Abstract
Phosphorus (P) limitation has been regarded as the rule in freshwater systems and the basis for phytoplankton growth management. We hypothesised that P would be the limiting nutrient for phytoplankton growth in Grahamstown Dam, a shallow, mesotrophic reservoir, across different seasons and on different experimental time scales. Seven fully factorial microcosm assays with additions of nitrogen (N) and P were conducted in situ during different seasons. The influence of longer experimental duration was examined in two 18-day mesocosm assays. Additions of N and P in combination evoked significantly higher phytoplankton biomass and biovolumes of individual algal genera compared with controls and other treatments in both types of experiment. There were some significant responses to P additions in the microcosm assays in winter. Some genera first responded to combined P and N addition and then to P only addition during the mesocoms assays. Our results show that P was not the limiting nutrient across all seasons but that phytoplankton was mostly co-limited by N and P. A longer experimental time scale did not change this outcome at the biomass level. This implies that input of N as well as of P should be considered in the management of phytoplankton growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton blooms, especially if dominated by toxic cyanobacteria, can have serious consequences for the supply of drinking water due to the production of potent toxins (Bowling, 1994; Qin et al., 2010). Hence, one goal of drinking water management is to reduce the main cause of phytoplankton blooms—the enrichment of water supplies with the macro nutrients nitrogen (N) and phosphorus (P). Of these two main algal nutrients, P has traditionally been the focus of nutrient management in freshwater systems (Schindler, 1977; Lewis & Wurtsbaugh, 2008; Sterner, 2008) and the reduction of P loading to lakes and reservoirs has successfully led to a decrease in algal and cyanobacterial biomass in many cases (Willén, 2001; Jeppesen et al., 2005). However, N alone can also limit phytoplankton productivity (James et al., 2003; Bergström et al., 2008) and there is increasing evidence that phytoplankton co-limitation by both nutrients sometimes occurs (Elser et al., 2007; Harpole et al., 2011). Thus, in some lakes controlling N may be as important as controlling P.
Moreover, seasonal changes in nutrient limitation can occur and different scenarios have been observed in different lakes. Co-limitation by P and silicate in spring and P and N in autumn may occur although limitation by P prevails during the rest of the year (Moon & Carrick, 2007). No limitation by N or P in some months and limitation by N only in others has been observed in a mainly P limited lake (Xu et al., 2013). In contrast, lakes that are governed by N limitation may become P limited for short periods in early summer followed by co-limitation by N and P before switching back to N limitation (Bergström et al., 2008). As these changes in nutrient limitation fall into growth periods that may be critical for algal bloom formation, the management of certain nutrients may be necessary before or during these periods of change while the management of other nutrients may be more important throughout the rest of the year.
Whether phytoplankton growth is controlled by P or N is commonly assessed using in situ nutrient enrichment assays. Based on Liebig’s law of the minimum, the nutrient that evokes the greatest biomass increase is deemed the limiting nutrient for phytoplankton growth. Biomass increase is often determined as chlorophyll a and, in fewer cases, as cell counts. In situ enrichment assays vary in spatial and temporal scale, ranging from whole-lake fertilisation lasting for years (Schindler et al., 2008) to microcosm assays using small volumes over a few days (Burger et al., 2007). The extrapolation from microcosm assays to whole ecosystem processes has received criticism in the past. Schindler (1998) and Carpenter (1996) argue that downscaling on the temporal and spatial level, as occurs in microcosm experiments, leads to ecologically unrealistic responses. Reasons for this include the partial exclusion of communities and the failure to capture slowly evolving processes (Schindler, 1998). However, research has shown that in nutrient enrichment studies algal response does not vary greatly with container volume or surface to volume ratio even when comparing mesocosms ranging from several litres to small ponds (Spivak et al., 2011). Thus, it is likely that small scale assays examining the direct response of phytoplankton to nutrient addition realistically represent processes occurring at larger scales. In terms of environmental impact, cost and ability to replicate, microcosm assays are the preferred option for investigating nutrient limitation in drinking water storages.
Temporal scale plays an important role in the outcome of nutrient enrichment assays. Algal biomass has been observed to be first limited by N then to not be nutrient limited and finally to be limited by P in bottle assays over a 11-day period (Carignan & Planas, 1994). Spivak et al. (2011) also report strong variation of algal responses to nutrient enrichment with time during their seven-day experiments. The growth response at a species or genus level may be even more affected by time than the overall growth or biomass increase. Each phytoplankton species has its own optimum growth rate which depends on growth conditions, i.e. temperature, light and nutrient availability. Optimum growth rates can vary greatly between species (Lürling et al., 2013) leading to different species growing at different rates under the same conditions (Litchman, 2000; Schabhüttl et al., 2013). This is important to consider if the growth response at a species or genus level is to be used in predicting the growth of nuisance groups. In particular, many cyanobacteria have slower growth rates than chlorophyta under similar temperature regimes (Lürling et al., 2013) which implies that cyanobacterial responses to nutrient enrichment may not be detected as fast.
