Abstract
Ischemic cardiomyopathy (ICM) is the most prevalent cause of heart failure (HF) in developed countries, with significant morbidity and mortality, despite constant improvements in the management of coronary artery disease. Current literature on this topic remains fragmented. Therefore, this review aimed to summarize the most recent data on ICM, focusing on its definition, epidemiology, outcomes, and therapeutic options. The most widely accepted definition is represented by a left ventricular dysfunction in the presence of significant coronary artery disease. The prevalence of ICM is largely influenced by age and sex, with older individuals and males being more affected. Its pathophysiology is characterized by plaque buildup, thrombus formation, hypoperfusion, ischemic cell death, and left ventricular remodeling. Despite improvements in therapy, ICM still represents a public health burden, with a 1-year mortality rate of 16% and a 5-year mortality rate of approximately 40% in the USA and Europe. Therefore, optimization of cardiovascular function, prevention of progressive remodeling, reduction of HF symptoms, and improved survival are the main goals of treatment. Therapeutic options for ICM include lifestyle changes, optimal medical therapy, revascularization, device therapy, mechanical circulatory support, and cardiac transplantation. Personalized management strategies and tailored patient care are needed to improve the outcomes of patients with ICM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic cardiomyopathy (ICM) represents the leading cause of heart failure (HF) in developed countries, and despite consistent improvements in the management of coronary artery disease (CAD), it remains a significant contributor to morbidity and mortality on a global scale [1, 2]. The advances in the management of acute myocardial infarction (AMI) have resulted in increased patient survival but with an unavoidable rise in the incidence of left ventricular remodeling and dysfunction, as well as the incidence and prevalence of ICM [3, 4].
Despite therapies to improve symptoms, quality of life, and outcomes, HF remains the most frequent cause of hospitalization in the USA with an elevated mortality rate and a dismal prognosis [5, 72]. In the USA and Europe, ICM is associated with a 1-year mortality rate of about 16% and a 5-year mortality rate of about 40% [6]. Additionally, compared to patients with non-ischemic HF, patients with HF due to CAD exhibit a higher rate of unfavorable cardiac events and poorer prognosis, with increased all-cause mortality and sudden cardiac death (SCD) compared to the non-ischemic cardiomyopathy (NICM) group [4, 7]. Therefore, a better understanding of the fundamental causes of HF and customized patient care is becoming the current focus of HF therapy, with HF etiology assessment being considered the initial crucial step for planning the most appropriate management [8].
Patients with ischemic HF have been shown to benefit from several therapeutic options, spanning from lifestyle modifications and medical therapy to revascularization, device therapy (implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT)), mechanical circulatory support, and cardiac transplantation [2, 9].
Currently, the literature on ischemic cardiomyopathy is characterized by fragmentation; that is, most review articles have focused on a specific subtopic in ICM. Therefore, this paper aimed to summarize the most recent data on ICM, with a particular focus on its current definition, epidemiology, outcomes, and therapeutic options.
Clinical definition
To date, a standardized definition is still lacking. Based on the large-scale trial, Surgical Treatment for Ischemic Heart Failure (STICH), ICM was defined as a significant decline in left ventricular (LV) systolic function (specifically a left ventricular ejection fraction (LVEF) < 35%) in the presence of severe CAD [2]. A cross-sectional observational study from China defines ICM as a myocardial disease secondary to severe coronary atherosclerotic lesions leading to long-term myocardial ischemia, myocardial cell damage, necrosis, and myocardial fibrosis resulting in changes in heart structure and a decrease in cardiac function [10].
The definition most frequently applied involves LV dysfunction (expressed as reduced ejection fraction) in the presence of significant CAD and at least one of the following: prior revascularization or AMI, > 75% stenosis in the left main or left anterior descending artery, or two or more coronary vessels presenting a > 75% luminal stenosis [1, 11]. The presence of necrotic (non-viable) myocardium and hibernated (dysfunctional but still viable) myocardium represents the principal mechanisms contributing to the development of ischemic heart failure [4]. Therefore, current ischemic cardiomyopathy terminology implies the presence of ventricular enlargement and impaired myocardial contractility resulting from ischemia or infarction [2].
There is now the consideration to abandon the term “ischemic cardiomyopathy,” since left ventricular dysfunction caused by coronary artery disease does not comprise a true cardiomyopathy [4].
Epidemiology
Trends
Heart failure impacts approximately 1–3% of the overall population and is the primary reason for hospitalization both in the USA and Europe [14]. ICM is considered the largest single cause of HF (although causes are often multifactorial), with nearly 70% of all HF syndromes being attributable to underlying ischemic heart disease (IHD) [11, 12]. However, the scarcity of epidemiological studies focusing on ICM as a distinct clinical entity has led to an underestimation of its accurate prevalence and incidence. Consequently, most studies refer to this condition as IHD or its associated manifestations, such as angina pectoris (AP), AMI, and ischemic heart failure [2].
Owing to the improved survival of patients with CAD, there has been a noticeable increase in the prevalence of ICM over the last few decades. This increase can be linked to advances in the management of AMI, through thrombolytic therapy and percutaneous coronary intervention (PCI) with drug-eluting stents resulting in successful revascularizations, and of patients with chronic CAD through optimal medical therapy [1, 4].
Additionally, due to an aging population and increased prevalence of risk factors, HF prevalence is likely to rise even further, with an increase of 46% expected between 2012 and 2030 in the USA and the total population affected going from 2.42 to 2.97% [5].
The increased survival has also led to higher morbidity due to remodeling of the left ventricle, chronic myocardial dysfunction, and subsequent increased ischemic heart failure incidence [3].
Demographics
The prevalence of ischemic HF is also influenced by age. Indeed, it has been observed that the percentage of the population affected increases with age (particularly after 60 years) with 12.8% of males and 12.0% of females showing a diagnosis of HF after 80 years [13]. Additionally, there has been a notable increase in diagnoses among individuals under the age of 55, indicating an increasing incidence in younger age groups [14].
