Abstract
Despite the strict indications for cardiac resynchronization therapy (CRT) implantation, a significant proportion of patients will fail to adequately respond to the treatment. This systematic review aims to present the existing evidence about the role of cardiac magnetic resonance (CMR) in identifying patients who are likely to respond better to the CRT. A systematic search in the MedLine database and Cochrane Library from their inception to August 2021 was performed, without any limitations, by two independent investigators. We considered eligible observational studies or randomized clinical trials (RCTs) that enrolled patients > 18 years old with heart failure (HF) of ischaemic or non-ischaemic aetiology and provided data about the association of baseline CMR variables with clinical or echocardiographic response to CRT for at least 3 months. This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement). Following our search strategy, 47 studies were finally included in our review. CMR appears to have an additive role in identifying the subgroup of patients who will respond better to CRT. Specifically, the presence and the extent of myocardial scar were associated with increased non-response rates, while those with no scar respond better. Furthermore, existing data show that scar location can be associated with CRT response rates. CMR-derived markers of mechanical desynchrony can also be used as predictors of CRT response. CMR data can be used to optimize the position of the left ventricular lead during the CRT implantation procedure. Specifically, positioning the left ventricular lead in a branch of the coronary sinus that feeds an area with transmural scar was associated with poorer response to CRT. CMR can be used as a non-invasive optimization tool to identify patients who are more likely to achieve better clinical and echocardiographic response following CRT implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to current guidelines, cardiac resynchronization therapy (CRT) is recommended in patients with advanced heart failure (HF), impaired left ventricular ejection fraction (LVEF), and a wide QRS complex [1, 2]. However, a significant proportion of CRT patients will fail to respond to the treatment with the rates of success varying according to the definition of the response criteria [3]. Specifically, the CRT response rates can range from 32 to 91% [4]. The identification of baseline characteristics that are associated with a higher probability of CRT response is therefore of great importance.
Cardiac magnetic resonance (CMR) is an imaging modality that can provide valuable clinical data, and therefore its use has been expanded in the current clinical practice [5]. In the field of HF, CMR can provide the “gold standard” method for measurements of biventricular ejection fraction; characterize myocardial tissue and mainly identify presence, location, and burden of myocardial fibrosis; assess myocardial viability; and thus help diagnose specific cardiomyopathies [6]. Furthermore, the presence and degree of myocardial scar on CMR has been associated with an adverse prognosis, including arrhythmias in conditions like aortic stenosis [7] and dilated cardiomyopathy [8, 9]. It is therefore possible that the presence and extent of myocardial scar can adversely affect the benefit following CRT and indeed the use of CMR in the pre-procedural evaluation of CRT candidates in combination with other diagnostic tools is able to identify patients unlikely to respond to CRT [10]. This systematic review aims to present the existing evidence about the role of CMR in identifying patients who are likely to respond to the CRT.
Myocardial tissue characterization
CMR is the only radiation-free modality that enables accurate assessment of myocardial tissue characterization. Various methods exist for this as well as different commercial packages. However, the mainstay of tissue characterization relies on the administration of the paramagnetic agent gadolinium and imaging the myocardium some 10–20 min after the administration. This delayed (or late) phase of imaging after the administration of gadolinium, often referred to as “late gadolinium enhancement, LGE” enables to identify scarred (or dead) myocardium from alive myocardium (Fig. 1). The white areas following gadolinium administration represent muscle that has died, often referred to as replacement (or focal) fibrosis, and there has been extensive validation of the CMR findings with histology. This type of fibrosis/scar is irreversible and forms the mainstay of assessment for viability by CMR. The black areas represent healthy myocardium. This also allows the location of the scar to be identified, with subendocardial or transmural scar relating to myocardial infarction and ischaemic cardiomyopathy. Midwall or subepicardial scar in the context of cardiomyopathy would relate to the non-ischaemic cardiomyopathy. Non-ischaemic cardiomyopathy is an umbrella term, capturing a plethora of conditions, which include cardiomyopathy secondary to dilated cardiomyopathy, myocarditis, cancer (either cancer itself or chemotherapy-related), hypertension, and infiltrative and autoimmune processes. When it comes to the quantification of the scar, there are both visual and semi-automatic/artificial-intelligence-guided methods. A simple, but well-recognized and valuable method, is to simply use the 16 AHA segment model and for each segment allocate 0 if there is no scar, 1 if there is subendocardial non-transmural scar, and 2 if there is transmular scar. This will therefore give a total score of 32, or each of the 16 segments representing 6% of the myocardium. More recent methods such as those provided by Circle CVI (Calgary, Canada) or MASS (Leiden, Netherlands) allow the operator to select a region of interest of normal (or abnormal) myocardium and the software will then automatically select and quantify the total scar for each ventricular slice. The software will then add the scar in all the slices giving an overall percentage both as mass and as a percentage. The quantification relies either on the full width half maximum method using regions defined above 50% of maximal signal intensity of the enhanced area, or the standard deviations method, where LGE can be quantified using a threshold above 2–7 standard deviations above a remote reference region [11].
Imaging in the late phase following gadolinium administration of the mid ventricular level. The white part of the myocardium in the septal and anteroseptal areas, indicated by the yellow arrows, corresponds to hyperenhancement of the myocardium, and effectively dead muscle. This was as a result of a left anterior descending artery myocardial infarction. The remaining myocardium which is black in colour is healthy
More recently, different CMR sequences based on T1 mapping, have been histologically validated [12] to show diffuse (or interstitial) fibrosis, effectively a “finer” form of fibrosis that might not be irreversible. This relies on expansion of the extracellular matrix, which is one of the initial hallmarks of pathology and, if the underlying aetiology is left untreated, is thought that it will result to myocardial scar. The two most common forms of measuring this diffuse fibrosis are native T1 mapping (which does not require administration of gadolinium) and extracellular volume fraction (ECV), which relies on measuring myocardial and blood T1 values before and after the administration of gadolinium. Both LGE and T1 mapping are therefore methods that provide myocardial tissue characterization and will be reviewed in this paper.
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement) [13].
Search strategy
Two independent investigators performed a systematic search in the MedLine database and Cochrane Library from inception to August 2021 without any limitations. In addition, we manually searched the reference lists of the relevant review and research studies. The following algorithm was used to retrieve all relevant studies: “(cardiac magnetic resonance) AND (cardiac resynchronization therapy OR CRT)”. We first screened the titles and abstracts of each study, and when a study was judged as relevant, we reviewed through the full text. Disagreements were resolved by a third investigator.
Eligibility criteria
We considered eligible observational studies or randomized clinical trials (RCTs) that enrolled patients > 18 years old with HF of ischaemic or non-ischaemic aetiology and provided data about the association of baseline CMR variables with clinical or echocardiographic response to CRT for at least 3 months. We excluded studies that provided data about acute CRT response only, studies that did not offer a definition of CRT response, and studies written in a different language than English.
Data collection process
The following data were extracted for each included study: publication data (first author, year of publication), patient characteristics (number of patients in each group (responders/nonresponders), mean age, gender, type of cardiomyopathy, left ventricular ejection fraction, LBBB, sinus rhythm), and the crude data of the reported outcomes. Two independent investigators performed the data extraction.
Results
Search results
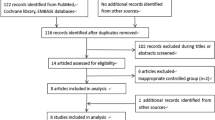
Our search strategy in electronic databases returned 489 possible relevant studies. Of them, 19 studies were excluded as duplicate records, and 373 studies were excluded at the title/abstract level while 50 studies were excluded at the full-text level. As a result, 47 studies were finally included in the review (Fig. 2, Table 1).
Association of CMR indices with CRT response
Impact of the myocardial scar on CRT response
The association of myocardial scar with CRT response has been studied in several studies. Both the presence of scar and scar extent/scar burden have been associated with increased non-response rates [14,15,16]. Also, the location of LV scar has been associated with CRT response [17, 18].
In the setting of ischaemic cardiomyopathy, a scar size ≥ 33%, a transmurality ≥ 51%, and pacing over a posterolateral scar have been associated with a suboptimal response to CRT [19]. By CMR imaging in the DCM group, the percentage of regional scar segments and percentage of regional scar score in the left ventricular inferior wall were significantly higher in the nonresponders than in the responders [20]. However, no statistical difference was found in the ICM, mainly because of the small sample size [20]. Midwall fibrosis seems to have a different impact on outcomes depending on the cause of cardiomyopathy. Specifically, it has been found that left ventricular reverse remodeling was observed in DCM without midwall hyperenhancement and in ICM but not in DCM with midwall hyperenhancement [21]. The same study showed that there were no differences between groups regarding the clinical response [21].