We examined the N and P limitation of phytoplankton in Grahamstown Dam, a shallow mesotrophic drinking water reservoir in south east Australia. The reservoir has a small natural forested catchment and water levels are maintained by pumping water from a nearby river when required. This process introduces nutrients into the lake as the river carries a comparatively higher nutrient concentration. Potentially toxic cyanobacteria are present in the lake but they do not dominate the phytoplankton. However, there is concern that cyanobacterial blooms may develop as a result of increased nutrient levels. Our aims were to find the limiting nutrient for phytoplankton growth at a community and genus level, to determine if nutrient limitation was seasonally different and to examine if the experimental period influenced the phytoplankton response to nutrient enrichment at a community and genus level.
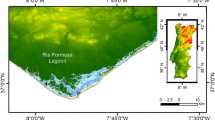
Location of the three experimental sites, marked with solid circles, within Grahamstown Dam. Coordinates of sites were S32°45′60″, E151°47′44″ (Site 1); S32°44′23″, E151°48′31″ (Site 2) and S32°42′41″, E151°49′1″ (Site 3). The solid circle on the inset shows the approximate position of the lake on the Australian continent
Methods
Study site
Grahamstown Dam is a shallow reservoir located 20 kilometres north of Newcastle on the east coast of New South Wales, Australia (Fig. 1). The lake is usually well mixed due to its surface area of 28 km2, which provides a large fetch, and its relatively mean shallow depth of 7 m. Short periods of thermal stratification can occur for a duration of up to 21 days during the summer months (Mueller and Mitrovic, unpublished data). The catchment comprises 73 km2 which at times may not be sufficient to maintain levels necessary for drinking water supply. To compensate, water levels can be increased by pumping water from the nearby Williams River through a canal (Balickera Canal) into the northern end of the lake. This procedure also increases nutrient levels in the reservoir as the Williams River carries a higher nutrient concentration than the lake. Currently, the lake has been classified as mesotrophic according to OECD guidelines (Cole & Williams, 2011).
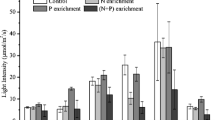
Chlorophyll a concentration for all three sites during the seasonal microcosm nutrient enrichment assays conducted from April 2009 until August 2011. Treatments with N only addition (N), P only addition (P) and N and P added in combination (PN) and the control (C) display values on day 4. Initial concentrations (i) were measured on day 0 prior to nutrient additions. Error bars are one standard error from the mean, n = 3
For this study, three different sites in the lake were chosen for their individual environmental characteristics. Site 3, close to Balickera Canal, is the most protected and shallowest (4–6 m, depending on capacity of the lake) while Site 2, closest to the centre of the lake, is the most wind exposed and deepest (9–10 m). Site 1, close to the drinking water off take, is between 7 and 9 m deep. Each site is permanently marked with a buoy (Fig. 1).
Concentrations of filtered oxidised nitrogen (\(\hbox {NO}_{x}\)) and soluble reactive phosphorus (srP) vary between 8 and 50 μg N/L and 5 and 30 μg P/L in Grahamstown Dam. Compared with the lake, concentrations of N and P are higher in the Williams River at Seaham Weir from where water is pumped into the dam via Balickera Canal. Concentrations of \(\hbox {NO}_{x}\) and srP vary between 66 and 450 μg N/L and 31 and 120 μg P/L.
The lake’s phytoplankton is dominated by Chlorophyceae and Bacillariophyceae by biovolume and by non toxic colonial cyanobacteria by cell counts. Potentially toxic cyanobacteria of the genera Microcystis, Anabaena and Aphanizomenon are present in the lake. Cell densities of Microcystis are often around 1,000/ml while Anabaena rarely reaches 1,000/ml. Counts have increased up to 30,000 for Microcystis and 20,000 cell/ml for Anabaena on two separate occasions since 1992 while Aphanizomenon has only been recorded intermittently since 2000 (Hunter Water monitoring data).
Microcosm enrichment assays
In order to identify the limiting nutrient for phytoplankton growth and potential changes with season, in situ microcosm nutrient enrichment assays were conducted in April 2009, August 2009, February 2010, May 2010, December 2010, May 2011 and August 2011 at three sites. Each microcosm enrichment experiment lasted for 4 days (from 12 pm on day 0 to 12 pm on day 4).
Three different nutrient treatments and one untreated control (C) were tested in triplicate for each of the three sites: 500 μg N/L in the form \(\hbox {KNO}_{3}\) (treatment N), 200 μg P/L in the form of \(\hbox {KH}_{2}\hbox {PO}_{4}\) (treatment P), a combination of both in the same concentration as in the single treatments (treatment PN). Nutrient concentrations were chosen so that nutrients were initially available in excess but within the natural range expected in Australian freshwater systems.
The experimental set up at each site consisted of two racks each holding six 1.25 L clear PET bottles parallel to the water surface in order to ensure an even light exposure. Racks were placed in the euphotic zone at half of the Secchi depth which approximated 25 % surface irradiance. At each site, about 25 l of surface water were filtered through a 63 μm plankton net and pooled in a plastic bin. Bottles were filled from the bin, leaving some space at the top. Nutrient solutions of P and N were added to make up intended concentrations, then the bottles were filled to the top and mixed by rotation. Lake water was filtered in order to exclude zooplankton and thus any changes in phytoplankton dynamics due to zooplankton grazing. The >63 μm fraction was examined and did not exclude cyanobacteria or other larger algae that were not present in the filtrate.