Sex also accounts for the differences in the prevalence of ischemic HF. A sub-analysis of the REDINSCOR II study revealed that, within the cohort of patients admitted with HF, approximately 20% of women and around 40% of males exhibited an ischemic etiology [15].
Another study demonstrated a higher prevalence of males and Caucasians in patients with HF due to ischemic etiology [16]. Also, it was demonstrated that women have a greater risk of developing symptomatic HF after an acute MI [17].
Several epidemiological data show that the role of CAD in HF varies depending on the geographic region: CAD is responsible for only 10% of all HF cases in sub-Saharan Africa, while it accounts for 50–70% of cases in the USA and Europe and for 30–40% of all cases in Latin America and Asia [12].
Incidence and prevalence after acute coronary syndromes (ACS)
HF remains a common complication in patients admitted for AMI, with high incidence rates (IRs) during the first months up to 1 year after discharge [18]. However, actual occurrence of HF in patients admitted for AMI varies across different clinical studies, spanning from 14 to 36% [19].
In a study assessing the link between clinically manifested myocardial infarction (CMI) and HF versus those without a history of MI, it was found that among 1 million individuals hospitalized for HF each year in the USA, up to one-third, experienced prior myocardial infarction [20]. Another study involving the enrollment of 483 incident cases of AMI from 1992 to 1996 showed that 4% of patients presented with HF upon admission, and 39% experienced HF during their hospital stay. The Global Registry of Acute Coronary Events (GRACE) study revealed that among 13,707 patients hospitalized for MI between 1999 and 2001, 13% presented with HF upon admission, and 5.6% developed HF during hospitalization. A more recent study enrolling 187,803 AMI patients hospitalized between 2007 and 2011 showed that 12% of patients had signs of HF on admission, while 4% developed HF while hospitalized [19]. In the Cardiovascular Disease in Norway (CVDNOR) project, it was demonstrated that, among 86,771 patients with a first AMI from 2001 to 2009, 18.7% of the patients presented with HF or developed HF during hospitalization [18]. Also, in the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) study, the incidence of HF within a 2-year period following PCI was reported to be 5.1% [21].
While the proportion of patients exhibiting signs of HF upon admission for AMI has been on the rise (increasing from 4% to approximately 12–13%), the percentage of those developing HF during hospitalization for AMI is decreasing, going from 39 to 4–8% [18]. Recent advancements in prehospital care leading to a reduction in out-of-hospital deaths could explain the increase in HF at MI presentation. In contrast, the decrease in in-hospital HF may be explained by the more substantial myocardial salvage provided by the use of PCI. Indeed, according to the SWEDEHEART registry, the incidence of HF complicating MI during hospitalization went from 46% in the thrombolytic era to 28% in the PCI era [22]. Regarding the incidence of HF after MI discharge, it was shown to be highest in the first months, followed by a drop and then a stable trend with a rate of 1.3–2.2%/year [22].
Despite that it comprises approximately half of all myocardial infarctions, the risk of heart failure in patients experiencing silent myocardial infarction (SMI) is still uncertain [20]. There is a lack of data regarding the incidence of HF after PCI for non-ST segment elevation myocardial infarction (NSTEMI) [21].
Incidence and prevalence in patients with stable coronary artery disease
Relatively limited information is available on the development of HF in patients with stable CAD. A study analyzing HF development in a population of outpatients with stable CAD showed that the latter were frequently hospitalized for HF and a high mortality was associated with it [23]. Although a higher occurrence of HF in patients with detected silent myocardial ischemia has never been reported in prospective studies, previously unrecognized CAD appears to be associated with HF. A study undertaking invasive coronary angiography over 99 out of 136 patients under 75 years hospitalized for HF in the UK showed that the etiology of HF was CAD in 71 of the patients, with the ischemic etiology not being recognized prior to angiography in 18 of them [24].
Moreover, data from the Framingham Heart Study showed that during the 118,000 person-years of follow-up, 5% of the cohort developed HF without a previously clinically recognized ischemic event. These studies strongly suggest a contribution of silent CAD [12].
Pathophysiology
Plaque buildup and thrombus formation can lead to a hypoperfused myocardium, unrestored blood flow which leads to ischemic cell death, and loss of contractile function of cardiac myocytes. However, normal contractility can be restored after reperfusion. This reversible contractile dysfunction is termed myocardial stunning [25]. The stunned myocardium may remain hypocontractile even after the reestablishment of normal blood flow and can be present longer than the duration of hypoperfusion [26]. Although not fully established, there are two proposed mechanisms, among several others, that are plausible in explaining the mechanism of stunning. These hypotheses include the generation of free oxygen radicals and the decreased sensitivity of contractile filaments to calcium. We believe that the two hypotheses are not mutually exclusive. During reperfusion, it is possible that a surge of free oxygen radicals can alter contractile filament sensitivity to calcium, resulting in decreased contractile function [27].
Repetitive episodes of myocardial stunning and chronic hypoperfusion yielded different results in the cardiac myocytes. Hibernating myocardium, unlike the stunned myocardium where contractility and basal blood flow are restored, shows a decrease in both [25]. The reduction of oxygen leads to the depletion of ATP, which is essential for calling molecular cells. In cardiomyocytes, depletion of ATP leads to an imbalance of ions, causing insufficient contractions and decreased cardiac function. Hibernating cardiac myocytes adapt to these physiological changes to allow their survival and possible reversion to normal cardiac function if sufficient perfusion and oxygen levels are reintroduced. However, a prolonged duration of insufficient oxygen can lead to irreversible damage of cardiac myocytes, leading to irreversible heart remodeling, such as thinning of the LV wall and deposition of fibrotic tissue [28].
