Abstract
Patients recovered from COVID-19 have an increased incidence of cardiovascular disease and heart structural changes. The aim of the present manuscript is to assess the risk of incident heart failure (HF) after COVID-19 infection. Data were obtained searching MEDLINE and Scopus for all studies published at any time up to September 1, 2022 reporting the risk of incident HF in COVID-19 recovered patients. The cumulative post-COVID-19 incidence and risk of incident HF were pooled using a random effects model and presented with the corresponding 95% confidence interval (CI). Statistical heterogeneity was measured using the Higgins I2 statistic. Overall, 21,463,173 patients (mean age 54.5 years, 58.7% males) were analyzed. Among them, 1,628,424 had confirmed COVID-19 infection while the remaining 19,834,749 represented the controls. The mean length of follow-up was 9.2 months. A random effect model revealed a pooled incidence of post COVID-19 HF in 1.1% of cases (95% CI: 0.7–1.6, I2: 99.8%). Moreover, recovered COVID-19 patients showed an increased risk of incident HF (HR: 1.90, 95% CI: 1.54–3.24, p < 0.0001, I2 = 96.5%) in the same follow-up period. Meta-regression showed a direct relationship for the risk of incident HF using age (p = 0.001) and hypertension (HT) (p = 0.02) as moderators, while an inverse association was observed when the follow-up length was adopted as moderating variable (p = 0.01). COVID-19 survivors had an additional 90% risk of developing HF after COVID-19 infection in the long-term period. This risk was directly related with age and previous history of HT especially in the early post-acute phase of the infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent analyses have demonstrated an increased incidence of cardiovascular disease and heart structural changes in recovered COVID-19 patients [1]. Although myocardial injury has been identified as a significant pathogenic feature of COVID-19 infection [2,3,4,5], associated with worse short-term prognosis, the long-term cardiovascular outcomes in COVID-19 survivors remain largely unclear. To this regard, few studies have investigated the risk of heart failure (HF) during the post-acute phase of COVID-19. Furthermore, a comprehensive assessment of this potential post-acute COVID-19 sequelae is still lacking. The aim of the present manuscript is to assess the risk of incident HF in COVID-19 recovered patients by performing a systematic review and meta-analysis of the available data.
Material and methods
Study design and eligibility criteria
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplementary file 1) [6]. Data were obtained searching MEDLINE and Scopus for all studies, either retrospective or prospective, published at any time up to September 1, 2022, and reporting the mid/long-term risk (defined as > 4 months) of incident HF in COVID-19 recovered patients. In the reviewed investigations, recovered COVID-19 subjects were compared to “contemporary cohorts” defined as subjects who did not experience the SARS-CoV-2 infection and developed incident HF in the same follow-up period.
Outcomes
The pooled incidence of incident HF in recovery COVID-19 patients was chosen as the primary outcome. Conversely, the secondary outcome was the risk of incident HF in the same period compared to contemporary cohorts, which were used by reviewed studies as control groups.
Data extraction and quality assessment
The selection of studies to be included in our analysis was independently conducted by two authors (M.Z., C.B.) in a blinded fashion. Any discrepancies in study selection were resolved by consulting a third author (G.R.). The following MeSH terms were used for the search: “Heart failure” AND “COVID-19 sequelae” OR “Heart failure” AND “COVID-19”. The full search strategy is presented in the Supplementary file 2. Moreover, we searched the bibliographies of the target studies for additional references. Specifically, inclusion criteria were (i) studies enrolling subjects with previous confirmed COVID-19 infection, and (ii) providing the hazard ratio (HR) and relative 95% confidence interval (CI) for the risk of incident HF after the infection compared to contemporary control cohorts. Conversely, case reports, review articles, abstracts, editorials/letters, and case series with less than 10 participants were excluded. Data extraction was independently conducted by two authors (M.Z., G.R.). Studies were excluded from the meta-analysis if they did not provide data regarding the risk of incident HF, reported as HR and relative 95% CI, after COVID-19 infection. For all studies reviewed, we extracted, when provided, the number of patients enrolled, the mean age, male gender, prevalence of cardiovascular comorbidities such as arterial hypertension (HT), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), obesity, pre-existing HF, cerebrovascular disease, and length of follow-up. The quality of included studies was graded using the Newcastle–Ottawa (NOS) quality assessment scale [7]. Any score ≥ 7 qualified as high quality with a low risk of bias, while a score < 5 is categorized as low quality with a high risk of inherent bias. Any score in between is rated as moderate quality [7].