Samples for phytoplankton, chlorophyll a and nutrient concentrations were collected on day 0 and day 4. Day 0 samples were taken in triplicate from the bin of filtered but otherwise untreated lake water. Phytoplankton samples were preserved in Lugol’s iodine. Concentrations of the added dissolved nutrients were measured in surrogate bottles on day 0. Surrogate bottles were prepared in triplicate and in the same way as the bottles for the PN treatment. Nutrient samples were filtered through a 0.45 μm filter. Dissolved oxygen, pH and temperature were measured in the bottles on day 0 and 4 at each site.
Mesocosm enrichment assays
In situ mesocosm assays were conducted to examine the effect of increasing incubation time on phytoplankton growth in nutrient enriched conditions. Assays were conducted from 17 January 2013 until 4 February 2013 and from 21 March 2013 until 8 April 2013. Mesocosm assays were deployed at Site 3 in Grahamstown Dam as the previous microcosm assays had shown that phytoplankton growth at this site was generally higher compared with the other sites and cyanobacterial numbers were often higher. Moreover, Site 3 was the most sheltered of the three. Simultaneously, microcosm assays were conducted at Site 3 as described above.
Nutrient treatments were the same as in the microcosm assays (C, N, P, PN, n = 3) . Mesocosms were made of polyethylene sheeting, heat-sealed into bags of 2 m length and 1.5 m width. A cylindrical shape (1 m diameter, 1.5 m length), open only at the water surface, was maintained by stiff plastic tubing attached at the top and bottom of the bags. The mesocosm bags were attached to three floating frames (2 × 2 × 2 m) and each level of nutrient treatment was represented within each frame. Mesocosms were filled with approximately 1,000 l of unfiltered lake water.
Integrated depth samples for phytoplankton, chlorophyll a and nutrient concentrations were collected on day 0, 4, 8, 12 and 18. Nutrient samples were taken before and after nutrient additions on day 0. Dissolved oxygen, pH and temperature were measured in the mesocosms on each sampling day.
Chlorophyll a analysis
Chlorophyll a samples were filtered via vacuum filtration onto glass fibre filters on site or in the laboratory on the same day of sampling. Chlorophyll a was analysed according to Gregor & Maršálek (2004) but omitting the acidification step. The glass fibre filters were extracted in 90 % boiling ethanol for five minutes. Remains of the filters were eliminated by centrifuging. The supernatant was analysed immediately using a Varian Cary 50 Bio UV spectrophotometer.
Phytoplankton enumeration
Phytoplankton numbers were determined microscopically at 200 times magnification using a Sedgwick-Rafter counting chamber. Samples were concentrated by factors 3.3, 5 or 10 prior to counting as required. Cyanobacteria and algae were identified to genus level using identification literature by Prescott (1978), Streble and Krauter (2008) and Entwistle et al. (1997). Biovolumes of the most abundant genera were determined by using the most appropriate conversion factors from Burch et al. (2006) and Olenina et al. (2005).
Nutrient analysis
Nutrient concentrations were determined photometrically with a QuikChem 8500 Lachat nutrient analyser. Soluble reactive phosphorus (srP) was measured with the molybdate blue method using ascorbic acid as reductant. Nitrate and nitrite \((\hbox {NO}_{x})\) were analysed as surrogate of nitrate (after establishing that nitrite concentrations were negligible) with the sulphanilamide method after reduction by a cadmium column (APHA, 1995).
Data analysis
Chlorophyll a concentrations from the seasonal microcosm assays were analysed with two factorial repeated measures analysis of variance (ANOVA) with a significance level of α = 0.05 using SPSS Statistics 19.0. The Wilk’s Lambda statistic was interpreted and interactions between treatments and sites were analysed using Tukey’s pairwise comparison. Data for phytoplankton genera and chlorophyll a were analysed with two factorial ANOVA and Tukey’s pairwise comparison for each assay. Homogeneity of variance was tested with the Levene statistic. Consequentially, chlorophyll a data from the seasonal microcosm assays were logarithmically transformed to satisfy the assumptions of ANOVA. Chlorophyll a concentrations on day 4 in the mesocosm assays and simultaneous microcosm assays were compared using two factorial ANOVA.
Results
Microcosm enrichment assays
Chlorophyll a response to nutrient enrichment
Chlorophyll a concentrations increased in the experimental bottles in each of the seven assays in at least one of the treatments compared with initial concentrations. Figure 2 shows that the PN treatment evoked significantly higher phytoplankton growth compared with all other treatments at all three sites in most assays.
There was a significant interaction between nutrient enrichment treatment and site in most assays. According to Tukey’s pairwise comparison, the PN treatment evoked significant responses P ≤ 0.05 compared to the controls and the remaining treatments on the following occasions: at all three sites in the assays conducted in February and May 2010, at Sites 2 and 3 in April 2009, at Sites 1 and 2 in December 2010, at Sites 1 and 2 in May 2011 and at Site 1 in August 2009. The remaining treatments did not lead to a significant growth response on these occasions.