Ischemic cell death arises within 20 min of occlusion of coronary blood flow, with severe cell death apparent at 60 min. The oxygen content within myocytes begins to deplete within 8–10 s of occlusion, and oxidative phosphorylation ceases and switches to anaerobic metabolism. Under hypoxic conditions, lactic acid is not metabolized, and ATP is depleted [29]. The lack of ATP influences several factors in contractile dysfunction, including insufficient intracellular calcium uptake into the sarcoplasmic reticulum and disruption of the cross-bridge cycle. During cardiomyocyte contraction, calcium is released from the sarcoplasmic reticulum, which exposes the myosin-binding site on the actin filaments. However, at low ATP concentrations, calcium cannot be actively pumped back into the SR, resulting in high intracellular calcium concentrations. This can lead to electrical and mechanical dysfunction, including arrhythmias and decreased contraction force [30]. In the cross-bridge cycle, ATP is utilized to break the actin-myosin cross-linkage and cock the myosin head back ready for another power stroke event. Without sufficient ATP, the actin-myosin complex becomes stuck in a state of muscle stiffening. Therefore, prolonged cardiomyocyte hypoxia may result in cell death.
Following an acute infarction, ventricular remodeling can result in thickening or thinning of the cardiac muscle tissue. The expansion of the initial infarct continues to degrade the cardiac muscle, leading to the thinning of the cardiac wall. Thinning and weakening of the cardiac wall cause contractile dysfunction, leading to a dilated physiology. On the other hand, deposition of scar or fibrotic tissue is another possible outcome following an infarction. A sudden surge in cell death stimulates the activation of myofibroblasts and deposits collagen and fibrotic tissue to prevent rupture of the already thin ventricular walls [31]. Exaggerated myofibroblast activation causes excessive scar tissue deposition on the cardiac wall. Scar tissue by nature has no contractile capability and serves only to prevent the rupture of the cardiac wall. Excessive deposition of fibrotic tissue enlarges and stiffens the heart, reducing contractile power, compliance, and stroke volume [32]. While the LV dilation secondary to myocardial scarring predominates in HFrEF (heart failure with reduced ejection fraction), the HFpEF (heart failure with preserved ejection fraction) phenotype secondary to ischemia is characterized by a more complex pathophysiology [12]. Indeed, despite improvements in addressing left ventricular adverse remodeling (LVAR) following STEMI, the risk for HF remains elevated. This suggests that patients may develop HF post-STEMI even in the absence of LVAR, indicating a shift in the classic paradigm and pointing to the emergence of a new HFpEF phenotype, influenced by a complex interplay of factors [84]. A decrease in cardiac reserve and a decline in cardiorespiratory fitness (CRF), resulting from impaired diastolic or systolic function during exercise due to acute myocardial damage, post-infarction response, individual risk factors, or subclinical cardiac abnormalities, are key contributors. Cardiomyocyte loss during initial injury contributes to a reduction in contractile function, even though systolic function appears normal at rest. This occurs due to compensatory mechanisms in play. Coronary vascular dysfunction and neurohormonal activation also seem to be involved in the pathophysiology of HFpEF [84]. Moreover, an increased systemic inflammation renders the myocardium vulnerable and unable to increase blood flow when needed, resulting in patchy areas of ischemia which lead to increased myocardial stiffness and elevated left ventricular filling pressures, and ultimately pulmonary congestion, even with preserved LV systolic function [12].
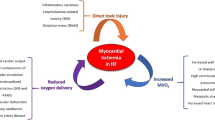
In Fig. 1, we report the key aspects concerning the pathophysiology of ICM [75].
Pathophysiology of ischemic cardiomyopathy. From Del Buono, M. G., et al. [75]. "Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction." Curr Cardiol Rep 24(10): 1505–1515. https://doi.org/10.1007/s11886-022-01766-6
Outcomes
Mortality
Despite improvements in therapy, heart failure remains associated with poor prognosis. Although a reduction in both short-term and long-term mortality after HF diagnosis has been seen, the rates remain high, with up to one-third of patients dying within 1 year from diagnosis [14, 33].
ICM is associated with a 1-year mortality rate of approximately 16% and a 5-year mortality rate of nearly 40% in the Western population, representing therefore a relevant public health burden [6].
Additionally, it has been shown that ICM is linked to higher rates of sudden cardiac death and all-cause mortality when compared to the NICM group [7]. An ischemic etiology was found to be an independent predictor of death in a study assessing mortality in systolic heart failure, with ischemic heart failure (IHF) patients seeing an almost 50% increase in mortality when compared to non-ischemic heart failure (NIHF) [34].
Moreover, a recent study revealed that ICM patients who underwent ICD implantation for primary prevention of SCD had a higher 1-year mortality rate than NICM patients [35].
Furthermore, it was demonstrated that the mortality risk was 43% higher in patients developing HF more than 3 days after MI than in those developing HF in the first 3 days after MI, suggesting that the timing of HF development is relevant in terms of adverse events [22, 36].
Hospitalizations
Heart failure continues to be the primary reason for hospitalization in the USA, accounting for approximately 1–2% of all hospital admissions. It also represents the most prevalent diagnosis among hospitalized patients aged 65 years [37, 73]. Due to an aging population, the number of hospital admissions is expected to rise even more, increasing by 50% over the next 25 years [38].
The SOLVD (Studies of Left Ventricular Dysfunction Treatment) trial reported a twofold higher hospitalization rate for decompensated HF and a fourfold higher mortality rate in patients with a history of MI and LVEF ≤ 35% compared to those without a prior MI [12].
Findings from the STICH trial indicate that surgical intervention can help reduce hospitalization rates. Specifically, it demonstrated that coronary artery bypass grafting (CABG) can effectively decrease both initial and recurrent hospitalizations among patients diagnosed with ICM and LVEF below 35% [39].
Moreover, after a first hospitalization for HF, around 20% of patients in the USA are readmitted within 30 days and around 66% within 1 year, suggesting that readmission rates remain high [5]. Given the high comorbidity burden in these patients, the reasons for readmissions are often other than heart failure [37]. A study enrolling 1082 patients with HFrEF reported that, in patients with ICM, the independent predictors of rehospitalization were frequent premature ventricular beats, diabetes mellitus (DM), previous HF hospitalization, and kidney injury [7].
