table 1) and probably harsher environmental conditions. As such, sites were optimal habitats for Chironomidae (i.e., Diamesinae), taxa that maintain viable populations even at stream temperatures below 2°C (Milner et al., 2001). Because of glacier retreat and the consequent decrease in glacial meltwater along with an increase in water temperature, our glacial-influenced sites have likely become favorable for other taxa for colonization (Milner et al., 2001; Snook & Milner, 2001; Brown et al., 2007; Finn et al., 2010). Indeed, other macroinvertebrates have shifted distributions upward in elevation, colonizing these sites (see Finn et al., 2010). Strictly cold-adapted taxa such as Diamesinae also shifted upstream to colonize recently emerged glacier streams as suitable habitats. As a result, lower chironomid drift densities occurred in downstream sites, but since these taxa were the most frequent taxa in our samples, their drift patterns still reflected total drift patterns (see Fig. 6). Downstream sites also had increased drift densities of Ephemeroptera and Plecoptera in 2008/2009. The highly synchronized inter-period drift patterns of Ephemeroptera and Plecoptera suggest that these taxa may be less responsive than alpine Chironomidae to the environmental changes associated with glacier retreat. Monitoring drift provided additional insight and mechanistic understanding of response patterns of aquatic macroinvertebrates to environmental change, such as those caused by glacial retreat and shifts in precipitation forms (e.g., snow to rain). Our findings provide a basis for future assessments of macroinvertebrate response (e.g., drift) to ongoing ecohydrological shifts in glacier-fed streams worldwide.
References
Allan, J. D., 1995. Stream Ecology. Chapman & Hall, New York.
Barnett, T. P., J. C. Adam & D. P. Lettenmaier, 2005. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438: 303–309.
Blaen, P. J., L. E. Brown, D. M. Hannah & A. M. Milner, 2014. Environmental drivers of macroinvertebrate communities in high Arctic rivers (Svalbard). Freshwater Biology 59: 378–391.
Borchardt, D., 1993. Effects of flow and refugia on drift loss of benthic macroinvertebrates: implications for habitat restoration in lowland streams. Freshwater Biology 29: 221–227.
Brittain, J. E., 1990. Life history strategies in Ephemeroptera and Plecoptera. In Campbell, I. (ed.), Mayflies and Stoneflies: Life Histories and Biology. Kluwer, Dordrecht: 1–12.
Brittain, J. E. & T. J. Eikeland, 1988. Invertebrate drift – a review. Hydrobiologia 166: 77–93.
Brittain, J. E. & A. M. Milner, 2001. Ecology of glacier-fed rivers: current status and concepts. Freshwater Biology 46: 1571–1578.
Brittain, J. E. & S. J. Saltveit, 1989. A review of the effect of river regulation on mayflies (Ephemeroptera). Regulated Rivers: Research & Management 3: 191–204.
Brown, L. E., D. M. Hannah & A. M. Milner, 2003. Alpine stream habitat classification: an alternative approach incorporating the role of dynamic water source contributions. Arctic, Antarctic, and Alpine Research 35: 313–322.
Brown, L. E., D. M. Hannah & A. M. Milner, 2007. Vulnerability of alpine stream biodiversity to shrinking glaciers and snowpacks. Global Change Biology 13: 958–966.
Burgherr, P. & J. V. Ward, 2000. Zoobenthos of kryal and lake outlet biotopes in a glacial flood plain. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 27: 1587–1590.
Burgherr, P. & J. V. Ward, 2001. Longitudinal and seasonal distribution patterns of the benthic fauna of an alpine glacial stream (Val Roseg, Swiss Alps). Freshwater Biology 46: 1705–1721.
Castella, E., H. Adalsteinsson, J. E. Brittain, G. M. Gislason, A. Lehmann, V. Lencioni, B. Lods-Crozet, B. Maiolini, A. M. Milner, J. S. Olafsson, S. J. Saltveit & D. L. Snook, 2001. Macrobenthic invertebrate richness and composition along a latitudinal gradient of European glacier-fed streams. Freshwater Biology 46: 1811–1831.