In August 2009, chlorophyll a values in the P and PN treatments were significantly greater than values in the remaining treatments at Sites 2 and 3. In December 2010, there was also a significant response to N additions at Site 3, albeit significantly lower than the response to the PN treatment.
There was a significant interaction P ≤ 0.001 between time, nutrient enrichment treatment and site (Table 1). This was due to a varying magnitude of the response to the PN treatment at different sites and different assays and a response mainly to the P treatment in August 2009. Also, a lack of a distinct response pattern at Site 3 in May and August 2011 likely contributed to the interaction. Site 3 generally had the highest chlorophyll a concentration in the PN treatment, followed by Site 1 and then Site 2. The magnitude of the response to the PN treatment was greater in the assays carried out during the warmer seasons, in particular in February and December 2010. The responses to nutrient treatments as well as overall growth were lowest in the August 2009 and 2011 assays, i.e. assays conducted in winter.
Time and site as well as time and treatment interaction terms were also significant and according to Tukey’s pairwise comparison the nutrient treatments can be grouped into the following three homogeneous subsets:
-
(1)
PN treatment,
-
(2)
P treatment and N treatment and
-
(3)
controls (C).
Genera response to nutrient enrichment
About 35 of the most abundant genera were counted in each assay of which 23 responded to the nutrient treatment. Responses of genera were similar at the three sites but often more pronounced at Site 3. Results of this site are summarised in Table 2.
An increase in biovolume as a response to the P, N or PN treatments was found in 218 cases. Biovolumes in these treatments were significantly higher than in the controls in 89 out of 218 responses (P ≤ 0.05).
Responses to the PN treatment were the most frequently recorded ones and were significantly higher than responses to the control and the other treatments on 63 occasions (P ≤ 0.05). Growth responses to the PN treatments and the P treatment were significantly higher than responses to the N treatment and the controls on 25 occasions. There was one significant growth response to the N treatment and the PN treatment when compared to the P treatment and the control.
Chlorophyceae and Bacillariophyceae mainly responded to the PN treatment or the P treatment with significantly increased growth but there were also responses by Cryptomonas, Mallomonas and Trachelomonas. Aphanothece and Aphanocapsa showed significant growth responses to the PN and the P treatments and thus were the only cyanobacteria to significantly respond to nutrient additions. The former responded to P and PN treatments in the April 2009, August 2009 and December 2010 assays and the latter to the PN treatment in the December 2009 assay.
Chlorophyll a concentration in μg/l in the mesocosm and microcosm assays conducted in January and March 2013. Treatments with N only addition (N), P only addition (P) and N and P added in combination (PN) and the control (C) display values on day 4. Values on day 0 (i) of the microcosm assays were measured in filtered and pooled lake water prior to filling the microcosms. Error bars are one standard error from the mean, n = 3
Mesocosm enrichment assays
Chlorophyll a response to nutrient enrichment
Similar to the seasonal microcosm assays, the greatest response to nutrient enrichment was found in the PN treatment in the two mesocosm and simultaneous microcosm assays conducted in 2013 (Fig. 3). In both mesocosm assays, biomass continued to increase in the PN treatment until day 8. After day 8, biomass declined in both assays.
In the January 2013 assay, chlorophyll a concentrations increased almost threefold between days 0 (5.2 μg/l) and 4 (14 μg/l) and more than threefold between days 4 and 8 (48 μg/l) in the PN treatment. In the March assay, biomass increase was more than threefold in the PN treatment between days 0 (9.4 μg/l) and 4 (33 μg/l) and almost doubled between days 4 and 8 (62 μg/l).
There was a small response to the N treatment, with the January assay reaching highest values on day 18 (12 μg/l) while there was no noticeable response to the P treatment compared with controls in either assay. Controls did not experience a biomass increase more than twofold in the January assays. In March, biomass declined in the controls from day 0 onwards. There was no statistical difference between chlorophyll a concentrations in treatments and controls on day 0 in either assay.
Biovolumes in mm3/l of some phytoplankton genera responding to additions of P and N in combination (PN treatment) in the mesocosm assays in January (Mougeotia) and March (Aphanocapsa, Chroomonas and Acanthoceras) 2013. Remaining treatments were additions of P (P treatment), additions of N (N treatment) and one untreated control (C). Error bars are one standard error from the mean, n = 3
Biovolumes in mm3/l of some phytoplankton genera responding to the PN and the N treatments (top row) or the PN and the P treatments (bottom row) mesocosm assays in January (Cryptomonas and Cyclotella) in March 2013 (Aphanizomenon and Anabaena). Remaining treatments were additions of P (P treatment) and one untreated control (C). Error bars are one standard error from the mean, n = 3
Chlorophyll a concentrations in the simultaneously conducted microcosm assay were statistically not different from the ones in the mesocosm assay on day 4 in the January assays (Table 3; Fig. 4). In the March assays, concentrations were higher in the microcosm assay but the response pattern was the same, i.e. highest response in the PN treatment (Table 3; Fig. 4).
Genera responses to nutrient enrichment
In both mesocosm assays, the most obvious response to nutrient additions by individual genera was observed in the PN treatment.