Quality of life
It has been reported that individuals with HF commonly experience the emergence of depressive symptoms, as well as lower quality of life (QoL) and a decline in long-term functional status. A study including 446 patients with HF (62.1% due to ischemia) and ICD reported that 43.2% had depressive symptoms. Furthermore, it was found that these symptoms were linked to diminished quality of life, irrespective of NYHA functional class [40]. Moreover, research has revealed that patients with congestive heart failure (CHF) have unsatisfactory self-care outcomes, low quality of life scores, and dissatisfaction with their overall health. Notably, IHD was found to be present in 72.5% of patients with CHF and diminished quality of life [41]. Numerous observational studies have demonstrated that CABG is an effective approach for improving both survival and quality of life in patients diagnosed with ICM [42].
Prognostic risk factors
Chronic ischemic cardiomyopathy is associated with a poor prognosis and significant morbidity and mortality, with HF representing one of the most devastating clinical outcomes. Therefore, it remains fundamental to predict the risk factors associated with adverse HF outcomes to closely monitor and manage them. At present, several variables affecting the prognosis of heart failure have emerged and serve, therefore, as predictors of survival in patients with ICM [2, 7].
In Table 1, we summarize the main prognostic risk factors associated with ischemic cardiomyopathy.
Therapeutic options
Therapeutic options for patients with ICM include lifestyle modifications, medical therapy, revascularization, device therapy, mechanical circulatory support, and cardiac transplantation, depending on the anatomy of coronary disease, symptoms severity, and associated cardiac and noncardiac comorbidities [2, 11]. Main objectives are represented by an optimization in cardiovascular function, prevention of progressive remodeling, reduction in heart failure symptoms, and survival improvement [1].
Lifestyle changes
Long-term lifestyle modifications are advised to effectively manage coronary artery disease (CAD) and mitigate the risk of complications. These changes encompass strategies such as avoidance of a sedentary lifestyle, smoking and alcohol cessation, and a healthy diet low in sodium and cholesterol [2].
Medication therapy
Several studies have provided evidence for enhanced long-term survival and reduced cardiovascular mortality with optimal medical therapy. Therefore, it represents the cornerstone for the treatment of patients with ischemic heart failure and is strongly recommended [1, 43]. It comprises a combination of medical treatment for heart failure plus secondary prevention for coronary artery disease [4]. Moreover, mounting evidence shows that targeting the intense inflammatory response occurring after AMI may contribute to preventing adverse cardiac remodeling and the development of HF following AMI. IL-1β and other IL-1 family cytokines are the main inflammatory mediators. Therefore, several immunosuppressive medications, anti-inflammatory agents, and immunomodulatory interventions have been explored in this context [76,77,78,79]. Indeed, the increasing research interest in understanding the inflammatory role in AMI has led to extensive and promising pre-clinical research and clinical observation evidence. However, despite the wealth of pre-clinical studies, these therapeutic interventions have faced significant challenges in translation to clinical applications over the past decades, mainly due to concerns related to impaired healing, elevated risk of cardiac rupture, and a failure to exhibit additional benefits beyond standard therapies. Therefore, as of now, there are no clinically effective anti-inflammatory treatments proven to prevent or treat adverse LV remodeling after AMI [83].
In Table 2, we report the currently available medications for patients with ICM as well as novel therapeutic targets, including inflammation.
Device therapy
Device therapies like ICD and CRT are recommended interventions for patients with severely impaired left ventricular systolic function and symptoms of heart failure refractory to medical therapy [4].
Individuals with reduced left ventricular function due to ischemic causes have a higher risk of life-threatening arrhythmias. The use of an ICD is an important tool to prevent SCD and improve survival in patients at high risk for ventricular tachycardia (VT) or ventricular fibrillation (VF) or those resuscitated from sustained VT/VF, and it is advised for both primary and secondary prevention purposes [2, 44]. ICD placement is recommended for primary prevention of SCD in patients with IHD who have LVEF < 35%. This recommendation applies to individuals with NYHA class II or III of HF, despite receiving guideline-directed management, who have surpassed a minimum of 40 days following a MI and 90 days following a revascularization procedure, and with an expected survival of more than 1 year [45].
CRT with biventricular pacing is associated with reduced morbidity and mortality rates. According to guidelines, it is recommended in heart failure patients in sinus rhythm, with left bundle branch block (LBBB), QRS duration ≥ 150 ms, and LVEF ≤ 35% who have moderate to severe symptoms despite maximally tolerated medical therapy (class IA) [46].
Furthermore, evidence indicates that this approach is linked to reverse remodeling, resulting in a decrease of 50% in SCD risk, even in the absence of an ICD [1].
Revascularization therapy
ICM sometimes includes areas of dysfunctional but viable myocardium. Therefore, revascularization therapy (whether performed via CABG or PCI), by aiming at restoring coronary blood flow in ischemic conditions, could potentially promote functional recovery and improve overall cardiac function [2, 47].
The evidence for surgical revascularization as an effective and beneficial treatment for ICM comes from several observational studies. The Coronary Artery Surgery Study (CASS) registry showed that prognosis is improved following CABG in patients with LV systolic dysfunction and more severe coronary disease [3]. Moreover, randomized controlled trials have provided evidence that patients with reduced LVEF and multivessel disease (MVD) experience improved long-term survival when undergoing CABG compared with optimal medical therapy (OMT) alone [48]. The STICH trial demonstrated a decrease in mortality rates and HF hospitalizations when CABG was combined with guideline-directed medical therapy (GDMT) in patients with LVEF < 35% and ICM. Notably, the trial highlighted greater benefits in those with more advanced ICM (lower EF or presence of 3-vessel disease) [49].