Cauvy-Fraunié, S., P. Andino, R. Espinosa, R. Calvez, F. Anthelme, D. Jacobsen & O. Dangles, 2014. Glacial flood pulse effects on benthic fauna in equatorial high-Andean streams. Hydrological Processes 28: 3008–3017.
Cellot, B., 1989. Macroinvertebrate movements in a large European river. Freshwater Biology 22: 45–55.
Di Sabatino, A., R. Gerecke & P. Martin, 2000. The biology and ecology of lotic water mites (Hydrachnidia). Freshwater Biology 44: 47–62.
Dyurgerov, M. B. & M. F. Meier, 2000. Twentieth century climate change: evidence from small glaciers. Proceedings of the National Academy of Sciences of the United States of America 97: 1406–1411.
Finn, D. S., K. Räsänen & C. T. Robinson, 2010. Physical and biological changes to a lengthening stream gradient following a decade of rapid glacial recession. Global Change Biology 16: 3314–3326.
Fuller, R. L. & K. W. Stewart, 1977. The food habits of stoneflies (Plecoptera) in the Upper Gunnison River, Colorado. Environmental Entomology 6: 293–302.
Füreder, L., C. Schütz, M. Wallinger & R. Burger, 2001. Physico-chemistry and aquatic insects of a glacier-fed and a spring-fed alpine stream. Freshwater Biology 46: 1673–1690.
Füreder, L., C. Vacha, K. Amprosi, S. Bühler, C. M. E. Hansen & C. Moritz, 2002. Reference conditions of alpine streams: physical habitat and ecology. Water, Air and Soil Pollution: Focus 2: 275–294.
Hieber, M., C. T. Robinson, U. Uehlinger & J. V. Ward, 2002. Are alpine lake outlets less harsh than other alpine streams? Archiv für Hydrobiologie 154: 199–223.
Hieber, M., C. T. Robinson & U. Uehlinger, 2003. Seasonal and diel patterns of invertebrate drift in different alpine stream types. Freshwater Biology 48: 1078–1092.
IAHS (ICSI) – UNEP – UNESCO, 2005. Fluctuations of Glaciers VIII. IAHS (ICSI) – UNEP – UNESCO, Paris.
Ilg, C. & E. Castella, 2006. Patterns of macroinvertebrate traits along three glacial stream continuums. Freshwater Biology 51: 840–853.
Ilg, C., E. Castella, B. Lods-Crozet & P. Marmonier, 2001. Invertebrate drift and physico-chemical variables in the tributaries of the Mutt, a Swiss glacial stream. Archiv für Hydrobiologie 151: 335–352.
Jacobsen, D. & O. Dangles, 2012. Environmental harshness and global richness patterns in glacier-fed streams. Global Ecology and Biogeography 21: 647–656.
Jacobsen, D., A. M. Milner, L. E. Brown & O. Dangles, 2012. Biodiversity under threat in glacier-fed river systems. Nature Climate Change 2: 361–364.
Jacobsen, D., P. Andino, R. Calvez, S. Cauvy-Fraunié, R. Espinosa & O. Dangles, 2014. Temporal variability in discharge and benthic macroinvertebrate assemblages in a tropical glacier-fed stream. Freshwater Science 33: 32–45.
Jansson, P., R. Hock & T. Schneider, 2003. The concept of glacier storage: a review. Journal of Hydrology 282: 116–129.
Jones, N. E., 2010. Incorporating lakes within the river discontinuum: longitudinal changes in ecological characteristics in stream–lake networks. Canadian Journal of Fisheries and Aquatic Sciences 67: 1350–1362.
Khamis, K., D. M. Hannah, L. E. Brown, R. Tiberti & A. M. Milner, 2014. The use of invertebrates as indicators of environmental change in alpine rivers and lakes. Science of the Total Environment 493: 1242–1254.