Biovolumes in mm3/l of phytoplankton genera responding to additions N (N treatment) in the mesocosm assay in January 2013. Remaining treatments were additions of P and N in combination (PN treatment), additions of P (P treatment) and one untreated control (C). Error bars are one standard error from the mean, n = 3
This treatment evoked distinctly higher growth in 13 of the 25 most abundant phytoplankton genera in the January assay and in 20 genera in the March assay. Three genera in the January assay and 11 genera in the March assay responded to the PN treatment with increased biovolumes but not to the other treatments or the control. Figure 5 illustrates this response pattern in four of the genera. Graphs showing the growth response of the remaining genera can be found in the supplementary material.
There was a response to the PN treatment and at the same time a small response to the N treatment in 10 genera in the January assay and in six genera in the March assay. Similarly, there was a response to the PN treatment and at the same time a small response to the P treatment in two genera in the January assay and three genera in the March assay. Responses of four of these genera are shown in Fig. 6 and graphs for the remaining genera can be found in the supplementary material.
Single additions of N also evoked growth responses in some genera while there was no distinct difference between the other treatments and the controls (Fig. 7).
Some genera responded to different nutrient treatments during the course of the experiment. The cyanobacteria Aphanizomenon and Anabaena responded to the PN treatment within the first 8 days of both experiments (Fig. 6 and graphs in the supplementary material). The P treatment evoked a 24-fold increase in biovolume by Aphanizomenon and a 28-fold increase in biovolume by Anabaena between days 12 and 18 in the January assay. Anabaena also responded to the P treatment between days 8 and 12 in the March assay but its growth response to the PN treatment exceeded the growth response to the P treatment.
Discussion
The most frequent outcome of our nutrient enrichment assays was a response to the PN treatment while addition of single nutrients did not lead to a significant growth response. This was observed at the biomass level and at the level of individual genera. The response pattern to factorial nutrient addition, where two simultaneously added nutrients evoke a greater growth response than their individual additions, is often referred to as co-limitation by both nutrients (Burger et al., 2007; Ogbebo et al., 2009; Harpole et al., 2011). Harpole et al. (2011) further distinguish between two types of co-limitation: simultaneous and independent. Responses to two resources in combination but not to their individual additions are classified as simultaneous co-limitation. In the case of independent co-limitation, two resources evoke an equal growth response when added individually. When added in combination, the growth response exceeds the ones caused by their individual additions.
Most results of our microcosm assays fall into the category of simultaneous co-limitation: community growth responses at all three sites in February and May 2010, at Sites 2 and 3 in April 2009, Sites 1 and 2 in December 2010, Sites 1 and 2 in May 2011 and at Site 1 in August 2009. Most of the genera responses to nutrient additions in these assays can also be classified as simultaneous co-limitation (e.g. Ankistrodesmus, Table 2).
A significant response to the PN treatment together with a significant response to addition of either N or P can be classified as “serial limitation” (Harpole et al., 2011). This type of response was observed at the community level in the microcosm assays at Sites 2 and 3 in August 2009, Site 1 in April 2009 and at Site 3 in December 2009. In August 2009, most genera responses reflected the community response, i.e. most genera were serially limited by P and then by N (Table 2). In December 2010 and April 2009, some genera responses could be classified as serially limited (e.g. Acanthoceras, limited first by N and then by P, Table 2) but most genera would be considered simultaneously co-limited. This indicates that in particular in August 2009, one nutrient was more important for the phytoplankton community and its individual genera than the other.
Similarly, the community response in the January mesocosm assay may fall into the category of serial limitation as concentrations in the N treatment were increased throughout the assay compared with the controls. In contrast, the biomass response in the March mesocosm assay could be considered as simultaneous co-limitation. Also, the community response was reflected by most genera in both assays.
Co-limitation of phytoplankton growth has been reported frequently in short term experiments using chlorophyll a or other composite measures to detect a growth response (Dzialowski et al., 2005; Burger et al., 2007; Quiblier et al., 2008; Ogbebo et al., 2009). We not only found phytoplankton growth to be co-limited by P and N at a community level but also at a genus level in two different types of nutrient enrichment assay, i.e. seasonal nutrient enrichment microcosm assays and two mesocosm assays.
According to Liebig’s Law of the Minimum, only one nutrient can be limiting at a given time and co-limitation by two nutrients at the same time would not be possible. Danger et al. (2008) argue that Liebig’s Law was developed for individual crop plants and could not be applied to naturally diverse communities which would adapt to the stoichiometry of their available resources. Ultimately, this would result in co-limitation at the community level. When individual taxa responses are not considered and a composite measure alone detects co-limitation, it seems obvious to explain co-limitation as the combined effect of individual taxonomic groups being limited by different single nutrients as suggested in some studies (Harpole et al., 2011). It has been demonstrated that the entire community can be co-limited by N and P while individual species or genera are limited by either nutrient (Burger et al., 2007; Quiblier et al., 2008).