The extended follow-up from STICH (STICH Extension Study-STICHES) also supports the significant survival advantage of combining CABG with medical therapy compared to medical therapy alone [4]. On the other hand, the impact of PCI on the same patient population has not been evaluated. Indeed, the REVIVED-BCIS2 (Study of Efficacy and Safety of Percutaneous Coronary Intervention to Improve Survival in Heart Failure) trial assessed the impact of PCI plus medical therapy in patients with extensive coronary disease, left ventricular systolic dysfunction, and viable myocardium compared to medical therapy alone over a median of 41 months. However, results showed that, in patients undergoing revascularization by PCI, there was no reduction in mortality from any cause or hospitalization for heart failure [70]. Nonetheless, given some limitations of this trial, such as doubts about viable myocardium-based patient selection, limited generalizability to more symptomatic cases, and potential bias toward surgery for patients with extensive coronary artery disease amenable to CABG, as well as the applicability of the angiographic eligibility measure for low LVEF patients potentially leading to incomplete revascularization, an extended follow-up and further investigation are necessary to validate these results [71].
In addition, a direct comparison between PCI and CABG has led to inconclusive results. Therefore, the ideal approach for revascularization remains uncertain [48]. Evidence from observational studies suggests equivalence in survival between PCI and CABG in patients with ischemic HF. Analysis of the subgroup of patients in the AWESOME trial with LVEF < 35% did not show any mortality difference. However, given the limited patient population, definitive conclusions from this study cannot be drawn [4].
According to current guidelines, revascularization is indicated to improve prognosis in patients with LV systolic dysfunction and significant two- or three-vessel disease, in addition to OMT, with no need to perform viability assessment prior to revascularization [1, 3].
In patients with multivessel disease and a satisfactory surgical risk, CABG is the recommended and preferred approach. When complete revascularization can be achieved, PCI can be regarded as a suitable alternative to CABG in subjects with one- or two-vessel disease. In patients with three-vessel disease, the choice between CABG and PCI depends on coronary anatomy, comorbidities, and completeness of revascularization. If comorbidities, high surgical risk, or unfavorable anatomy for CABG, PCI can be offered [4].
Furthermore, in the context of AMI, it is possible to reduce the extent of the infarct by unloading the LV with a mechanical circulatory support device prior to revascularization [75]. An experimental translational study in 14 adult male Yorkshire swine demonstrated that mechanically reducing LV wall stress through an intracorporeal axial flow catheter and delaying reperfusion for 1 h results in decreased infarct size during an AMI [80]. More recently, a pilot trial (DTU-STEMI pilot trial) demonstrated the safety and feasibility of LV unloading through the use of Impella CP for 30 min prior to reperfusion in 50 patients with anterior ST-elevation myocardial infarction (STEMI) [81]. A comparative study in four UK hospitals showed that, in patients with STEMI, the use of pressure-controlled intermittent coronary sinus occlusion (PiCSO) as an adjunct to PCI resulted in a lower infarct size at 5 days compared to patients undergoing PCI alone [82].
Mechanical circulatory support (MCS) and cardiac transplantation
Mechanical circulatory support (MCS) has been demonstrated to be effective in extending lifespan and enhancing the functional capacities of individuals with advanced HFrEF. It represents a useful alternative in critically ill patients with cardiogenic shock or refractory heart failure. It can provide short-term (hours to days) or long-term support (months to years) [9, 50].
Examples of short-term MCS include venoarterial extracorporeal membrane oxygenation (VA-ECMO), percutaneous ventricular assist devices, and intra-aortic balloon pumps (IABP) [51].
As per guidelines, the utilization of temporary MCS is beneficial in stabilizing patients during the evaluation phase for determining the appropriate transition to definitive management, such as durable MCS as a bridge or destination therapy, stabilization until cardiac transplantation, or device removal, if there is observed improvement and recovery [9].
The utilization of durable left ventricular assist devices (LVAD) as destination therapy in patients with stage D heart failure is widely increasing [52]. Current guidelines show that durable MCS can be beneficial for symptom improvement, functional class improvement, and mortality reduction in patients with advanced HFrEF with NYHA class IV symptoms despite GDMT [9].
Cardiac transplantation serves as the primary treatment option for individuals with stage D HF who failed guideline-directed medical therapy, device, and surgical optimization, and evidence has demonstrated its efficacy in improving functional status and quality of life [9]. One of the most common indications is represented by ischemic cardiomyopathy, accounting for 38% of heart transplantations [53]. Furthermore, a study conducted in Japan demonstrated that approximately 70% of patients with refractory HF secondary to advanced ICM presented with an improvement in LVEF after autologous skeletal myoblast transplantation, pointing toward a new potential therapy for advanced ICM patients. However additional research is required to validate these findings [54].
Conclusions
This comprehensive review provides insights into the multifaceted nature of ICM. Despite advancements in the management of CAD and AMI, the incidence and prevalence of ICM continue to rise. The current understanding of ICM is still fragmented, and there is a need for a standardized definition. However, the most frequently applied definition involves left ventricular dysfunction in the presence of significant CAD. The pathophysiology of ICM involves plaque buildup, thrombus formation, and subsequent myocardial ischemia, leading to reversible contractile dysfunction (myocardial stunning) or chronic hypoperfusion (hibernating myocardium). Prolonged hypoperfusion can cause irreversible damage to cardiac myocytes, leading to heart remodeling. The prevalence of ICM is influenced by age and sex, with a higher prevalence among older individuals and males. The incidence and prevalence of ICM have been increasing owing to improved survival rates in CAD patients, an aging population, and the presence of risk factors. HF is a common complication in patients with AMI, with varying incidence rates reported in different studies. In patients with chronic coronary syndrome, there is limited information on the development of HF; however, unrecognized CAD is associated with HF. The outcomes of ICM are poor, with elevated mortality rates and a significant impact on quality of life. The growing burden of ICM points, therefore, toward an urgent need for effective management strategies. Customized patient care and a better understanding of the underlying causes of HF are crucial for improving outcomes. The exploration of lifestyle modifications, medical therapy, device interventions, revascularization therapy, MCS, and cardiac transplantation highlights the range of therapeutic options available to improve outcomes in patients with ICM. Future research should focus on refining treatment strategies, exploring emerging therapies, and advancing our understanding of this complex condition to further optimize patient care and outcomes.
Availability of data and materials
Not applicable.