Focal scar burden detected by LGE CMR has been associated with a poor echocardiographic response to CRT, while diffuse interstitial fibrosis assessment by T1 mapping was not associated with CRT response [22]. A high scar tissue burden is more pronounced in nonresponders [23]. Specifically, in an observational study, scar burden as indicated by the total scar burden score and the number of transmurally infarcted segments was significantly higher in nonresponders [24]. Additionally, another study including ischaemic and non-ischaemic patients, defined scar burden on a 5-point scale, whereby 0 had no scar, and each incremental point corresponded to a group of 25% of transmurality, 1 = 1–25%, 2 = 26 = 50%, 3 = 51–75%, 4 = 76–100%. It was found that all patients with high scar burden score of > 1.2 failed to respond to CRT [24].
In a prospective, multicentre study, the septal scar was found to be a significant predictor of reverse remodeling defined as at least 15% reduction in LVESV indexed to body surface area at 6 months follow-up. In comparison, any scar in the septum showed a sensitivity of 81% for non-response to CRT [25]. In the same study, the combined assessment of septal viability and lateral wall to septal work difference performed better in predicting CRT response than work difference alone [25]. In a small cohort study of both ischaemic and non-ischaemic patients, both global and lateral wall scar burden were significantly associated with reverse remodeling [26]. Specifically, a cutoff value of 36.5% for global LV scar burden showed a sensitivity of 81.8% and specificity of 68.4% for predicting non-response. In comparison, a cutoff for lateral wall scar burden 40.5% of the whole lateral wall had a sensitivity of 72.7% and specificity of 68.4% [26]. Similarly, another study showed that patients with a transmural posterolateral scar had low clinical response rates [17, 18]. A significant positive correlation was found between myocardial scar mass and the evolution of LVESV at 12-month follow-up [27]. On the other hand, in a small observational study, the absence of scar in the posterolateral area (in the region of LV lead placement) was associated with CRT response only in univariate analysis but not in the multivariate analysis [28]. Interestingly, LV dyssynchrony remained the most critical determinant of response to CRT, even in the presence of posterolateral scar.
LGE ≥ 14% is associated with non-response, defined as no death or hospitalization for a major cardiovascular event, and a significant decrease in left ventricular end-systolic volume of 15% or more in a study with mixed ischaemic and non-ischaemic patients [29]. The percent of LGE was higher, regional vector of circumferential strain variance was lower, and uniformity of radial strain was higher in nonresponders vs. responders [30]. The same study showed that transmurality of LGE was an important predictor of lack of response to CRT in ischaemic HF [30]. In a prospective database of patients, it was found that the presence of subendocardial scar on CMR predicted CRT clinical non-response but was not associated with echocardiographic response [10]. Another study which aimed to investigate the role of the delayed enhancement MRI in predicting CRT response showed that a total scar of 15% or septal scar of 40% accurately identified patients with clinical response to CRT [31]. Similarly, in another observational study, the percentage of responders tended to be lower among patients with identifiable areas of delayed enhancement on CMR while scar mass and scar as a percentage of LV myocardium were not significantly different between responders and nonresponders [32].
Scar quantification can have an incremental value in the prediction of CRT response. In this setting, incorporating scar quantification to QRS duration and presence of LBBB was found to improve the prediction of CRT response [33]. The same study also found that an increase in scar burden was associated with worse outcomes in any location [33]. Native T1 mapping and extracellular volume have also been associated with poor response, while Scar2SD and Gray2SD markers of focal scar core were substantially better at predicting CRT response [34]. By combining QRS area and CMR focal scar assessment, CRT response prediction improves beyond that by either vectorcardiography or scar parameters alone [34].
The impact of scar in the left ventricular pacing site on CRT response
Scar in the left ventricular pacing site has been associated with adverse outcomes following CRT implantation [15]. Interestingly, the chronic response has been found to be significantly better in patients paced in a CMR target segment [35]. Pacing in the scar-free segments was found to lead to a greater reverse remodeling [16]. CMR-guided lead placement has been associated with significantly better clinical response rates compared to electrophysiological-guided placement in patients with more advanced HF [36]. However, in another study, left ventricular reverse remodeling was less pronounced in the CMR-guided and pacing scar group than in the non-CMR-guided group [37]. In the CMR-guided and non-pacing scar group, a scar burden of < 10% was not associated with a better LV reverse remodeling or clinical response compared with a scar burden of ≥ 10% [37].
Scar presence, total scar extent, transmural scar, and positioning the left ventricular lead in a branch of the coronary sinus that corresponds to an area with transmural scar were associated with non-response to CRT. Interestingly, the non-transmural scar was not associated with CRT response [38]. Another study showed that the presence of septal scar was associated with a poor acute and chronic response to CRT in ischaemic cardiomyopathy patients. At the same time, this finding may be related to the inability to achieve a right ventricular septal lead placement [39]. Beyond the impact on CRT response, a myocardial scar in the region of the left ventricular pacing lead has been associated with worse long-term survival in ischaemic heart failure patients treated with CRT [40]. A left ventricular lead position over a segment with scar emerged has also been found to be a strong predictor of cardiac mortality and cardiac mortality or HF hospitalizations [41].
On the other hand, results of another prospective study showed that pacing outside of scar compared to pacing within scar did not result in a significant improvement in clinical composite score or reduction in left ventricular end-systolic volume [10]. However, it should be noted that most patients in this study had a subendocardial scar.
A multimodality cardiac imaging using speckle tracking echocardiography and CMR imaging to guide CRT implantation was found to increase the response rates [42]. These findings highlight the potential use of CMR in guiding the LV lead placement during CRT implantation procedure.
Other variables
Combined CMR scar and dyssynchrony imaging prior to CRT identified those patients who subsequently had a clinical response to CRT [43]. A systolic dyssynchrony index derived from volume change can predict reverse remodeling following CRT while a 16-segment systolic dyssynchrony index of regional strain did not [44]. Measures of dyssynchrony (SD-TTPLV) and discoordination (ISFLV) were strongly related to CRT response when using myocardial tagging [45]. The end-systolic septal strain parameter showed a consistent high correlation with LVESV change for all techniques [45]. Magnetic resonance myocardial tagging (MR-MT) assessment of circumferential mechanical dyssynchrony was found to achieve an excellent predictive value for clinical response following CRT. At the same time, its accuracy could be further improved by combining MR-MT with scar imaging by delayed enhancement magnetic response imaging [46]. Circumferential uniformity ratio estimate, delayed circumferential contraction onset at left ventricular lead position, absent left ventricular lead position scar, and time from QRS onset to left ventricular lead position electrogram have been associated with echocardiographic response [47]. Recently, circumferential uniformity ratio estimated with singular value decomposition with Displacement Encoding with Stimulated Echoes strain imaging was significantly correlated with echocardiographic CRT response [48]. Septal flash predicted increased response to CRT while the presence of septal flash with no scar was a highly specific predictor of CRT response [49]. The left ventricular dyssynchrony and interventricular dyssynchrony were significantly longer in responders, while it was found that the use of the optimal cutoff of left ventricular dyssynchrony ≥ 65 ms can differentiate CRT responders from nonresponders with a sensitivity and a specificity of 100% [50].