Although co-limitation of the phytoplankton community seems to be common, it has rarely been shown that growth of individual genera can also be co-limited. Hyenstrand et al. (2001) demonstrated that growth of the cyanobacterium Gloeotrichia echinulata was co-limited by trace metal nutrients and macronutrients (N and P) in combination. Different mechanisms for co-limitation of algal growth rates by trace metals have been suggested and shown, e.g. biochemical substitution where two trace metals can fulfil the same biological function, or biochemically dependent co-limitation where the lack of one element prevents the acquisition of another (Saito et al., 2008). However, these mechanisms cannot explain co-limitation by biochemically mutually exclusive nutrients such as N and P at the genus or species level.
In our assays, many genera from different families were simultaneously co-limited in both types of nutrient enrichment assay. Harpole et al. (2011) found that simultaneous and independent co-limitation were often observed in nutrient enrichment assays where environmental nutrient concentrations were low. This may also apply to our case as concentrations of srP and \(\hbox {NO}_{x}\) in Grahamstown Dam are usually low (8–50 μg N/L and 5–30 μg P/L). The response evoked by additions of single nutrients might have been too small to be statistically significant and thus simultaneous co-limitation may have masked serial limitation.
Simultaneous co-limitation does not contradict Liebig’s Law, if indeed small growth responses and low statistical power are responsible for this response and serial limitation was the actual type of nutrient limitation. Serial limitation may be interpreted as the limitation by one nutrient, i.e. the nutrient that evokes a response when added on its own, N or P in our case. This would alleviate limitation but it might also induce limitation by the second nutrient. When the second nutrient is also present, as in the PN treatment, further growth can occur.
Different container sizes and the inclusion or exclusion of zooplankton, in mesocosms and microcosms, respectively, may also have influenced phytoplankton responses to nutrient additions in our enrichment assays. However, container size had only an effect in the experiments conducted in March 2013. Chlorophyll a concentrations were higher in all treatments of the microcosm assay compared with treatments of the mesocosm assay on day 4. Different mean light levels may have affected algal biomass in mesocosm and microcosm assays. The microcosm bottles were suspended at the same depth (at 25 % surface irradiance) during the course of the assay, while the mesocosms covered the first 1.5 m of the water column (including the depth where microcosm bottles where placed). It is also likely that the exclusion of zooplankton from the microcosms affected algal growth positively. In turn, zooplankton grazing in the mesocosms may have caused the delay of growth responses in some genera. It is difficult to tease apart the specific effects of zooplankton grazing and container size on our experiments without quantifying zooplankton densities. Generally, mesocosms would provide a more natural environment than microcosms. However, the fact that the pattern of the nutrient limitation response of the phytoplankton community was the same in both types of enrichment asssay—mesocosm and microcosm—within the first four days, confirms and validates the results of the seasonal microcosm assays. Further, it suggests that the results from the seasonal microcosm assays are a good indicator of the nutrients limiting algal biomass in Grahamstown Dam.
Co-limitation may not have prevailed throughout the year in Grahamstown Dam as there were signs of P limitation of the phytoplankton community as well as individual genera during winter. Although this may indicate a greater importance of P compared with N, growth responses were also much lower in winter, hinting at a combination of nutrient and light or temperature limitation. Seasonal changes in nutrient limitation have been shown in other studies (Moon & Carrick, 2007; Bergström et al., 2008; Xu et al., 2013) and in contrast to the present results, they often occur during the phytoplankton growing season. It is possible that the sampling frequency was too low to pick up switches in nutrient limitation in Grahamstown Dam. However, several experiments were conducted during the main growing season in the lake (December to May) and all of those resulted in co-limitation of phytoplankton biomass.
Not only seasonality but also experimental duration influenced the phytoplankton response to nutrient enrichment in Grahamstown Dam. Specifically, there were three different effects of experimental duration on the response to nutrient enrichment, as shown in the two mesocosm experiments. Firstly, some genera switched from responding to one nutrient treatment to responding to another during the course of the experiment. This was most evident in the potentially toxic cyanobacteria Anabaena and Aphanizomenon in the January experiment. Both genera are capable of fixing atmospheric N and may have used this trait to their advantage in the P treatments. It has been observed, that low N:P ratios can result in increased N fixation rates (Piehler et al., 2009) and dominance of the phytoplankton by N-fixing cyanobacteria (Vrede et al., 2009; Tezanos & Litchman, 2010). In contrast, switching from responding to the PN treatment to the N treatment may be explained by the utilisation of internal P storages. The formation of polyphosphate storage granules may take place after periods of P starvation (Eixler et al., 2006) and may increase growth when additional N is available. As srP concentrations are often low in Grahamstown Dam, this is not unlikely to occur.
Secondly, some genera showed a delay in the response to nutrient additions. Again, an explanation may be found in zooplankton grazing. Initial cell densities of these genera may have been at the detection limit but in balance, i.e. growth and loss would have been in balance so that a stable population would have been present. Added nutrients would have increased the growth of these sparse genera. Eventually, the exponential nature of algal growth would have led to growth exceeding loss and thus would have made the growth response detectable.
The day the highest growth response was measured varied between individual genera, i.e. the day the highest growth response was measured varied between day 4, 8, 12 or 18. This may have been due to different growth rates of different taxa within the phytoplankton community, preferences of zooplankton grazing or a combination of both factors. In contrast, at the community level, the highest magnitude of the growth response occurred on day 8 (or shortly thereafter as biomass on day 12 was lower than on day 8).