References
Cabac-Pogorevici I et al (2020) Ischaemic cardiomyopathy. Pathophysiological insights, diagnostic management and the roles of revascularisation and device treatment. Gaps and dilemmas in the era of advanced technology. Eur J Heart Fail 22(5):789–799. https://doi.org/10.1002/ejhf.1747
Albakri A (2018) Ischemic cardiomyopathy: a review of literature on clinical status and meta-analysis of diagnostic and clinical management. Biol Eng Med. https://doi.org/10.15761/BEM.1000151
Briceno N et al (2016) Ischaemic cardiomyopathy: pathophysiology, assessment and the role of revascularisation. Heart 102(5):397–406. https://doi.org/10.1136/heartjnl-2015-308037
Konstantinos Aznaouridis MD (2022) PCMM, PhD2 Charalambos Vlachopoulos MD, PhD1 The patient with ischemic heart failure, in heart failure: an essential clinical guide. CRC Press, Boca Raton. https://doi.org/10.1201/9780429244544
Lampros Papadimitriou PG, Kalogeropoulos Andreas (2022) Epidemiology of heart failure, in Heart failure: an essential clinical guide. CRC Press, Boca Raton. https://doi.org/10.1201/9780429244544
Wongpraparut N et al (2020) Impact of guideline-recommended versus non-guideline-recommended beta-blocker and Doppler echocardiographic parameters on 1-year mortality in Thai ischemic cardiomyopathy patients: a prospective multicenter registry. BMC Cardiovasc Disord 20(1):8. https://doi.org/10.1186/s12872-019-01311-4
Zhang ZH et al (2020) Clinical characteristics and long-term prognosis of ischemic and non-ischemic cardiomyopathy. Indian Heart J 72(2):93–100. https://doi.org/10.1016/j.ihj.2020.04.004
McDonagh TA et al (2022) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed) 75(6):523. https://doi.org/10.1016/j.rec.2022.05.005
Heidenreich PA et al (2022) AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol 79(17):e263–e421. https://doi.org/10.1016/j.jacc.2021.12.012
Xi R et al (2018) Increased serum interleukin-34 levels are related to the presence and severity of cardiac dysfunction in patients with ischemic cardiomyopathy. Front Physiol 9:904. https://doi.org/10.3389/fphys.2018.00904
Bakaeen FG et al (2021) The American Association for Thoracic Surgery expert consensus document: coronary artery bypass grafting in patients with ischemic cardiomyopathy and heart failure. J Thorac Cardiovasc Surg 162(3):829–850 e1. https://doi.org/10.1016/j.jtcvs.2021.04.052
Elgendy IY, Mahtta D, Pepine CJ (2019) Medical therapy for heart failure caused by ischemic heart disease. Circ Res 124(11):1520–1535. https://doi.org/10.1161/CIRCRESAHA.118.313568
Virani SS et al (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circ 141(9):e139–e596. https://doi.org/10.1161/CIR.0000000000000757
Chan DZL, Kerr AJ, Doughty RN (2021) Temporal trends in the burden of heart failure. Intern Med J 51(8):1212–1218. https://doi.org/10.1111/imj.15253
Vicent L et al (2021) Ischemic etiology and prognosis in men and women with acute heart failure. J Clin Med 10(8). https://doi.org/10.3390/jcm10081713
Balmforth C et al (2019) Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail 7(6):457–465. https://doi.org/10.1016/j.jchf.2019.02.015
Divoky L, Maran A, Ramu B (2018) Gender differences in ischemic cardiomyopathy. Curr Atheroscler Rep 20(10):50. https://doi.org/10.1007/s11883-018-0750-x
Sulo G et al (2016) Heart failure complicating acute myocardial infarction; burden and timing of occurrence: a nation-wide analysis including 86 771 patients from the Cardiovascular Disease in Norway (CVDNOR) project. J Am Heart Assoc 5(1). https://doi.org/10.1161/JAHA.115.002667
Bahit MC, Kochar A, Granger CB (2018) Post-myocardial infarction heart failure. JACC Heart Fail 6(3):179–186. https://doi.org/10.1016/j.jchf.2017.09.015
Qureshi WT et al (2018) Silent myocardial infarction and long-term risk of heart failure: the ARIC study. J Am Coll Cardiol 71(1):1–8. https://doi.org/10.1016/j.jacc.2017.10.071
Auer J, Auer L (2021) Stent selection in patients with NSTEMI and risk of incident heart failure. Atherosclerosis 316:66–68. https://doi.org/10.1016/j.atherosclerosis.2020.10.889
Jenca D et al (2021) Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail 8(1):222–237. https://doi.org/10.1002/ehf2.13144
Lamblin N et al (2018) First hospitalization for heart failure in outpatients with stable coronary artery disease: determinants, role of incident myocardial infarction, and prognosis. J Card Fail 24(12):815–822. https://doi.org/10.1016/j.cardfail.2018.09.013
Valensi P, Meune C (2019) Congestive heart failure caused by silent ischemia and silent myocardial infarction : diagnostic challenge in type 2 diabetes. Herz 44(3):210–217. https://doi.org/10.1007/s00059-019-4798-3
Vaidya Y, Cavanaugh SM, Dhamoon AS (2022) Myocardial stunning and hibernation. StatPearls Treasure Island (FL)
Kalogeropoulos A, Skopicki H, Butler J (2023) Heart failure : an essential clinical guide. CRC Press, Boca Raton, FL. p. 1 online resource.
Bolli R (1998) Basic and clinical aspects of myocardial stunning. Prog Cardiovasc Dis 40(6):477–516. https://doi.org/10.1016/s0033-0620(98)80001-7
Zaret, BL, Beller G (2010) Clinical nuclear cardiology : state of the art and future directions. 4th ed. Philadelphia: Mosby/Elsevier. xviii, 878 pages.