Data synthesis and analysis
Continues variables were expressed as mean while categorical variables were presented as numbers and relative percentages. The cumulative post-COVID-19 incidence of HF (n/N), defined as the ratio between post COVID-19 patients experiencing HF (n) and the number of all recovered COVID-19 patients (N) over the follow-up period, was pooled using a random effects model and presented with the corresponding 95% confidence interval (CI). Similarly, for the secondary outcome, the HRs with the related 95% confidence interval (CI) were pooled using a random-effect model. Furthermore, a predefined sensitivity analysis (leave-one-out analysis) was performed removing one study at the time, to evaluate the stability of our results regarding both the HF incidence and risk. Statistical heterogeneity was measured using the Higgins I2 statistic and Q value. Specifically, a I2 = 0 indicated no heterogeneity while we considered low, moderate, and high degrees of heterogeneity based on the values of I2 as < 25%, 25–75%, and above 75% respectively. The presence of potential publication bias was verified by visual inspection of the funnel plot. Due to the low number of the included studies (< 10), small-study bias was not examined as our analysis was underpowered to detect such bias. To further appraise the impact of potential baseline confounders, a meta-regression analysis was also performed to explain the observed heterogeneity related to the risk of incident HF, if any. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, USA).
Results
Search results and included studies
A total of 10,908 articles were obtained using our search strategy. After excluding duplicates and preliminary screening, 4431 full-text articles were assessed for eligibility. Among them, 4326 studies were excluded for not meeting the inclusion criteria, leaving 5 retrospective investigations fulfilling the inclusion criteria (Fig. 1) [7,8,9,10,11,12].
Characteristics of the population and quality assessment
Overall, 21,463,173 patients (mean age 54.5 years, 58.7% males) were included in this analysis [8,9,10,11,12]. Among them, 1,628,424 had confirmed COVID-19 infection while the remaining 19,834,749 represented the controls. The general characteristics of the studies included are presented in Table 2. Although the demographic characteristics and concomitant comorbidities were not systematically recorded in all investigations, the cohorts mainly consisted of middle-aged patients. No data regarding the severity of the infection or vaccination status were provided. The mean length of follow-up was 9.2 months ranging between 4 and 12 months. The reviewed investigations identified the occurrence of AMI by screening the medical records of enrolled patients using the International Classification of Diseases 10th Revision (ICD-10) codes I50 [8,9,10,11,12]. Quality assessment showed that all studies were of moderate-high quality according to the NOS scale (Table 1).
Pooled incidence of incident heart failure
The cumulative post-acute COVID-19 rate of incident HF in COVID-19 survivors ranged between 0.4 and 2% among the reviewed studies [8,9,10,11,12]. A random effect model revealed a pooled incidence of post COVID-19 HF in 1.1% of cases (95% CI: 0.7–1.6, I2: 99.8%) (Fig. 2). The relative sensitivity analysis slightly changed the combined incidence rate, which remained statistically significant across a range from 0.9% of cases (95% CI: 0.5–1.4, I2: 99.8%) to 1.3% of cases (95% CI: 0.9–1.8, I2: 99.7%), suggesting that no single investigation had an undue impact on the study outcome. The visual inspection of the funnel plot is showed in supplementary file 3, panel A.
Mid-long-term risk of incident HF
After a mean follow-up of 9.2 months, recovered COVID-19 patients presented an increased risk of incident HF (HR: 1.90, 95% CI: 1.54–3.24, p < 0.0001, I2 = 96.5%) compared to those patients that not experienced COVID-19 infection in the same period (Fig. 3). The funnel plot disclosed the presence of potential publication bias (Supplementary file 3, panel B). The sensitivity analysis confirmed yielded results reporting an HR ranging between 2.04 (95% CI: 1.67–2.49, p < 0.0001, I2: 96.7) and 1.80 (95% CI: 1.43–2.25, p < 0.0001, I2: 98.7%), indicating that the obtained results were not driven by any single study.
Meta-regression for the risk of incident heart failure
A meta-regression analysis showed a significant direct relationship for the risk of incident HF using age (p = 0.001) and HT (p = 0.02) as moderators, while an indirect association was observed when the follow-up length was adopted as moderating variable (p = 0.01) (Table 2).
Discussion
Our results, based on a large population of more than 20 million people, demonstrated that COVID-19 recovery subjects had an additional 90% risk of developing HF within 9 months from the acute infection. This risk was directly influenced by age and previous history of HT and resulted higher in the early post-acute phase of the infection, as demonstrated by the meta-regression. Furthermore, the absence of any correlation with other cardiovascular risk factors or comorbidities suggested that the risk of incident HF might manifest even in subjects at relatively low cardiovascular risk. The high heterogeneity observed may be partly explained by the differences in baseline characteristics of the population enrolled, immunization against COVID-19 infection, pre-existing cardiovascular risk factors, or previous HF history. Although sensitivity analysis confirmed the robustness of our results, our estimates should be carefully considered.