On the other hand, internal stretch fraction, defined as the ratio of stretch to shortening during ejection, has been found to differ significantly between responders and non-responders [46] significantly. The findings of this study show that discoordination rather than dyssynchrony can predict the reserve contractile capacity recruited by CRT [51]. An observational study showed that LV shape might play a role in selecting CRT patients [52]. Specifically, it was revealed that nonresponders had a relatively shorter septal wall and longer lateral wall, while in responders, thicker walls in the lateral and basal regions were revealed compared with nonresponders [52]. Time to the onset of circumferential shortening (TOS)/QRS, scar at the LV pacing site, and circumferential uniformity ratio estimate calculated using singular value decomposition have been found to be significantly associated with the change in LVESV 6 months after CRT [53]. Furthermore, the regional vector of strain variance and uniformity of radial strain was found to be lower in nonresponders than responders [30]. In patients with non-ischaemic cardiomyopathy, a wide pattern of systolic left ventricular volume/time curves measured using CMR was significantly associated with CRT response compared to patients with a narrow pattern [54]. Summed maximum vortex flow can predict CRT response in DCM, while circumferential temporal delay and longitudinal temporal delay were not associated with CRT response [55]. Furthermore, a standard deviation of 16-segment time-to-peak radial thickness was significantly larger in responders compared with nonresponders [38]. Segment length in cine strain analysis can be used for the prediction of CRT response [56]. Specifically, of all parameters, the end-systolic septal strain showed the strongest correlation with reverse remodeling after CRT [56]. In patients with strict LBBB and with an indication of CRT implantation, a “U-shaped” contraction pattern was found to be strongly predictive for reverse remodeling and super-response [57]. Additionally, a “U-shaped” wall motion pattern compared to a homogenous (type I) wall motion pattern and a concordant LV lead can predict CRT response [44, 58]. These findings may help to improve patient selection by evaluating wall motion pattern and targeting LV lead placement.
The right ventricular function may have a significant role in predicting response following CRT implantation. Specifically, right ventricular dysfunction evaluated by CMR has been significantly associated with non-response to CRT. At the same time, the same study showed that late enhancement presence, lateral wall fibrosis, and the percentage of LV fibrosis were not associated with CRT response [59]. Likewise, another small retrospective study confirmed these results showing that except myocardial scar burden, CMR-derived right ventricular dysfunction was an independent predictor of non-response and adverse outcomes in patients on CRT [60].
Discussion
This systematic review shows the additive role of CMR in the selection of potential CRT candidates and the CMR role in guiding the left ventricular lead placement away from areas of transmural scar, for achieving a better outcome. Furthermore, we confirm that for individuals with high burden of myocardial scar there is less likelihood of having a good response. CRT is mainly recommended in patients with HF and LVEF ≤ 35% in sinus rhythm who have a QRS duration ≥ 130 ms and remain symptomatic despite optimal medical therapy. Additionally, CRT may be considered in patients with HF and LVEF ≤ 35% in AF who have a QRS duration ≥ 130 ms and remain in New York Heart Association (NYHA) class III or IV despite optimal medical therapy, provided biventricular capture can be ensured or the patient is expected to return to sinus rhythm [61]. Occasionally, CRT may be used as an upgrade from a conventional pacemaker or an ICD in patients with HFrEF who develop worsening HF attributable to a high rate of right ventricular pacing [61]. Although it has been shown that CRT improves cardiac function, symptoms, and quality of life, as well as it reduces morbidity and mortality in appropriately selected patients with HFrEF, not all patients who have an indication for CRT according to current guidelines respond favourably to this therapeutic modality [61]. Therefore, novel imaging techniques such as CMR that can assess additional factors to LVEF including myocardial scar, cardiac dyssynchrony, and site of latest activation of the LV are increasingly being studied as utilities for the better selection of patients appropriate for CRT, as well as for optimal lead placement [61]. While identification of areas of myocardial scar can guide the branch positioning of the left ventricular lead in the coronary sinus, it should also be appreciated that complex venous anatomy might prevent pacing the optimal scar-free area [62].
It is already known that the type of cardiomyopathy is a significant predictor of CRT response. Most of the included studies in this review consisted mainly of a mixed population. However, six studies [14, 16,17,18,19, 23] included ischaemic cardiomyopathy patients and one study [55] included only dilated cardiomyopathy patients. In ischaemic cardiomyopathy patients, a posterolateral scar imaged using LGE-CMR was an independent predictor of cardiovascular death or hospitalizations for HF [18]. Furthermore, scar size of 33%, transmurality of 51%, and pacing over a posterolateral scar were associated with a suboptimal response to CRT in ischaemic cardiomyopathy patients [17, 19]. Scar burden has also been associated with higher non-response rates in the setting of ischaemic HF [14, 23]. Additionally, LV reverse remodeling following CRT implantation was affected by the total left ventricular scar burden [16]. In the setting of dilated cardiomyopathy, summed maximum vortex flow has been found to predict CRT response [55].
According to our systematic literature review, both the presence and burden of scar as identified by CMR, as well as scar in certain locations, may be useful markers of non-response to CRT. Focal scar burden identified by LGE in CMR, numerically represented by the total scar burden score and/or the number of transmurally infarcted segments, has been shown to be a good marker of poor CRT response. Furthermore, the presence of either any septal scar or increased lateral wall scar burden was associated with less induction of reverse ventricular remodeling, while reports regarding scar in the posterolateral area are mixed, and LV dyssynchrony seems to remain the most important determinant of response to CRT, even in the presence of posterolateral scar. Of note, the combined assessment of septal viability and lateral wall to septal work difference has been reported to be a better marker of CRT response than work difference alone, and regional vector of circumferential strain variance was lower, and uniformity of radial strain was higher in nonresponders. By combining scar quantification and QRS duration in the setting of LBBB, or QRS area and CMR focal scar assessment, CRT response prediction significantly improved. Even further, the available evidence summarized in this review suggests that CMR may have an ancillary role in guiding the LV lead placement during CRT implantation procedure. Indeed, pacing in the scar-free segments has been shown to induce reverse remodeling to a greater extent, and CMR-guided lead placement has been associated with a better chronic response and higher BNP response rates. Additionally, pacing over a transmural scar has been associated with non-response to CRT, and pacing over a myocardial scar in the region of the left ventricle has been associated with worse long-term survival in ischaemic heart failure patients treated with CRT, and may portend a worse prognosis with regard to cardiac mortality and/or HF hospitalizations. However, CMR-guided lead placement has not been found to be superior to electrophysiologically guided procedures in some studies, and therefore further research is needed before reaching definitive conclusions. Finally, other CMR modalities such as dyssynchrony and strain imaging, as well as discoordination measures may also have an additive role on CRT candidate selection.
Except for chronic CRT response outcome, acute response following CRT implantation is another outcome of interest. CMR-identified scar had been found to predict acute CRT response adversely [63]. Specifically, septum-to-lateral wall myocardial work ratio at baseline is significantly related to acute response to CRT defined as acute left ventricular pump function improvement [64]. Furthermore, it has been found that pacing within scar on electroanatomic mapping and CMR (both epicardial and endocardial positions) resulted in failure to capture and a poor acute hemodynamic response [65]. Interestingly, patients with a postero-lateral scar as identified on CMR can be benefited from a multi-site left ventricular pacing as it can increase acute response by 16% compared to single-site pacing [65].
Beyond the role of CMR in predicting CRT response, it can be used to predict clinically important outcomes during follow-up. Specifically, the presence and size of myocardial scar have been associated with malignant arrhythmic events irrespective of CRT response [27]. Additionally, the presence of myocardial scar has been associated with all-cause mortality [27], while myocardial dyssynchrony assessed by CMR-tissue synchronization index was an independent predictor of mortality and morbidity after CRT [66]. An index derived by a combination of dyssynchrony, posterolateral scar location, and creatinine has been significantly associated with cardiovascular mortality [67].
As already discussed, pacing in the scar-free segments has led to a greater reverse remodeling and better long-term survival [16, 40]. Overlaying the CMR and CT dataset onto live fluoroscopy during left ventricular lead placement is a feasible technique that may lead to a greater reverse remodeling during follow-up [68]. A double-blind, randomized controlled trial showed the clinical benefit of multimodality imaging-guided left ventricular lead placement in CRT [69]. Specifically, it was found that imaging-guided LV lead placement using cardiac computed tomography venography, 99mTechnetium myocardial perfusion imaging, and speckle-tracking echocardiography radial strain reduced the number of clinical nonresponders [69]. However, a recent randomized study failed to demonstrate the benefit of the evaluation of delayed activation on echocardiography combined with anatomic information from computed tomography (coronary sinus tributary anatomy) and CMR (large scar preventing lead placement) in terms of clinical, echocardiographic response or in a significant reduction of death or heart failure hospitalization [70].
Several echocardiographic and clinical definitions of CRT response have been used to assess the efficacy of CRT [71]. CMR has also been used to evaluate CRT response, providing very high-quality data on the right/left ventricular function and strain/synchrony before and after CRT [72].