Assessing responses of potentially toxic cyanobacteria to nutrient enrichment in a lake where those cyanobacteria occur in low densities proved to be difficult. Although potentially toxic cyanobacteria were present during the seasonal microcosm experiments, there were no significant growth responses to any nutrient treatment. Despite even lower densities of the potentially toxic cyanobacteria in the mesocosm assays, the mesocosm assays enabled us to record the growth response of the two genera that were present in the lake at the time the assays were conducted. This can be attributed to the longer duration of the mesocosm assays.
The results of the present study confirm that one has to choose the experimental period of nutrient enrichment assays carefully. Although micro- and mesocosm assays conducted in 2013 revealed the same response pattern in terms of biomass within the first 4 days, the mesocosm assays have also shown that longer time frames showed some temporal response patterns.
For the management of external nutrient loading to Grahamstown Dam, our results imply that the input of N and P needs to be addressed to prevent excessive phytoplankton growth. Currently, water quality management is based on concentration of total phosphorus (TP) and cyanobacterial numbers in Grahamstown Dam (Cole & Williams, 2011). Under this management plan, water can be extracted from the Williams River, if the concentration of TP is below 200 μg/l and toxic cyanobacteria do not exceed 10,000 cells/ml. Thus, it would be advisable to include N in the management plan and define limits of external N loading. As P is likely to be more important for potentially toxic N-fixing cyanobacteria, the control of P should be continued.
References
APHA (ed.). 1995. Standard Methods for the Examination of Water and Wastewater, 19th edn. American Public Health Association/American Water Works Association/Water Pollution Control Federation, Washington, DC.
Bergström, A. K., A. Jonsson & M. Jansson, 2008. Phytoplankton responses to nitrogen and phosphorus enrichment in unproductive Swedish lakes along a gradient of atmospheric nitrogen deposition. Aquatic Biology 4: 55–64.
Bowling, L. C., 1994. Occurrence and possible causes of a severe cyanobacterial bloom in Lake Cargelligo, New South Wales. Australian Journal of Marine and Freshwater Research 45: 737–745.
Burch, M. D., F. L. Harvey, P. D. Baker & G. Jones, 2006. National protocol for the monitoring of cyanobacteria and their toxins in surface fresh waters Draft V6.0.
Burger, D. F., D. P. Hamilton, J. A. Hall & E. F. Ryan, 2007. Phytoplankton nutrient limitation in a polymictic eutrophic lake: community versus species-specific responses. Archiv für Hydrobiologie 169(1): 57–68.
Carignan, R. & D. Planas, 1994. Recognition of nutrient and light limitation in turbid mixed layers: three approaches compared in the Paraná floodplain (Argentina). Limnology and Oceanography 39(3): 580–596.
Carpenter, S. R., 1996. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology 77(3): 677–680.
Cole, B. & B. Williams, 2011. Grahamstown Dam Water Quality & Aquatic Ecological Functioning. A Compilation of Scientific and Adaptive Management Studies. Hunter Water Corporation, Newcastle, NSW.
Danger, M., T. Daufresne, F. Lucas, S. Pissard & G. Lacroix, 2008. Does Liebig’s law of the minimum scale up from species to communities? Oikos 117(11): 1741–1751.
De Tezanos Pinto, P. & E. Litchman, 2010. Interactive effects of N:P ratios and light on nitrogen-fixer abundance. Oikos 119: 567–575.
Dzialowski, A. R., S. H. Wang, N. C. Lim, W. W. Spotts & D. G. Huggins, 2005. Nutrient limitation of phytoplankton growth in central plains reservoirs, USA. Journal of Plankton Research 27(6): 587–595.
Eixler, S., U. Karstens & U. Selig, 2006. Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 45: 53–60.
Elser, J. J., M. E. Bracken, E. E. Cleland, D. S. Gruner, W. S. Harpole, H. Hillebrand, J. T. Ngai, E. W. Seabloom, J. B. Shurin & J. E. Smith, 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142.
Entwistle, T. J., J. A. Sonneman & S. H. Lewis, 1997. Freshwater Algae in Australia. A Guide to Conspicuous Genera. Sainty and Associates, Sydney.
Gregor, J. & B. Maršálek, 2004. Freshwater phytoplankton quantification by chlorophyll a: a comparative study of in vitro, in vivo and in situ methods. Water Research 38: 517–522.
Harpole, W. S., J. T. Ngai, E. E. Cleland, E. W. Seabloom, E. T. Borer, M. E. Bracken, J. J. Elser, D. S. Gruner, H. Hillebrand, J. B. Shurin & J. E. Smith, 2011. Nutrient co-limitation of primary producer communities. Ecology Letters 14: 852–862.
Hyenstrand, P., E. Rydin, M. Gunnerhed, J. Linder & P. Blomqvist, 2001. Response of the cyanobacterium Gloeotrichia echinulata to iron and boron additions – an experiment from Lake Erken. Freshwater Biology 46: 735–741.
