Abstract
Targeting the renin-angiotensin system (RAS) pathways has been considered a logical intervention for patients with heart failure with preserved ejection fraction (HFpEF), due to its hypothesized link to left ventricular (LV) remodeling. Although the effects of RAS inhibitors including angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), and direct renin inhibitors (DRIs) on LV structure and function and exercise capacity in HFpEF patients have been examined in multiple randomized controlled trials (RCTs), results are inconsistent due partly to limited power. We conducted a meta-analysis of RCTs on the effects of RAS inhibitors on LV structure and function as well as exercise capacity in HFpEF patients. The search of electronic databases identified 7 trials including 569 patients; 4 trials were on ACE-Is; 2 on ARBs; and 1 on DRIs. Follow-up duration ranged across trials from 12 to 52 weeks. The pooled analysis showed that RAS inhibitors significantly increased EF compared with control (weighted mean difference [95% CI] = 2.182 [0.462, 3.901] %). In contrast, RAS inhibitors did not significantly change the ratio of peak early to late diastolic mitral inflow velocities (weighted mean difference [95% CI] = 0.046 [− 0.012, 0.105]), early diastolic mitral annular velocity (0.327 [− 0.07, 0.725] cm/s), the ratio of early diastolic mitral inflow to annular velocities (0.291 [− 0.937, 1.518]), LV mass (− 6.254 [− 15.165, 2.656] g), or 6-min walk distance (1.972 [− 14.22, 18.163] m) compared with control. The present meta-analysis suggests that RAS inhibitors may increase LVEF in HFpEF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly half of patients with heart failure (HF) in the community have preserved ejection fraction (EF) and the mortality and morbidity of patients with HF with preserved (HFpEF) are high [1,2,3]. However, there is no established therapy to improve survival in these patients [4,5,6,7,8]. Patients with HFpEF are often elderly and their primary chronic symptom is severe exercise intolerance [9, 10]. Improvement of exercise capacity presents another important clinical outcome in HFpEF patients.
Targeting the renin–angiotensin system (RAS) pathways has been considered a logical intervention for HFpEF, due to its hypothesized link to left ventricular (LV) remodeling [11, 12]. The effects of RAS inhibitors including angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor blockers (ARBs), and direct renin inhibitors (DRIs) on LV structure and function or exercise capacity in HFpEF patients have been examined in multiple randomized controlled trials (RCTs) [13,14,15,16,17,18]. However, the results are inconsistent due partly to limited power. Accordingly, we aimed to conduct a meta-analysis of RCTs on the effects of RAS inhibitors on LV structure and function as well as exercise capacity in HFpEF.
Methods
This meta-analysis was performed and reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [19]. Studies on the effect of RAS inhibitors on LV structure and function in patients with HFpEF published until November 30, 2019, were identified using PubMed and EMBASE databases. For the search of the eligible studies, the following keywords and medical subject heading were used: diastolic heart failure, heart failure with normal (preserved) ejection fraction, and randomized controlled trial. Our literature search was limited to studies involving human subjects and those published in English. Additionally, we manually searched the references that were cited in other relevant publications. Studies were considered eligible if they (1) included HFpEF; (2) were RCT; (3) used RAS inhibitors (ACE-Is, ARBs, or DRIs); (4) compared with standard medical care or placebo control group; and (5) assessed at least one of the following outcome measures: LV systolic or diastolic function, LV mass, and exercise capacity.
Primary outcomes of interest were LV structure and function. In the measure of LV structure, LV mass was extracted. In the measure of LV systolic function, LVEF was extracted. In the measures of LV diastolic function, the ratio of peak early to late diastolic mitral inflow velocities (E/A), early diastolic mitral annular velocity (e’), and the ratio of early diastolic mitral inflow to annular velocities (E/e’) were extracted. Secondary outcome of interest was exercise capacity. In the measure of exercise capacity, 6-min walk distance (6MWD) was extracted. Other outcomes of interest were systolic and diastolic blood pressure.
Information on the study and patient characteristics, methodological quality, intervention strategies, and clinical outcomes was systematically extracted separately by 2 reviewers (TG and KW). Disagreements were resolved by consensus.