This systematic literature review has several potential limitations. Firstly, our results were derived from observational studies, which are subject to selection biases and have a limited ability to determine causality. In addition, populations were heterogenous between the studies which prevented reaching definitive conclusions. Furthermore, as all the studies included patients who fulfilled the clinical guideline criteria for a CRT, we were unable to review non-traditional criteria such as pacing-induced cardiomyopathy. Finally, the included studies provided dissimilar CMR data and how the scar was quantified and therefore a quantitative synthesis was not feasible.
Conclusions
The clinical significance of CMR is to identify scar regions that should be avoided during the coronary sinus LV lead implantation. Furthermore, CMR can be used as an optimization tool for identifying those patients with extensive myocardial scar who might achieve a sub-optimal clinical and echocardiographic response following CRT implantation. However, further research is needed to elucidate the role of CMR in predicting important outcomes in CRT patients.
References
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128(16):1810–1852. https://doi.org/10.1161/CIR.0b013e31829e8807
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. https://doi.org/10.1093/eurheartj/ehab368
Bonakdar HR, Jorat MV, Fazelifar AF, Alizadeh A, Givtaj N, Sameie N, Sadeghpour A, Haghjoo M (2009) Prediction of response to cardiac resynchronization therapy using simple electrocardiographic and echocardiographic tools. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 11(10):1330–1337. https://doi.org/10.1093/europace/eup258
Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, Fyfe DA, Leon AR, Oshinski JN (2010) Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation 121(18):1985–1991. https://doi.org/10.1161/CIRCULATIONAHA.109.910778
von Knobelsdorff-Brenkenhoff F, Schulz-Menger J (2016) Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J Cardiovasc Magn Reson 18:6. https://doi.org/10.1186/s12968-016-0225-6
Yoneyama K, Kitanaka Y, Tanaka O, Akashi YJ (2018) Cardiovascular magnetic resonance imaging in heart failure. Expert Rev Cardiovasc Ther 16(4):237–248. https://doi.org/10.1080/14779072.2018.1445525
Vassiliou VS, Pavlou M, Malley T, Halliday BP, Tsampasian V, Raphael CE, Tse G, Vieira MS, Auger D, Everett R, Chin C, Alpendurada F, Pepper J, Pennell DJ, Newby DE, Jabbour A, Dweck MR, Prasad SK (2021) A novel cardiovascular magnetic resonance risk score for predicting mortality following surgical aortic valve replacement. Sci Rep 11(1):20183. https://doi.org/10.1038/s41598-021-99788-7
Vassiliou VS, Perperoglou A, Raphael CE, Joshi S, Malley T, Everett R, Halliday B, Pennell DJ, Dweck MR, Prasad SK (2017) Midwall fibrosis and 5-year outcome in moderate and severe aortic stenosis. J Am Coll Cardiol 69(13):1755–1756. https://doi.org/10.1016/j.jacc.2017.01.034
Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, Arzanauskaite M, Lota A, Tayal U, Vassiliou VS, Gregson J, Alpendurada F, Frenneaux MP, Cook SA, Cleland JGF, Pennell DJ, Prasad SK (2019) Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 12(8 Pt 2):1645–1655. https://doi.org/10.1016/j.jcmg.2018.07.015
Sidhu BS, Gould J, Elliott MK, Mehta VS, Niederer SA, Carr-White G, Rinaldi CA (2021) Clinical effectiveness of a dedicated cardiac resynchronization therapy pre-assessment clinic incorporating cardiac magnetic resonance imaging and cardiopulmonary exercise testing on patient selection and outcomes. Int J Cardiol Heart Vasc 34:100800. https://doi.org/10.1016/j.ijcha.2021.100800
Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG, Jerosch-Herold M, Ghoshhajra BB, Kwong RY (2013) CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging 6(9):944–954. https://doi.org/10.1016/j.jcmg.2013.05.013
Vassiliou VS, Wassilew K, Cameron D, Heng EL, Nyktari E, Asimakopoulos G, de Souza A, Giri S, Pierce I, Jabbour A, Firmin D, Frenneaux M, Gatehouse P, Pennell DJ, Prasad SK (2018) Identification of myocardial diffuse fibrosis by 11 heartbeat MOLLI T 1 mapping: averaging to improve precision and correlation with collagen volume fraction. MAGMA 31(1):101–113. https://doi.org/10.1007/s10334-017-0630-3
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
D’Andrea A, Caso P, Scarafile R, Riegler L, Salerno G, Castaldo F, Gravino R, Cocchia R, Del Viscovo L, Limongelli G, Di Salvo G, Ascione L, Iengo R, Cuomo S, Santangelo L, Calabro R (2009) Effects of global longitudinal strain and total scar burden on response to cardiac resynchronization therapy in patients with ischaemic dilated cardiomyopathy. Eur J Heart Fail 11(1):58–67. https://doi.org/10.1093/eurjhf/hfn010
Cochet H, Denis A, Ploux S, Lumens J, Amraoui S, Derval N, Sacher F, Reant P, Lafitte S, Jais P, Laurent F, Ritter P, Montaudon M, Bordachar P (2013) Pre- and intra-procedural predictors of reverse remodeling after cardiac resynchronization therapy: an MRI study. J Cardiovasc Electrophysiol 24(6):682–691. https://doi.org/10.1111/jce.12101
Gathier WA, Salden OAE, van Ginkel DJ, van Everdingen WM, Mohamed Hoesein FAA, Cramer MJM, Doevendans PA, Meine M, Chamuleau SAJ, van Slochteren FJ (2020) Feasibility and potential benefit of pre-procedural CMR imaging in patients with ischaemic cardiomyopathy undergoing cardiac resynchronisation therapy. Neth Heart J 28(2):89–95. https://doi.org/10.1007/s12471-019-01360-6
Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ (2006) Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 113(7):969–976. https://doi.org/10.1161/CIRCULATIONAHA.105.543678
Chalil S, Stegemann B, Muhyaldeen SA, Khadjooi K, Foley PW, Smith RE, Leyva F (2007) Effect of posterolateral left ventricular scar on mortality and morbidity following cardiac resynchronization therapy. Pacing Clin Electrophysiol 30(10):1201–1209. https://doi.org/10.1111/j.1540-8159.2007.00841.x
Chalil S, Foley PW, Muyhaldeen SA, Patel KC, Yousef ZR, Smith RE, Frenneaux MP, Leyva F (2007) Late gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace 9(11):1031–1037. https://doi.org/10.1093/europace/eum133
Yokokawa M, Tada H, Toyama T, Koyama K, Naito S, Oshima S, Taniguchi K (2009) Magnetic resonance imaging is superior to cardiac scintigraphy to identify nonresponders to cardiac resynchronization therapy. Pacing Clin Electrophysiol 32(Suppl 1):S57-62. https://doi.org/10.1111/j.1540-8159.2008.02227.x
Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith RE, Prasad SK (2012) Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 60(17):1659–1667. https://doi.org/10.1016/j.jacc.2012.05.054
Chen Z, Sohal M, Sammut E, Child N, Jackson T, Claridge S, Cooklin M, O’Neill M, Wright M, Gill J, Chiribiri A, Schaeffter T, Carr-White G, Razavi R, Rinaldi CA (2016) Focal but not diffuse myocardial fibrosis burden quantification using cardiac magnetic resonance imaging predicts left ventricular reverse modeling following cardiac resynchronization therapy. J Cardiovasc Electrophysiol 27(2):203–209. https://doi.org/10.1111/jce.12855
Storkas HS, Hansen TF, Tahri JB, Lauridsen TK, Olsen FJ, Borgquist R, Vinther M, Lindhardt TB, Bruun NE, Sogaard P, Risum N (2020) Left axis deviation in patients with left bundle branch block is a marker of myocardial disease associated with poor response to cardiac resynchronization therapy. J Electrocardiol 63:147–152. https://doi.org/10.1016/j.jelectrocard.2019.04.007