Unfortunately, the revised studies did not systematically report data regarding a previous history of HF, as well as no data related to the left ventricular ejection fraction, limiting the possibility to further characterize the reported risk [9,10,11,12]. However, to the best of our knowledge, our analysis represents the first attempt to comprehensively assess the risk of incident HF in the post-acute phase of COVID-19 subjects. Notably, we observed that the HF risk was comparable between men and women, although this result had to be cautiously considered. Indeed, the comparison of rates between gender is highly dependent on the age range of the population under study. Furthermore, when the death rate is high from causes other than the disease of interest, the incidence rates of the disease are generally overestimated in traditional Kaplan–Meier survival analysis due to competing risks. Nevertheless, sensitivity analysis confirmed the robustness of our results [13].
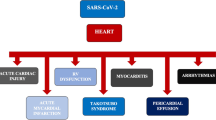
The exact pathophysiological mechanism underling the higher risk of HF in COVID-19 survivors has not yet been completely established. The direct viral invasion of cardiomyocytes and subsequent cell death or endothelial cell infection and subsequent dysfunction or the complement-mediated coagulopathy and microangiopathy may represent some of potential triggers [14,15,16,17]. Indeed, we cannot evaluate if COVID-19 infection represented just a trigger or the underlying cause of HF during the follow-up period.
Our findings are in accordance with the results provided by Zhang et al. [18] which observed an increased risk of HF in COVID-19 survivors within 89 days, but their results were presented as risk ratio and therefore they were not included in our meta-analysis. Indeed, it would be incorrect to pool risk ratio and hazard ratio together since they evaluate different outcomes from different study designs.
Doubtless, our preliminary findings have several implications for daily clinical practice and future research. The HF risk is not limited just to the acute phase of the infection but extends in the medium/long-term period, indicating the need to prevent the infection and to consider post COVID-19 patients at future risk of HF. As consequence, the epidemiology of the disease may change in term of rates of relative hospitalizations and associated outcomes as well as in the related costs for the public health care systems. While clinicians are wondering whether the pandemic will be followed by a cardiovascular after shock [19], our findings, for the first time, provide preliminary comprehensive evidence regarding the higher risk of HF in COVID-19 survivors which emphasizes the need of specific clinical follow-up for these subjects to prevent long-term cardiovascular consequences.
Limitations
Our study has several limitations related to the observational nature of the studies reviewed and their own limitations with all inherited bias. Potential underestimation could derive from detection bias considering that some of the articles reviewed were based on data derived from medical records with relative ICD-19 codes; therefore, we cannot exclude that miscoding may have biased our results. Moreover, sampling bias by the competing risk of death may also have led to underestimation of the real cumulative incidence of HF. Probably, reviewed data may have underestimated the real impact of HF after COVID-19 infection especially during the early phase of the pandemic, for the presence of undiagnosed cases and for patients lost during the follow-up period. Furthermore, we cannot exclude that some bias regarding the diagnosis of HF may derived from the misinterpretation of respiratory symptoms, especially during the recovery phase. However, previous investigations have reported a relatively higher sensitivity, specificity, and positive predictive value for the HF diagnosis using ICD-10 codes [20, 21]. Doubtless, the rates of underling comorbidities, sex ratios, and age distribution in the observed analyses may be different from those present in other regions of the world, limiting the generalizability of our findings. Notably, no data regarding the different treatment strategies administered to enrolled COVID-19 recovered patients as well as the potential influence of vaccinations were provided, limiting the possibility of further sub-analyses. Finally, no data were available regarding the LVEF, limiting the characterization of HF patients [22].
Conclusion
After reviewing a large population of more than 20 million people, we observed that COVID-19 survivors had a higher risk of developing HF after in the long-term period, especially in the early post-acute phase. Our findings suggest that HF represents an important post-acute COVID-19 sequelae that might benefit from an adequate primary prevention against SARS-CoV-2 infection and an appropriate follow-up in COVID-19 patients.
Data availability
Not applicable.