Jennings RB, Reimer KA (1991) The cell biology of acute myocardial ischemia. Annu Rev Med 42:225–46. https://doi.org/10.1146/annurev.me.42.020191.001301
Vassalle M, Lin CI (2004) Calcium overload and cardiac function. J Biomed Sci 11(5):542–65. https://doi.org/10.1007/BF02256119
Frangogiannis NG (2021) Cardiac fibrosis. Cardiovasc Res 117(6):1450–1488. https://doi.org/10.1093/cvr/cvaa324
Talman V, Ruskoaho H (2016) Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 365(3):563–81. https://doi.org/10.1007/s00441-016-2431-9
Conrad N et al (2019) Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol 4(11):1102–1111. https://doi.org/10.1001/jamacardio.2019.3593
Gajanana D et al (2016) Mortality in systolic heart failure revisited: ischemic versus non-ischemic cardiomyopathy. Int J Cardiol 224:15–17. https://doi.org/10.1016/j.ijcard.2016.08.316
Higgins AY et al (2020) Comparison of mortality and readmission in non-ischemic versus ischemic cardiomyopathy after implantable cardioverter-defibrillator implantation. Am J Cardiol 133:116–125. https://doi.org/10.1016/j.amjcard.2020.07.035
Gerber Y et al (2016) Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail 9(1):e002460. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002460
Groenewegen A et al (2020) Epidemiology of heart failure. Eur J Heart Fail 22(8):1342–1356. https://doi.org/10.1002/ejhf.1858
National Guideline C (2018) National Institute for Health and Care Excellence: guidelines. Chronic heart failure in adults: diagnosis and management. London: National Institute for Health and Care Excellence (NICE)
Howlett JG et al (2019) CABG improves outcomes in patients with ischemic cardiomyopathy: 10-year follow-up of the STICH trial. JACC Heart Fail 7(10):878–887. https://doi.org/10.1016/j.jchf.2019.04.018
Zormpas C et al (2022) Depressive symptoms and quality of life in patients with heart failure and an implantable cardioverter-defibrillator. Front Psychiatry 13:827967. https://doi.org/10.3389/fpsyt.2022.827967
Wisnicka A, Lomper K, Uchmanowicz I (2022) Self-care and quality of life among men with chronic heart failure. Front Public Health 10:942305. https://doi.org/10.3389/fpubh.2022.942305
Jose R et al (2019) Early and mid-term outcomes of patients undergoing coronary artery bypass grafting in ischemic cardiomyopathy. J Am Heart Assoc 8(10)
Farsky PS et al (2021) Optimal medical therapy with or without surgical revascularization and long-term outcomes in ischemic cardiomyopathy. J Thorac Cardiovasc Surg. https://doi.org/10.1016/j.jtcvs.2020.12.094
Park KH et al (2017) Effectiveness of implantable cardioverter-defibrillator therapy for heart failure patients according to ischemic or non-ischemic etiology in Korea. Korean Circ J 47(1):72–81. https://doi.org/10.4070/kcj.2016.0242
Sazonova SI et al (2022) Prediction of appropriate ICD therapy in patients with ischemic heart failure. J Nucl Cardiol 29(2):680–691. https://doi.org/10.1007/s12350-020-02321-y
Ponikowski P et al (2016) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18(8):891–975. https://doi.org/10.1002/ejhf.592
Panza JA, Chrzanowski L, Bonow RO (2021) Myocardial viability assessment before surgical revascularization in ischemic cardiomyopathy: JACC review topic of the week. J Am Coll Cardiol 78(10):1068–1077. https://doi.org/10.1016/j.jacc.2021.07.004
Volz S et al (2021) Long-term mortality in patients with ischaemic heart failure revascularized with coronary artery bypass grafting or percutaneous coronary intervention: insights from the Swedish Coronary Angiography and Angioplasty registry (SCAAR). Eur Heart J 42(27):2657–2664. https://doi.org/10.1093/eurheartj/ehab273
Murphy SP, Ibrahim NE, Januzzi JL Jr (2020) Heart failure with reduced ejection fraction: a review. JAMA 324(5):488–504. https://doi.org/10.1001/jama.2020.10262
Garzon-Rodriguez JD et al (2018) Mechanical circulatory support as bridge therapy for heart transplant: case series report. BMC Res Notes 11(1):430. https://doi.org/10.1186/s13104-018-3515-2
Zhou AL et al (2021) Bridge to transplantation from mechanical circulatory support: a narrative review. J Thorac Dis 13(12):6911–6923. https://doi.org/10.21037/jtd-21-832
Brown MA et al (2021) Intra-aortic balloon pump as a bridge to durable left ventricular assist device. J Am Heart Assoc 10(15):e019376. https://doi.org/10.1161/JAHA.120.019376
Alraies MC, Eckman P (2014) Adult heart transplant: indications and outcomes. J Thorac Dis 6(8):1120–8. https://doi.org/10.3978/j.issn.2072-1439.2014.06.44
Kainuma S et al (2021) Long-term outcomes of autologous skeletal myoblast cell-sheet transplantation for end-stage ischemic cardiomyopathy. Mol Ther 29(4):1425–1438. https://doi.org/10.1016/j.ymthe.2021.01.004
Martinez-Selles M et al (2018) Influence of sex and pregnancy on survival in patients admitted with heart failure: data from a prospective multicenter registry. Clin Cardiol 41(7):924–930. https://doi.org/10.1002/clc.22979
Postigo A, Martínez-Sellés M (2020) Sex influence on heart failure prognosis. Front Cardiovasc Med 7:616273. https://doi.org/10.3389/fcvm.2020.616273
Nayak A, Hicks AJ, Morris AA (2020) Understanding the complexity of heart failure risk and treatment in black patients. Circ Heart Fail 13(8):e7264. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007264
Oh GC, Cho HJ (2020) Blood pressure and heart failure. Clin Hypertens 26:1–213. https://doi.org/10.1186/s40885-019-0132-x
Corbalan R et al (2019) Analysis of outcomes in ischemic vs nonischemic cardiomyopathy in patients with atrial fibrillation: a report from the GARFIELD-AF registry. JAMA Cardiol 4(6):526–548. https://doi.org/10.1001/jamacardio.2018.4729
Chery G et al (2020) Prognostic value of myocardial fibrosis on cardiac magnetic resonance imaging in patients with ischemic cardiomyopathy: a systematic review. Am Heart J 229:52–60. https://doi.org/10.1016/j.ahj.2020.08.004