Stankovic I, Aarones M, Smith HJ, Voros G, Kongsgaard E, Neskovic AN, Willems R, Aakhus S, Voigt JU (2014) Dynamic relationship of left-ventricular dyssynchrony and contractile reserve in patients undergoing cardiac resynchronization therapy. Eur Heart J 35(1):48–55. https://doi.org/10.1093/eurheartj/eht294
Aalen JM, Donal E, Larsen CK, Duchenne J, Lederlin M, Cvijic M, Hubert A, Voros G, Leclercq C, Bogaert J, Hopp E, Fjeld JG, Penicka M, Linde C, Aalen OO, Kongsgard E, Galli E, Voigt JU, Smiseth OA (2020) Imaging predictors of response to cardiac resynchronization therapy: left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur Heart J 41(39):3813–3823. https://doi.org/10.1093/eurheartj/ehaa603
Ahmed W, Samy W, Tayeh O, Behairy N, Abd El Fattah A (2016) Left ventricular scar impact on left ventricular synchronization parameters and outcomes of cardiac resynchronization therapy. Int J Cardiol 222:665–670. https://doi.org/10.1016/j.ijcard.2016.07.158
Linhart M, Doltra A, Acosta J, Borras R, Jauregui B, Fernandez-Armenta J, Anguera I, Bisbal F, Marti-Almor J, Tolosana JM, Penela D, Soto-Iglesias D, Villuendas R, Perea RJ, Ortiz JT, Bosch X, Auricchio A, Mont L, Berruezo A (2020) Ventricular arrhythmia risk is associated with myocardial scar but not with response to cardiac resynchronization therapy. Europace 22(9):1391–1400. https://doi.org/10.1093/europace/euaa142
Jansen AH, Bracke F, van Dantzig JM, Peels KH, Post JC, van den Bosch HC, van Gelder B, Meijer A, Korsten HH, de Vries J, van Hemel NM (2008) The influence of myocardial scar and dyssynchrony on reverse remodeling in cardiac resynchronization therapy. Eur J Echocardiogr 9(4):483–488. https://doi.org/10.1016/j.euje.2007.07.002
Andre C, Piver E, Perault R, Bisson A, Pucheux J, Vermes E, Pierre B, Fauchier L, Babuty D, Clementy N (2018) Galectin-3 predicts response and outcomes after cardiac resynchronization therapy. J Transl Med 16(1):299. https://doi.org/10.1186/s12967-018-1675-4
Petryka J, Misko J, Przybylski A, Spiewak M, Malek LA, Werys K, Mazurkiewicz L, Gepner K, Croisille P, Demkow M, Ruzyllo W (2012) Magnetic resonance imaging assessment of intraventricular dyssynchrony and delayed enhancement as predictors of response to cardiac resynchronization therapy in patients with heart failure of ischaemic and non-ischaemic etiologies. Eur J Radiol 81(10):2639–2647. https://doi.org/10.1016/j.ejrad.2011.10.003
White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M (2006) Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol 48(10):1953–1960. https://doi.org/10.1016/j.jacc.2006.07.046
Fernandez-Armenta J, Berruezo A, Mont L, Sitges M, Andreu D, Silva E, Ortiz-Perez JT, Tolosana JM, de Caralt TM, Perea RJ, Calvo N, Trucco E, Borras R, Matas M, Brugada J (2012) Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace 14(11):1578–1586. https://doi.org/10.1093/europace/eus104
Harb SC, Toro S, Bullen JA, Obuchowski NA, Xu B, Trulock KM, Varma N, Rickard J, Grimm R, Griffin B, Flamm SD, Kwon DH (2019) Scar burden is an independent and incremental predictor of cardiac resynchronisation therapy response. Open Heart 6(2):e001067. https://doi.org/10.1136/openhrt-2019-001067
Nguyen UC, Claridge S, Vernooy K, Engels EB, Razavi R, Rinaldi CA, Chen Z, Prinzen FW (2018) Relationship between vectorcardiographic QRSarea, myocardial scar quantification, and response to cardiac resynchronization therapy. J Electrocardiol 51(3):457–463. https://doi.org/10.1016/j.jelectrocard.2018.01.009
Shetty AK, Duckett SG, Ginks MR, Ma Y, Sohal M, Bostock J, Kapetanakis S, Singh JP, Rhode K, Wright M, O’Neill MD, Gill JS, Carr-White G, Razavi R, Rinaldi CA (2013) Cardiac magnetic resonance-derived anatomy, scar, and dyssynchrony fused with fluoroscopy to guide LV lead placement in cardiac resynchronization therapy: a comparison with acute haemodynamic measures and echocardiographic reverse remodelling. Eur Heart J Cardiovasc Imaging 14(7):692–699. https://doi.org/10.1093/ehjci/jes270
Kockova R, Sedlacek K, Wichterle D, Sikula V, Tintera J, Jansova H, Praveckova A, Langova R, Kryze L, El-Husseini W, Segetova M, Kautzner J (2018) Cardiac resynchronization therapy guided by cardiac magnetic resonance imaging: A prospective, single-centre randomized study (CMR-CRT). Int J Cardiol 270:325–330. https://doi.org/10.1016/j.ijcard.2018.06.009
Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, Auricchio A (2011) Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 13:29. https://doi.org/10.1186/1532-429X-13-29
Marsan NA, Westenberg JJ, Ypenburg C, van Bommel RJ, Roes S, Delgado V, Tops LF, van der Geest RJ, Boersma E, de Roos A, Schalij MJ, Bax JJ (2009) Magnetic resonance imaging and response to cardiac resynchronization therapy: relative merits of left ventricular dyssynchrony and scar tissue. Eur Heart J 30(19):2360–2367. https://doi.org/10.1093/eurheartj/ehp280
Duckett SG, Ginks M, Shetty A, Kirubakaran S, Bostock J, Kapetanakis S, Gill J, Carr-White G, Razavi R, Rinaldi CA (2012) Adverse response to cardiac resynchronisation therapy in patients with septal scar on cardiac MRI preventing a septal right ventricular lead position. J Interv Card Electrophysiol 33(2):151–160. https://doi.org/10.1007/s10840-011-9630-9
Delgado V, van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR, Ypenburg C, Boersma E, Holman ER, Schalij MJ, Bax JJ (2011) Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation 123(1):70–78. https://doi.org/10.1161/CIRCULATIONAHA.110.945345
Taylor RJ, Umar F, Panting JR, Stegemann B, Leyva F (2016) Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: a feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm 13(2):481–489. https://doi.org/10.1016/j.hrthm.2015.10.024
Bertini M, Mele D, Malagu M, Fiorencis A, Toselli T, Casadei F, Cannizzaro T, Fragale C, Fucili A, Campagnolo E, Benea G, Ferrari R (2016) Cardiac resynchronization therapy guided by multimodality cardiac imaging. Eur J Heart Fail 18(11):1375–1382. https://doi.org/10.1002/ejhf.605
Taylor AJ, Elsik M, Broughton A, Cherayath J, Leet A, Wong C, Iles L, Butler M, Pfluger H (2010) Combined dyssynchrony and scar imaging with cardiac magnetic resonance imaging predicts clinical response and long-term prognosis following cardiac resynchronization therapy. Europace 12(5):708–713. https://doi.org/10.1093/europace/euq047
Sohal M, Shetty A, Duckett S, Chen Z, Sammut E, Amraoui S, Carr-White G, Razavi R, Rinaldi CA (2013) Noninvasive assessment of LV contraction patterns using CMR to identify responders to CRT. JACC Cardiovasc Imaging 6(8):864–873. https://doi.org/10.1016/j.jcmg.2012.11.019
Zweerink A, van Everdingen WM, Nijveldt R, Salden OAE, Meine M, Maass AH, Vernooy K, de Lange FJ, Vos MA, Croisille P, Clarysse P, Geelhoed B, Rienstra M, van Gelder IC, van Rossum AC, Cramer MJ, Allaart CP (2018) Strain imaging to predict response to cardiac resynchronization therapy: a systematic comparison of strain parameters using multiple imaging techniques. ESC Heart Fail 5(6):1130–1140. https://doi.org/10.1002/ehf2.12335