References
Tobler DL, Pruzansky AJ, Naderi S, Ambrosy AP, Slade JJ (2022) Long-term cardiovascular effects of COVID-19: emerging data relevant to the cardiovascular clinician. Curr Atheroscler Rep 24:563–570
Izquierdo-Marquisá A, Cubero-Gallego H, Aparisi Á, Vaquerizo B, Ribas-Barquet N (2022) Myocardial injury in COVID-19 and its implications in short- and long-term outcomes. Front Cardiovasc Med 9:901245
Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A (2020) Myocardial injury and COVID-19: Possible mechanisms. Life Sci 253:117723
Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, Thiemann DR, Trost JC, Hasan RK (2021) Myocardial injury in severe COVID-19 compared with non-COVID-19 acute respiratory distress syndrome. Circulation 143:553–565
Zuin M, Rigatelli G, Zuliani G, Bilato C, Zonzin P, Roncon L (2020) Incidence and mortality risk in coronavirus disease 2019 patients complicated by acute cardiac injury: systematic review and meta-analysis. J Cardiovasc Med (Hagerstown) 21:759–764
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 29 Aug 2022
Cohen K, Ren S, Heath K, Dasmariñas MC, Jubilo KG, Guo Y, Lipsitch M, Daugherty SE (2022) Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 376:e068414
Wang W, Wang CY, Wang SI, Wei JC (2022) Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine 53:101619
Xie Y, Xu E, Bowe B, Al-Aly Z (2022) Long-term cardiovascular outcomes of COVID-19. Nat Med 28:583–590
Salah HM, Fudim M, O’Neil ST, Manna A, Chute CG, Caughey MC (2022) Post-recovery COVID-19 and incident heart failure in the National COVID Cohort Collaborative (N3C) study. Nat Commun 13:4117
Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, Lipsitch M, Cohen K (2021) Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 373:n1098
Lacny S, Wilson T, Clement F, Roberts DJ, Faris P, Ghali WA, Marshall DA (2018) Kaplan-Meier survival analysis overestimates cumulative incidence of health-related events in competing risk settings: a meta-analysis. J Clin Epidemiol 93:25–35
Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC (2020) COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 17:543–558
Carfì A, Bernabei R, Landi F (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324:603–605
Bader F, Manla Y, Atallah B, Starling RC (2021) Heart failure and COVID-19. Heart Fail Rev 26:1–10
Cowie MR, Mourilhe-Rocha R, Chang HY, Volterrani M, Ban HN, Campos de Albuquerque D, Chung E, Fonseca C, Lopatin Y, Magaña Serrano JA, Mircheva L, Moncada-Paz GA, Pagava Z, Reyes EB, Saldarriaga C, Schwartzmann P, Sim Kheng Leng D, Trivi M, Yotov YT, Zieroth S, Heart Failure Care OPTIMIZE, program coordinators (2022) The impact of the COVID-19 pandemic on heart failure management: Global experience of the OPTIMIZE Heart Failure Care network. Int J Cardiol 363:240–246
Zhang HG, Dagliati A, Shakeri Hossein Abad Z, Xiong X, Bonzel CL, Xia Z, Tan BWQ, Avillach P, Brat GA, Hong C, Morris M, Visweswaran S, Patel LP, Gutiérrez-Sacristán A, Hanauer DA, Holmes JH, Samayamuthu MJ, Bourgeois FT, L’Yi S, Maidlow SE, Moal B, Murphy SN, Strasser ZH, Neuraz A, Ngiam KY, Loh NHW, Omenn GS, Prunotto A, Dalvin LA, Klann JG, Schubert P, Vidorreta FJS, Benoit V, Verdy G, Kavuluru R, Estiri H, Luo Y, Malovini A, Tibollo V, Bellazzi R, Cho K, Ho YL, Tan ALM, Tan BWL, Gehlenborg N, Lozano-Zahonero S, Jouhet V, Chiovato L, Aronow BJ, Toh EMS, Wong WGS, Pizzimenti S, Wagholikar KB, Bucalo M; Consortium for clinical characterization of COVID-19 by EHR (4CE), Cai T, South AM, Kohane IS, Weber GM (2022) International electronic health record-derived post-acute sequelae profiles of COVID-19 patients. NPJ Digit Med 5:81
Sidik SM (2022) Heart disease after COVID: what the data say. Nature 608:26–28
Cozzolino F, Montedori A, Abraha I, Eusebi P, Grisci C, Heymann AJ, Lombardo G, Mengoni A, Orso M, Ambrosio G (2019) A diagnostic accuracy study validating cardiovascular ICD-9-CM codes in healthcare administrative databases. The Umbria Data-Value Project PLoS One 14:e0218919
Delekta J, Hansen SM, AlZuhairi KS, Bork CS, Joensen AM (2018) The validity of the diagnosis of heart failure (I50.0-I50.9) in the Danish National Patient Register. Dan Med J 65:A5470
Greene SJ, Lautsch D, Yang L, Tan XI, Brady JE (2022) Prognostic interplay between COVID-19 and heart failure with reduced ejection fraction. J Card Fail 28:1287–1297
Author information
Authors and Affiliations
Contributions
Zuin Marco: conception; original draft; statistical analysis; data collection. Rigatelli Gianluca: original draft; statistical analysis; data collection; Loris Roncon: data collection, Revision and editing; Gianpaolo Pasquetto: data collection; Claudio Bilato: revision, editing and supervision. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuin, M., Rigatelli, G., Roncon, L. et al. Risk of incident heart failure after COVID-19 recovery: a systematic review and meta-analysis. Heart Fail Rev 28, 859–864 (2023). https://doi.org/10.1007/s10741-022-10292-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10292-0