Kowarc, V. A., 1992. Depth distribution and mobility of a harpacticoid copepod within the bed sediment of an alpine brook. Regulated Rivers: Research & Management 7: 57–63.
Kownacka, M. & A. Kownacki, 1975. Gletscherbach-Zuckmücken der Ötztaler Alpen in Tirol (Diptera: Chironomidae: Diamesinae). Entomologica Germanica 2: 35–43.
Lisi, P. J., D. E. Schindler, K. T. Bentley & G. R. Pess, 2013. Association between geomorphic attributes of watersheds, water temperature, and salmon spawn timing in Alaskan streams. Geomorphology 185: 78–86.
Lytle, D. A. & N. L. Poff, 2004. Adaptation to natural flow regimes. Trends in Ecology & Evolution 19: 94–100.
Maiolini, B., V. Lencioni, A. Boggero, B. Thaler, A. F. Lotter & B. Rossaro, 2006. Zoobenthic communities of inlets and outlets of high altitude alpine lakes. Hydrobiologia 562: 217–229.
Malard, F., K. Tockner & J. V. Ward, 1999. Shifting dominance of subcatchment water sources and flow paths in a glacial floodplain, Val Roseg, Switzerland. Arctic, Antarctic, and Alpine Research 31: 135–150.
Malard, F., K. Tockner & J. V. Ward, 2000. Physicochemical heterogeneity in a glacial riverscape. Landscape Ecology 15: 679–695.
Milner, A. M. & G. E. Petts, 1994. Glacial rivers: physical habitat and ecology. Freshwater Biology 32: 295–307.
Milner, A. M., J. E. Brittain, E. Castella & G. Petts, 2001. Trends in macroinvertebrate community structure in glacier-fed rivers in relation to environmental conditions: a synthesis. Freshwater Biology 46: 1833–1847.
Milner, A. M., A. E. Robertson, K. Monaghan, A. J. Veal & E. A. Flory, 2008. Colonization and development of a stream community over 28 years; Wolf Point Creek in Glacier Bay, Alaska. Frontiers in Ecology and the Environment 6: 413–419.
Milner, A. M., L. E. Brown & D. M. Hannah, 2009. Hydroecological effects of shrinking glaciers. Hydrological Processes 23: 62–77.
Müller, K., 1974. Stream drift as a chronobiological phenomenon in running water ecosystems. Annual Review of Ecology, Evolution, and Systematics 5: 309–323.
Oesch, D. C., J.-M. Jaquet, A. Hauser & S. Wunderle, 2005. Lake surface water temperature retrieval using advanced very high resolution radiometer and Moderate Resolution Imaging Spectroradiometer data: validation and feasibility study. Journal of Geophysical Research: Oceans 110: 1–17.
Pringle, C. M. & A. Ramírez, 1998. Use of both benthic and drift sampling techniques to assess tropical stream invertebrate communities along an altitudinal gradient, Costa Rica. Freshwater Biology 39: 359–373.
Richardson, J. S. & R. J. Mackay, 1991. Lake outflow streams and the distribution of filter feeders: an assessment of hypotheses. Oikos 62: 370–380.
Robinson, C. T., U. Uehlinger & M. Hieber, 2001. Spatiotemporal variation in macroinvertebrate assemblages of glacial streams in the Swiss Alps. Freshwater Biology 46: 1663–1672.
Robinson, C. T., K. Tockner & P. Burgherr, 2002. Seasonal patterns in macroinvertebrate drift and seston transport in streams of an alpine glacial flood plain. Freshwater Biology 47: 985–993.
Robinson, C. T., K. Tockner & P. Burgherr, 2004. Drift benthos relationships in the seasonal colonization dynamics of alpine streams. Archiv für Hydrobiologie 160: 447–470.
Roy, J. W., B. Zaitlin, M. Hayashi & S. B. Watson, 2011. Influence of groundwater spring discharge on small-scale spatial variation of an alpine stream ecosystem. Ecohydrology 4: 661–670.