Bilchick KC, Dimaano V, Wu KC, Helm RH, Weiss RG, Lima JA, Berger RD, Tomaselli GF, Bluemke DA, Halperin HR, Abraham T, Kass DA, Lardo AC (2008) Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging 1(5):561–568. https://doi.org/10.1016/j.jcmg.2008.04.013
Bilchick KC, Kuruvilla S, Hamirani YS, Ramachandran R, Clarke SA, Parker KM, Stukenborg GJ, Mason P, Ferguson JD, Moorman JR, Malhotra R, Mangrum JM, Darby AE, Dimarco J, Holmes JW, Salerno M, Kramer CM, Epstein FH (2014) Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol 63(16):1657–1666. https://doi.org/10.1016/j.jacc.2014.02.533
Bilchick KC, Auger DA, Abdishektaei M, Mathew R, Sohn MW, Cai X, Sun C, Narayan A, Malhotra R, Darby A, Mangrum JM, Mehta N, Ferguson J, Mazimba S, Mason PK, Kramer CM, Levy WC, Epstein FH (2020) CMR DENSE and the Seattle Heart Failure Model inform survival and arrhythmia risk after CRT. JACC Cardiovasc Imaging 13(4):924–936. https://doi.org/10.1016/j.jcmg.2019.10.017
Sohal M, Amraoui S, Chen Z, Sammut E, Jackson T, Wright M, O’Neill M, Gill J, Carr-White G, Rinaldi CA, Razavi R (2014) Combined identification of septal flash and absence of myocardial scar by cardiac magnetic resonance imaging improves prediction of response to cardiac resynchronization therapy. J Interv Card Electrophysiol 40(2):179–190. https://doi.org/10.1007/s10840-014-9907-x
Kawakubo M, Nagao M, Kumazawa S, Chishaki AS, Mukai Y, Nakamura Y, Honda H, Morishita J (2013) Evaluation of cardiac dyssynchrony with longitudinal strain analysis in 4-chamber cine MR imaging. Eur J Radiol 82(12):2212–2216. https://doi.org/10.1016/j.ejrad.2013.06.014
Kirn B, Jansen A, Bracke F, van Gelder B, Arts T, Prinzen FW (2008) Mechanical discoordination rather than dyssynchrony predicts reverse remodeling upon cardiac resynchronization. Am J Physiol Heart Circ Physiol 295(2):H640-646. https://doi.org/10.1152/ajpheart.00106.2008
Warriner DR, Jackson T, Zacur E, Sammut E, Sheridan P, Hose DR, Lawford P, Razavi R, Niederer SA, Rinaldi CA, Lamata P (2018) An asymmetric wall-thickening pattern predicts response to cardiac resynchronization therapy. JACC Cardiovasc Imaging 11(10):1545–1546. https://doi.org/10.1016/j.jcmg.2018.01.022
Auger DA, Bilchick KC, Gonzalez JA, Cui SX, Holmes JW, Kramer CM, Salerno M, Epstein FH (2017) Imaging left-ventricular mechanical activation in heart failure patients using cine DENSE MRI: validation and implications for cardiac resynchronization therapy. J Magn Reson Imaging 46(3):887–896. https://doi.org/10.1002/jmri.25613
Aimo A, Valleggi A, Barison A, Salerni S, Emdin M, Aquaro GD (2021) Morphologies and prognostic significance of left ventricular volume/time curves with cardiac magnetic resonance in patients with non-ischaemic heart failure and left bundle branch block. Int J Cardiovasc Imaging 37(7):2245–2255. https://doi.org/10.1007/s10554-021-02194-3
Nakao R, Nagao M, Fukushima K, Sakai A, Watanabe E, Kawakubo M, Sakai S, Hagiwara N (2019) Prediction of cardiac resynchronization therapy response in dilated cardiomyopathy using vortex flow mapping on cine magnetic resonance imaging. Circ Rep 1(8):333–341. https://doi.org/10.1253/circrep.CR-18-0024
Zweerink A, Nijveldt R, Braams NJ, Maass AH, Vernooy K, de Lange FJ, Meine M, Geelhoed B, Rienstra M, van Gelder IC, Vos MA, van Rossum AC, Allaart CP (2021) Segment length in cine (SLICE) strain analysis: a practical approach to estimate potential benefit from cardiac resynchronization therapy. J Cardiovasc Magn Reson 23(1):4. https://doi.org/10.1186/s12968-020-00701-4
Jackson T, Sohal M, Chen Z, Child N, Sammut E, Behar J, Claridge S, Carr-White G, Razavi R, Rinaldi CA (2014) A U-shaped type II contraction pattern in patients with strict left bundle branch block predicts super-response to cardiac resynchronization therapy. Heart Rhythm 11(10):1790–1797. https://doi.org/10.1016/j.hrthm.2014.06.005
Hartlage GR, Suever JD, Clement-Guinaudeau S, Strickland PT, Ghasemzadeh N, Magrath RP, Parikh A, Lerakis S, Hoskins MH, Leon AR, Lloyd MS, Oshinski JN (2015) Prediction of response to cardiac resynchronization therapy using left ventricular pacing lead position and cardiovascular magnetic resonance derived wall motion patterns: a prospective cohort study. J Cardiovasc Magn Reson 17(1):57. https://doi.org/10.1186/s12968-015-0158-5
Manca P, Cossa S, Matta G, Scalone A, Tola G, Schintu B, Setzu A, Melis M, Giardina A, Corda M, Sinagra G, Porcu M (2020) Right ventricular function assessed by cardiac magnetic resonance predicts the response to resynchronization therapy. J Cardiovasc Med (Hagerstown) 21(4):299–304. https://doi.org/10.2459/JCM.0000000000000931
Alpendurada F, Guha K, Sharma R, Ismail TF, Clifford A, Banya W, Mohiaddin RH, Pennell DJ, Cowie MR, McDonagh T, Prasad SK (2011) Right ventricular dysfunction is a predictor of non-response and clinical outcome following cardiac resynchronization therapy. J Cardiovasc Magn Reson 13:68. https://doi.org/10.1186/1532-429X-13-68
Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabes JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylen I, Tolosana JM, Group ESCSD (2021) 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 42(35):3427–3520. https://doi.org/10.1093/eurheartj/ehab364
Catanzaro JN, Makaryus JN, Jadonath R, Makaryus AN (2014) Planning and guidance of cardiac resynchronization therapy-lead implantation by evaluating coronary venous anatomy assessed with multidetector computed tomography. Clin Med Insights Cardiol 8(Suppl 4):43–50. https://doi.org/10.4137/CMC.S18762
Okafor O, Umar F, Zegard A, van Dam P, Walton J, Stegemann B, Marshall H, Leyva F (2020) Effect of QRS area reduction and myocardial scar on the hemodynamic response to cardiac resynchronization therapy. Heart Rhythm 17(12):2046–2055. https://doi.org/10.1016/j.hrthm.2020.07.025
Zweerink A, de Roest GJ, Wu L, Nijveldt R, de Cock CC, van Rossum AC, Allaart CP (2016) Prediction of acute response to cardiac resynchronization therapy by means of the misbalance in regional left ventricular myocardial work. J Card Fail 22(2):133–142. https://doi.org/10.1016/j.cardfail.2015.10.020
Behar JM, Jackson T, Hyde E, Claridge S, Gill J, Bostock J, Sohal M, Porter B, O’Neill M, Razavi R, Niederer S, Rinaldi CA (2016) Optimized left ventricular endocardial stimulation is superior to optimized epicardial stimulation in ischemic patients with poor response to cardiac resynchronization therapy: a combined magnetic resonance imaging, electroanatomic contact mapping, and hemodynamic study to target endocardial lead placement. JACC Clin Electrophysiol 2(7):799–809. https://doi.org/10.1016/j.jacep.2016.04.006
Chalil S, Stegemann B, Muhyaldeen S, Khadjooi K, Smith RE, Jordan PJ, Leyva F (2007) Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: a study using cardiovascular magnetic resonance tissue synchronization imaging. J Am Coll Cardiol 50(3):243–252. https://doi.org/10.1016/j.jacc.2007.03.035
Leyva F, Foley PW, Stegemann B, Ward JA, Ng LL, Frenneaux MP, Regoli F, Smith RE, Auricchio A (2009) Development and validation of a clinical index to predict survival after cardiac resynchronisation therapy. Heart 95(19):1619–1625. https://doi.org/10.1136/hrt.2009.173880
Salden OAE, van den Broek HT, van Everdingen WM, Mohamed Hoesein FAA, Velthuis BK, Doevendans PA, Cramer MJ, Tuinenburg AE, Leufkens P, van Slochteren FJ, Meine M (2019) Multimodality imaging for real-time image-guided left ventricular lead placement during cardiac resynchronization therapy implantations. Int J Cardiovasc Imaging 35(7):1327–1337. https://doi.org/10.1007/s10554-019-01574-0