Fantini F et al (2021) Restrictive filling pattern in ischemic cardiomyopathy: insights after surgical ventricular restoration. J Thorac Cardiovasc Surg 161(2):651–660. https://doi.org/10.1016/j.jtcvs.2019.09.173
Powell AC et al (2017) An exploration of the association between ischemic etiology and the likelihood of heart failure hospitalization following cardiac resynchronization therapy. Clin Cardiol 40(11):1090–1094. https://doi.org/10.1002/clc.22779
Tyminska A et al (2022) Ischemic cardiomyopathy versus non-ischemic dilated cardiomyopathy in patients with reduced ejection fraction- clinical characteristics and prognosis depending on heart failure etiology (data from European Society of Cardiology Heart Failure Registries). Biology (Basel) 11(2). https://doi.org/10.3390/biology11020341
van Dongen IM et al (2018) Evaluation of the impact of a chronic total coronary occlusion on ventricular arrhythmias and long-term mortality in patients with ischemic cardiomyopathy and an implantable cardioverter-defibrillator (the eCTOpy-in-ICD study). J Am Heart Assoc 7(10). https://doi.org/10.1161/JAHA.118.008609
Di Biase L et al (2021) Catheter ablation of ventricular tachycardia in ischemic cardiomyopathy: impact of concomitant amiodarone therapy on short- and long-term clinical outcomes. Heart Rhythm 18(6):885–893. https://doi.org/10.1016/j.hrthm.2021.02.010
Klenke S et al (2018) Circulating miR-192 is a prognostic marker in patients with ischemic cardiomyopathy. Future Cardiol 14(4):283–289. https://doi.org/10.2217/fca-2017-0108
Yancy CW et al (2017) ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Card Fail 23(8):628–651. https://doi.org/10.1016/j.cardfail.2017.04.014
Jering KS et al (2021) Prospective ARNI vs. ACE inhibitor trial to determine superiority in reducing heart failure events after myocardial infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail 23(6):1040–1048. https://doi.org/10.1002/ejhf.2191
Zannad F et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet 396(10254):819–829. https://doi.org/10.1016/S0140-6736(20)31824-9
Perera D et al (2022) Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med 387(15):1351–1360. https://doi.org/10.1056/NEJMoa2206606
Vergallo R, Liuzzo G (2022) The REVIVED-BCIS2 trial: percutaneous coronary intervention vs. optimal medical therapy for stable patients with severe ischaemic cardiomyopathy. Eur Heart J 43(46):4775–4776. https://doi.org/10.1093/eurheartj/ehac568
Zehnder AR, Pedrosa Carrasco AJ, Etkind SN (2022) Factors associated with hospitalisations of patients with chronic heart failure approaching the end of life: a systematic review. Palliat Med 36(10):1452–1468. https://doi.org/10.1177/02692163221123422
Lahoz R et al (2020) Recurrent heart failure hospitalizations are associated with increased cardiovascular mortality in patients with heart failure in Clinical Practice Research Datalink. ESC Heart Fail 7(4):1688–1699. https://doi.org/10.1002/ehf2.12727
Oikawa T et al (2018) Prognostic impact of statin intensity in heart failure patients with ischemic heart disease: a report from the CHART-2 (Chronic Heart Failure registry and Analysis in the Tohoku District 2) study. J Am Heart Assoc 7(6):231–123. https://doi.org/10.1161/JAHA.117.007524
Del Buono MG et al (2022) Ischemic cardiomyopathy and heart failure after acute myocardial infarction. Curr Cardiol Rep 24(10):1505–1515. https://doi.org/10.1007/s11886-022-01766-6
Viola M, de Jager SC, Sluijter JP (2021) Targeting inflammation after myocardial infarction: a therapeutic opportunity for extracellular vesicles? Int J Mol Sci 22(15). https://doi.org/10.3390/ijms22157831
Abbate A et al (2022) Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Heart J Cardiovasc Pharmacother 8(5):503–510. https://doi.org/10.1093/ehjcvp/pvab075
Everett BM et al (2019) Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circ 139(10):1289–1299. https://doi.org/10.1161/CIRCULATIONAHA.118.038010
Broch K et al (2021) Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 77(15):1845–1855. https://doi.org/10.1016/j.jacc.2021.02.049
Kapur NK et al (2015) Mechanical pre-conditioning with acute circulatory support before reperfusion limits infarct size in acute myocardial infarction. JACC Heart Fail 3(11):873–82. https://doi.org/10.1016/j.jchf.2015.06.010
Kapur NK et al (2019) Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction. Circ 139(3):337–346. https://doi.org/10.1161/CIRCULATIONAHA.118.038269
Egred M et al (2020) Effect of pressure-controlled intermittent coronary sinus occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. Int J Cardiol Heart Vasc 28:100526. https://doi.org/10.1016/j.ijcha.2020.100526
Seropian IM et al (2014) Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 63(16):1593–603. https://doi.org/10.1016/j.jacc.2014.01.014
Del Buono MG et al (2023) Heart failure after ST-elevation myocardial infarction: beyond left ventricular adverse remodeling. Curr Probl Cardiol 48(8):101215. https://doi.org/10.1016/j.cpcardiol.2022.101215
Author information
Authors and Affiliations
Contributions
Dr. Pastena has led literature review and drafting, Dr. Frye has contributed to editing and drafting, Mr. Ho has contributed to review and drafting (pathophysiology), Dr. Goldschmidt has contributed to editing and drafting, and Dr. Kalogeropoulos contributed to conceptualization, structure, drafting, and oversight.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pastena, P., Frye, J.T., Ho, C. et al. Ischemic cardiomyopathy: epidemiology, pathophysiology, outcomes, and therapeutic options. Heart Fail Rev 29, 287–299 (2024). https://doi.org/10.1007/s10741-023-10377-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10377-4