James, C., J. Fisher & B. Moss, 2003. Nitrogen driven lakes: the Shropshire and Cheshire Meres? Archiv für Hydrobiologie 158: 249–266.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Kohler, E. H. H. R. Lammens, T. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Noges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. L. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lake responses to reduced nutrient loading – an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771
Lewis, W. M. & W. A. Wurtsbaugh, 2008. Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. Interational Review of Hydrobiology 93(4): 446–465.
Litchman, E., 2000. Growth rates of phytoplankton under fluctuating light. Freshwater Biology 44: 223–235.
Lürling, M., F. Eshetu, E. J. Faassen, S. Kosten, V. L. M. Huszar, 2013. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshwater Biology 58(3): 552–559.
Moon, J. B. & H. J. Carrick, 2007. Seasonal variation of phytoplankton nutrient limitation in Lake Erie. Aquatic Microbial Ecology 48: 61–71.
Ogbebo, F. E., M. S. Evans, M. J. Waiser, V. P. Tumber & J. J. Keating, 2009. Nutrient limitation of phytoplankton growth in Arctic lakes of the lower Mackenzie River Basin, northern Canada. Canadian Journal of Fisheries and Aquatic Sciences 66(2): 247–260.
Olenina, I., S. Hajdu, L. Edler, A. Andersson, N. Wasmund, S. Busch, J. Göbel, S. Gromisz, S. Huseby, M. Huttunen, A. Jaanus, P. Kokkonen, I. Ledaine & E. Niemkiewicz, 2005. Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Baltic Sea Environment Proceedings 106: 1–144.
Piehler, M. F., J. Dyble, P. H. Moisander, A. D. Chapman, J. Hendrickson & H. W. Paerl, 2009. Interactions between nitrogen dynamics and the phytoplankton community in Lake George, Florida, USA. Lake and Reservoir Management 25: 1–14.
Prescott, G. W., 1978. How to Know the Freshwater Algae, 3rd edn. Wm.C. Brown Company Publishers, Dubuque, IA.
Qin, B., G. Zhu, G. Gao, Y. Zhang, W. Li, H. W. Paerl & W. W. Carmichael, 2010. A drinking water crisis in Lake Taihu, China: linkage to climatic variability and lake management. Environmental Management 45: 105–112.
Quiblier, C., C. Leboulanger, S. Sané & P. Dufour, 2008. Phytoplankton growth control and risk of cyanobacterial blooms in the lower senegal river delta region. Water Research 42: 1023–1034.
Saito, M. A., T. J. Goepfert & J. T. Ritt, 2008. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnology and Oceanography 53(1): 276–290.
Schabhüttl, S., P. Hingsamer, G. Weigelhofer, T. Hein, A. Weigert & M. Striebel, 2013. Temperature and species richness effects in phytoplankton communities. Oecologia 171: 527–536.
Schindler, D. W., 1977. Evolution of phosphorus limitation in lakes. Science 195(4275): 260–262.
Schindler, D. W., 1998. Replication versus realism: need for ecosystem-scale experiments. Ecosystems 1: 323–334.
Schindler, D. W., R. E. Hecky, D. L. Findlay, M. P. Stainton, B. R. Parker, M. J. Paterson, K. G. Beaty, M. Lyng & S. E. M. Kasian, 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences of the United States of America 105(32): 11254–11258.
Spivak, A. C., M. J. Vanni & E. M. Mette, 2011. Moving on up: can results from simple aquatic mesocosm experiments be applied across broad spatial scales? Freshwater Biology 56: 279–291.
Sterner, R. W., 2008. On the phosphorus limitation paradigm for lakes. International Review of Hydrobiology 93(4–5): 433–445.
Streble, H. & D. Krauter, 2008. Das Leben im Wassertropfen, 10th edn. Franckh-Kosmos Verlags-GmbH & Co. KG, Stuttgart.
Vrede, T., A. Ballantyne, C. Mille-Lindblom, G. Algesten, C. Gudasz, S. Lindahl & A. K. Brunberg, 2009. Effects of N:P loading ratios on phytoplankton community composition, primary production and N fixation in a eutrophic lake. Freshwater Biology 54: 331–344.
Willén, E., 2001. Phytoplankton and water quality characterization: experiences from the Swedish Large Lakes Mälaren, Hjälmaren, Vättern and Vänern. Ambio 30: 529–538.
Xu, S., B. Huang, Z. B. Wei, J. Luo, A. J. Miao & L.-Y.Yang, 2013. Seasonal variation of phytoplankton nutrient limitation in Lake Taihu, China: a monthly study from year 2011 to 2012. Ecotoxicology and Environmental Safety 94: 190–196.
Acknowledgments
We would like to thank Hunter Water for funding this project under the Grahamstown Dam Adaptive Management Strategy, in particular Bruce Cole for management of the project and Ian Graham, Stuart Bailey, Jim Carter, Greg Mason and Paul for their help with boat operations and sampling. Further, we are grateful for the assistance of James Hitchcock and Martin Pfeil in the field and we owe Dr Darren Baldwin thanks for useful comments on this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David Philip Hamilton
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Müller, S., Mitrovic, S.M. Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: progressing from the phosphorus limitation paradigm. Hydrobiologia 744, 255–269 (2015). https://doi.org/10.1007/s10750-014-2082-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2082-3