Sommer A, Kronborg MB, Nørgaard BL, Poulsen SH, Bouchelouche K, Böttcher M, Jensen HK, Jensen JM, Kristensen J, Gerdes C, Mortensen PT, Nielsen JC (2016) Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Eur J Heart Fail 18(11):1365–1374. https://doi.org/10.1002/ejhf.530
Borgquist R, Carlsson M, Markstad H, Werther-Evaldsson A, Ostenfeld E, Roijer A, Bakos Z (2020) Cardiac resynchronization therapy guided by echocardiography, MRI, and CT imaging. JACC: Clinical Electrophysiology 6 (10):1300–1309. https://doi.org/10.1016/j.jacep.2020.05.011
Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, Fyfe DA, León AR, Oshinski JN (2010) Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation 121(18):1985–1991. https://doi.org/10.1161/CIRCULATIONAHA.109.910778
Gao X, Abdi M, Auger DA, Sun C, Hanson CA, Robinson AA, Schumann C, Oomen PJ, Ratcliffe S, Malhotra R, Darby A, Monfredi OJ, Mangrum JM, Mason P, Mazimba S, Holmes JW, Kramer CM, Epstein FH, Salerno M, Bilchick KC (2021) Cardiac magnetic resonance assessment of response to cardiac resynchronization therapy and programming strategies. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2021.06.015
Aalen JM, Donal E, Larsen CK et al (2020) Imaging predictors of response to cardiac resynchronization therapy: left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur Heart J 41(39):3813–3823. https://doi.org/10.1093/eurheartj/ehaa603[publishedOnlineFirst:2020/09/13]
Ahmed W, Samy W, Tayeh O et al (2016) Left ventricular scar impact on left ventricular synchronization parameters and outcomes of cardiac resynchronization therapy. Int J Cardiol 222:665–670. https://doi.org/10.1016/j.ijcard.2016.07.158[publishedOnlineFirst:2016/08/16]
Aimo A, Valleggi A, Barison A et al (2021) Morphologies and prognostic significance of left ventricular volume/time curves with cardiac magnetic resonance in patients with non-ischaemic heart failure and left bundle branch block. Int J Cardiovasc Imaging 37(7):2245–2255. https://doi.org/10.1007/s10554-021-02194-3[publishedOnlineFirst:2021/02/27]
Alpendurada F, Guha K, Sharma R et al (2011) Right ventricular dysfunction is a predictor of non-response and clinical outcome following cardiac resynchronization therapy. J Cardiovasc Magn Reson 13:68. https://doi.org/10.1186/1532-429X-13-68[publishedOnlineFirst:2011/11/02]
Andre C, Piver E, Perault R et al (2018) Galectin-3 predicts response and outcomes after cardiac resynchronization therapy. J Transl Med 16(1):299. https://doi.org/10.1186/s12967-018-1675-4[publishedOnlineFirst:2018/11/06]
Auger DA, Bilchick KC, Gonzalez JA et al (2017) Imaging left-ventricular mechanical activation in heart failure patients using cine DENSE MRI: Validation and implications for cardiac resynchronization therapy. J Magn Reson Imaging 46(3):887–896. https://doi.org/10.1002/jmri.25613[publishedOnlineFirst:2017/01/10]
Bertini M, Mele D, Malagu M et al (2016) Cardiac resynchronization therapy guided by multimodality cardiac imaging. Eur J Heart Fail 18(11):1375–1382. https://doi.org/10.1002/ejhf.605[publishedOnlineFirst:2016/11/05]
Bilchick KC, Dimaano V, Wu KC et al (2008) Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging 1(5):561–568. https://doi.org/10.1016/j.jcmg.2008.04.013[publishedOnlineFirst:2009/04/10]
Bilchick KC, Kuruvilla S, Hamirani YS et al (2014) Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol 63(16):1657–1666. https://doi.org/10.1016/j.jacc.2014.02.533[publishedOnlineFirst:2014/03/04]
Bilchick KC, Auger DA, Abdishektaei M et al (2020) CMR DENSE and the Seattle Heart Failure Model Inform Survival and Arrhythmia Risk After CRT. JACC Cardiovasc Imaging 13(4):924–936. https://doi.org/10.1016/j.jcmg.2019.10.017[publishedOnlineFirst:2019/12/23]
Bleeker GB, Kaandorp TA, Lamb HJ et al (2006) Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 113(7):969–976. https://doi.org/10.1161/CIRCULATIONAHA.105.543678[publishedOnlineFirst:2006/02/16]
Chalil S, Stegemann B, Muhyaldeen SA et al (2007) Effect of posterolateral left ventricular scar on mortality and morbidity following cardiac resynchronization therapy. Pacing Clin Electrophysiol 30(10):1201–1209. https://doi.org/10.1111/j.1540-8159.2007.00841.x[publishedOnlineFirst:2007/09/28]
Chalil S, Foley PW, Muyhaldeen SA et al (2007) Late gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace 9(11):1031–1037. https://doi.org/10.1093/europace/eum133[publishedOnlineFirst:2007/10/16]
Chen Z, Sohal M, Sammut E et al (2016) Focal but not diffuse myocardial fibrosis burden quantification using cardiac magnetic resonance imaging predicts left ventricular reverse modeling following cardiac resynchronization therapy. J Cardiovasc Electrophysiol 27(2):203–209. https://doi.org/10.1111/jce.12855[publishedOnlineFirst:2015/10/16]
Cochet H, Denis A, Ploux S et al (2013) Pre- and intra-procedural predictors of reverse remodeling after cardiac resynchronization therapy: an MRI study. J Cardiovasc Electrophysiol 24(6):682–691. https://doi.org/10.1111/jce.12101[publishedOnlineFirst:2013/02/27]
D’Andrea A, Caso P, Scarafile R et al (2009) Effects of global longitudinal strain and total scar burden on response to cardiac resynchronization therapy in patients with ischaemic dilated cardiomyopathy. Eur J Heart Fail 11(1):58–67. https://doi.org/10.1093/eurjhf/hfn010[publishedOnlineFirst:2009/01/17]
Duckett SG, Ginks M, Shetty A et al (2012) Adverse response to cardiac resynchronisation therapy in patients with septal scar on cardiac MRI preventing a septal right ventricular lead position. J Interv Card Electrophysiol 33(2):151–160. https://doi.org/10.1007/s10840-011-9630-9[publishedOnlineFirst:2011/12/01]
Fernandez-Armenta J, Berruezo A, Mont L et al (2012) Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace 14(11):1578–1586. https://doi.org/10.1093/europace/eus104[publishedOnlineFirst:2012/05/09]
Gathier WA, Salden OAE, van Ginkel DJ et al (2020) Feasibility and potential benefit of pre-procedural CMR imaging in patients with ischaemic cardiomyopathy undergoing cardiac resynchronisation therapy. Neth Heart J 28(2):89–95. https://doi.org/10.1007/s12471-019-01360-6[publishedOnlineFirst:2020/01/19]
Harb SC, Toro S, Bullen JA et al (2019) Scar burden is an independent and incremental predictor of cardiac resynchronisation therapy response. Open Heart 6(2):e001067. https://doi.org/10.1136/openhrt-2019-001067[publishedOnlineFirst:2019/07/30]
Hartlage GR, Suever JD, Clement-Guinaudeau S et al (2015) Prediction of response to cardiac resynchronization therapy using left ventricular pacing lead position and cardiovascular magnetic resonance derived wall motion patterns: a prospective cohort study. J Cardiovasc Magn Reson 17(1):57. https://doi.org/10.1186/s12968-015-0158-5
Jackson T, Sohal M, Chen Z et al (2014) A U-shaped type II contraction pattern in patients with strict left bundle branch block predicts super-response to cardiac resynchronization therapy. Heart Rhythm 11(10):1790–1797. https://doi.org/10.1016/j.hrthm.2014.06.005[publishedOnlineFirst:2014/06/10]
Jansen AH, Bracke F, van Dantzig JM et al (2008) The influence of myocardial scar and dyssynchrony on reverse remodeling in cardiac resynchronization therapy. Eur J Echocardiogr 9(4):483–488. https://doi.org/10.1016/j.euje.2007.07.002[publishedOnlineFirst:2007/09/11]