Saltveit, S. J., I. Haug & J. E. Brittain, 2001. Invertebrate drift in a glacial river and its non-glacial tributary. Freshwater Biology 46: 1777–1789.
Sandlund, O. T., 1982. The drift of zooplankton and microzoobenthos in the river Strandaelva, western Norway. Hydrobiologia 94: 33–48.
Sartori, M. & P. Landolt, 1999. Atlas de Distribution des Éphémères de Suisse (Insecta, Ephemeroptera). Fauna Helvetica, Neuchâtel: 1–214.
Schütz, C., M. Wallinger, R. Burger & L. Füreder, 2001. Effects of snow cover on the benthic fauna in a glacier-fed stream. Freshwater Biology 46: 1691–1704.
Sertić Perić, M., M. Miliša, R. Matoničkin Kepčija, B. Primc-Habdija & I. Habdija, 2011. Seasonal and fine-scale spatial drift patterns in a tufa-depositing barrage hydrosystem. Fundamental & Applied Limnology 178: 131–145.
Smith, B. P. G., D. M. Hannah, A. M. Gurnell & G. E. Petts, 2001. A hydrogeomorphological framework for ecological research on alpine glacial rivers. Freshwater Biology 46: 1579–1596.
Snook, D. L. & A. M. Milner, 2001. The influence of glacial runoff on stream macroinvertebrates in the Taillon catchment, French Pyrenees. Freshwater Biology 46: 1609–1624.
Stahl, K., R. D. Moore, J. M. Shea, D. Hutchinson & A. J. Cannon, 2008. Coupled modelling of glacier and streamflow response to future climate scenarios. Water Resources Research 44: W02422.
StatSoft, Inc., 2010. STATISTICA (data analysis software system), version 9.1. www.statsoft.com.
Tockner, K., F. Malard, P. Burgherr, C. T. Robinson, U. Uehlinger, R. Zah & J. V. Ward, 1997. Physicochemical characterization of channel types in a glacial floodplain (Val Roseg, Switzerland). Archiv für Hydrobiologie 140: 433–463.
Uehlinger, U., M. Maisch, C. Rothenbühler & R. Zah, 2003. Val Roseg: a high alpine catchment. In Ward, J. V. & U. Uehlinger (eds), Ecology of a Glacial Flood Plain. Kluwer, Dordrecht: 1–16.
Ward, J. V., 1994. Ecology of alpine streams. Freshwater Biology 32: 277–294.
Waringer, J. A., 1992. The drifting of invertebrates and particulate organic matter in an Austrian mountain brook. Freshwater Biology 27: 367–378.
Waters, T. F., 1972. The drift of stream insects. Annual Review of Entomology 17: 253–272.
Zar, J. H., 1984. Biostatistical Analysis. Prentice Hall, Englewood Cliffs, NJ.
Zah, R., 2001. Patterns, pathways and trophic transfer of organic matter in a glacier stream ecosystem in the Alps. PhD thesis, Diss. ETH Nr. 13998, ETH, Zürich.
Acknowledgements
We thank R. Illi for chemical analysis of the water samples. Special thanks to K. Tockner who provided 1997/1998 drift data. Much thanks to D. Finn, C. Jolidon, C. Baumgartner, R. Freimann, E. Snyder, W. K. Dodds, P. Turko, and M. Perić for field assistance. Special thanks to Deb Finn for constructive suggestions that helped during the winter sampling in the field, and intense discussions that helped during the shaping of this manuscript. We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions. We are grateful to the village of Pontresina for providing access to the study area. This research was supported by the Swiss Government, through the Federal Commission for Scholarships for Foreign Students (FCS), as well as Project No. 119-0000000-1205 of the Ministry of Science, Education and Sports of the Republic of Croatia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sonja Stendera
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perić, M.S., Robinson, C.T. Spatio-temporal shifts of macroinvertebrate drift and benthos in headwaters of a retreating glacier. Hydrobiologia 751, 25–41 (2015). https://doi.org/10.1007/s10750-014-2149-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2149-1