Kawakubo M, Nagao M, Kumazawa S et al (2013) Evaluation of cardiac dyssynchrony with longitudinal strain analysis in 4-chamber cine MR imaging. Eur J Radiol 82(12):2212–2216. https://doi.org/10.1016/j.ejrad.2013.06.014[publishedOnlineFirst:2013/08/06]
Kirn B, Jansen A, Bracke F et al (2008) Mechanical discoordination rather than dyssynchrony predicts reverse remodeling upon cardiac resynchronization. Am J Physiol Heart Circ Physiol 295(2):H640–H646. https://doi.org/10.1152/ajpheart.00106.2008[publishedOnlineFirst:2008/06/03]
Kockova R, Sedlacek K, Wichterle D et al (2018) Cardiac resynchronization therapy guided by cardiac magnetic resonance imaging: A prospective, single-centre randomized study (CMR-CRT). Int J Cardiol 270:325–330. https://doi.org/10.1016/j.ijcard.2018.06.009[publishedOnlineFirst:2018/06/18]
Leyva F, Foley PW, Chalil S et al (2011) Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 13:29. https://doi.org/10.1186/1532-429X-13-29[publishedOnlineFirst:2011/06/15]
Leyva F, Taylor RJ, Foley PW et al (2012) Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 60(17):1659–1667. https://doi.org/10.1016/j.jacc.2012.05.054[publishedOnlineFirst:2012/10/02]
Manca P, Cossa S, Matta G et al (2020) Right ventricular function assessed by cardiac magnetic resonance predicts the response to resynchronization therapy. J Cardiovasc Med (Hagerstown) 21(4):299–304. https://doi.org/10.2459/JCM.0000000000000931[publishedOnlineFirst:2020/02/29]
Marsan NA, Westenberg JJ, Ypenburg C et al (2009) Magnetic resonance imaging and response to cardiac resynchronization therapy: relative merits of left ventricular dyssynchrony and scar tissue. Eur Heart J 30(19):2360–2367. https://doi.org/10.1093/eurheartj/ehp280[publishedOnlineFirst:2009/07/07]
Nakao R, Nagao M, Fukushima K et al (2019) Prediction of cardiac resynchronization therapy response in dilated cardiomyopathy using vortex flow mapping on cine magnetic resonance imaging. Circ Rep 1(8):333–341. https://doi.org/10.1253/circrep.CR-18-0024[publishedOnlineFirst:2019/06/26]
Petryka J, Misko J, Przybylski A et al (2012) Magnetic resonance imaging assessment of intraventricular dyssynchrony and delayed enhancement as predictors of response to cardiac resynchronization therapy in patients with heart failure of ischaemic and non-ischaemic etiologies. Eur J Radiol 81(10):2639–2647. https://doi.org/10.1016/j.ejrad.2011.10.003[publishedOnlineFirst:2011/11/08]
Shetty AK, Duckett SG, Ginks MR et al (2013) Cardiac magnetic resonance-derived anatomy, scar, and dyssynchrony fused with fluoroscopy to guide LV lead placement in cardiac resynchronization therapy: a comparison with acute haemodynamic measures and echocardiographic reverse remodelling. Eur Heart J Cardiovasc Imaging 14(7):692–699. https://doi.org/10.1093/ehjci/jes270[publishedOnlineFirst:2012/11/24]
Sidhu BS, Gould J, Elliott MK et al (2021) Clinical effectiveness of a dedicated cardiac resynchronization therapy pre-assessment clinic incorporating cardiac magnetic resonance imaging and cardiopulmonary exercise testing on patient selection and outcomes. Int J Cardiol Heart Vasc 34:100800. https://doi.org/10.1016/j.ijcha.2021.100800[publishedOnlineFirst:2021/06/24]
Sohal M, Shetty A, Duckett S et al (2013) Noninvasive assessment of LV contraction patterns using CMR to identify responders to CRT. JACC Cardiovasc Imaging 6(8):864–873. https://doi.org/10.1016/j.jcmg.2012.11.019[publishedOnlineFirst:2013/06/06]
Sohal M, Amraoui S, Chen Z et al (2014) Combined identification of septal flash and absence of myocardial scar by cardiac magnetic resonance imaging improves prediction of response to cardiac resynchronization therapy. J Interv Card Electrophysiol 40(2):179–190. https://doi.org/10.1007/s10840-014-9907-x[publishedOnlineFirst:2014/06/12]
Stankovic I, Aarones M, Smith HJ et al (2014) Dynamic relationship of left-ventricular dyssynchrony and contractile reserve in patients undergoing cardiac resynchronization therapy. Eur Heart J 35(1):48–55. https://doi.org/10.1093/eurheartj/eht294[publishedOnlineFirst:2013/08/07]
Storkas HS, Hansen TF, Tahri JB et al (2020) Left axis deviation in patients with left bundle branch block is a marker of myocardial disease associated with poor response to cardiac resynchronization therapy. J Electrocardiol 63:147–152. https://doi.org/10.1016/j.jelectrocard.2019.04.007[publishedOnlineFirst:2019/04/21]
Taylor AJ, Elsik M, Broughton A et al (2010) Combined dyssynchrony and scar imaging with cardiac magnetic resonance imaging predicts clinical response and long-term prognosis following cardiac resynchronization therapy. Europace 12(5):708–713. https://doi.org/10.1093/europace/euq047[publishedOnlineFirst:2010/03/02]
Taylor RJ, Umar F, Panting JR et al (2016) Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm 13(2):481–489. https://doi.org/10.1016/j.hrthm.2015.10.024[publishedOnlineFirst:2015/10/27]
Nguyen UC, Claridge S, Vernooy K et al (2018) Relationship between vectorcardiographic QRSarea, myocardial scar quantification, and response to cardiac resynchronization therapy. J Electrocardiol 51(3):457–463. https://doi.org/10.1016/j.jelectrocard.2018.01.009[publishedOnlineFirst:2018/02/20]
Warriner DR, Jackson T, Zacur E et al (2018) An asymmetric wall-thickening pattern predicts response to cardiac resynchronization therapy. JACC Cardiovasc Imaging 11(10):1545–1546. https://doi.org/10.1016/j.jcmg.2018.01.022[publishedOnlineFirst:2018/03/20]
White JA, Yee R, Yuan X et al (2006) Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol 48(10):1953–1960. https://doi.org/10.1016/j.jacc.2006.07.046[publishedOnlineFirst:2006/11/23]
Yokokawa M, Tada H, Toyama T et al (2009) Magnetic resonance imaging is superior to cardiac scintigraphy to identify nonresponders to cardiac resynchronization therapy. Pacing Clin Electrophysiol 32(Suppl 1):S57-62. https://doi.org/10.1111/j.1540-8159.2008.02227.x[publishedOnlineFirst:2009/03/11]
Zweerink A, van Everdingen WM, Nijveldt R et al (2018) Strain imaging to predict response to cardiac resynchronization therapy: a systematic comparison of strain parameters using multiple imaging techniques. ESC Heart Fail 5(6):1130–1140. https://doi.org/10.1002/ehf2.12335[publishedOnlineFirst:2018/07/28]
Zweerink A, Nijveldt R, Braams NJ et al (2021) Segment length in cine (SLICE) strain analysis: a practical approach to estimate potential benefit from cardiac resynchronization therapy. J Cardiovasc Magn Reson 23(1):4. https://doi.org/10.1186/s12968-020-00701-4[publishedOnlineFirst:2021/01/12]
Linhart M, Doltra A, Acosta J et al (2020) Ventricular arrhythmia risk is associated with myocardial scar but not with response to cardiac resynchronization therapy. Europace 22(9):1391–1400. https://doi.org/10.1093/europace/euaa142[publishedOnlineFirst:2020/09/09]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bazoukis, G., Hui, J.M.H., Lee, Y.H.A. et al. The role of cardiac magnetic resonance in identifying appropriate candidates for cardiac resynchronization therapy — a systematic review of the literature. Heart Fail Rev 27, 2095–2118 (2022). https://doi.org/10.1007/s10741-022-10263-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10263-5