For each outcome, the effect size for the intervention was calculated by the difference between the means of the intervention and control groups at the end of the intervention. If the outcome was measured on the same scale, the weighted mean difference (WMD) and 95% confidence interval (CI) were calculated. For each outcome, heterogeneity was assessed using Cochran’s Q and I2 statistic; for Cochran’s Q and I2 statistic, a p value of < 0.1 and I2 > 50% were considered significant, respectively [20]. When there was significant heterogeneity, the data were pooled using a random-effects model; otherwise, a fixed-effects model was used. All analyses were based on intension-to-treat data. All the included studies did not report the standard deviation of the change or the correlation of the pre and post measurements and did only the pre and post measurements. Accordingly, the correlation was conservatively set at 0.5 as previously reported [21]. Meta-regression was used to determine the factors that impact on the effect size. A one-study-removed analysis was performed to assess the influence of any one particular study on the overall meta-analysis result. Publication bias was assessed graphically using a funnel plot and mathematically using the Egger test. For all analyses, Comprehensive Meta-Analysis Software version 2 (Biostat, Englewood, NJ, USA) was used.
Results
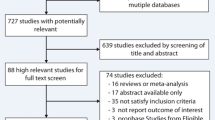
The study identification and selection process is summarized in Fig. 1. A total of 7 trials including 569 patients were included in the present meta-analysis. The Hong Kong diastolic heart failure study was split into two sub-trials because the included patients were randomized to two different drug interventions (irbesartan and ramipril), both compared with usual medication [15].
The characteristics of the included trials are summarized in Table 1. Of the included trials, 4 trials were on ACE-Is; 2 on ARBs; and 1 on DRIs. Follow-up duration ranged across trials from 12 to 52 weeks. As to the primary outcomes of interest in the present meta-analysis, 6 trials reported the effect of RAS inhibitors on LV mass, EF, and E/A and 4 trials on E/e’ and e’. As to the secondary outcomes of interest, 5 trials reported the effect of RAS inhibitors on 6MWD.
Baseline patient characteristics of the included trials are summarized in Table 2. Many patients were taking HF standard medications such as diuretics from 54 to 100%. Baseline measures of the primary and secondary outcomes of interest in the present meta-analysis are shown in supplement Tables 1 and 2.
The effects of RAS inhibitors on LV structure and function are shown in Fig. 2. RAS inhibitors significantly increased EF compared with control (WMD [95% CI] = 2.182 [0.462, 3.901] %; pfix < 0.05). In contrast, RAS inhibitors did not significantly change LV mass (WMD [95% CI] = − 6.254 [− 15.165, 2.656] g; pfix > 0.10), E/A (0.046 [− 0.012, 0.105]; pfix > 0.10), e’ (0.327 [− 0.07, 0.725] cm/s; pfix > 0.10), or E/e’ (0.291 [− 0.937, 1.518]; pfix > 0.10) compared with control. In meta-regression, no variables listed in Table 2 were significantly associated with the increase in EF (all p > 0.1).
Forest plots showing the effects of renin–angiotensin system inhibitors (RAS-I) on left ventricular (LV) mass (g; a), ejection fraction (EF; %, b), the ratio of peak early to late diastolic mitral inflow velocities (E/A; c), early diastolic mitral annular velocity (e’; cm/s, d), and the ratio of early diastolic mitral inflow to annular velocities (E/e’; e)
The effects of RAS inhibitors on exercise capacity and blood pressure are shown in Fig. 3. RAS inhibitors did not significantly change 6MWD (WMD [95% CI] = 1.972 [− 14.22, 18.163] m; pfix > 0.10) compared with control. RAS inhibitors significantly decreased systolic blood pressure (WMD [95% CI] = − 5.686 [− 10.84, − 0.532] mmHg; prandom < 0.05) and diastolic blood pressure (− 4.343 [− 6.750, − 1.936] mmHg; prandom < 0.001) compared with control.
No evidence of publication bias was found for each outcome either at visual inspection of funnel plots or the Egger test (all p > 0.1). A one-study-removed analysis showed that none of the individual study substantially influenced the pooled estimate for the differences in outcomes of interest between RAS inhibitors and control groups.
Among the included trials, 6 trials reported adverse outcomes during drug intervention. Although minor or moderate events including cough [14, 15, 17] and hypotension [14, 16, 17] were reported to be possibly related to RAS inhibitors, there were no serious adverse events judged related to RAS inhibitors.
Discussion
In the present study, we conducted a meta-analysis of RCTs examining the effects of RAS inhibitors on LV structure and function as well as exercise capacity in HFpEF patients. We observed that RAS inhibitors increased EF compared with control. However, there was no significant difference in changes in LV mass, LV diastolic function measures, or 6MWD between RAS inhibitors and control groups. Thus, our meta-analysis suggests that RAS inhibitors may improve LV systolic function in HFpEF patients.
Several previous meta-analyses of RCTs have reported that RAS inhibitors do not improve clinical outcomes, including cardiovascular mortality, heart failure hospitalization, all-cause mortality, or health-related quality of life in HFpEF patients [22,23,24]. To the best of our knowledge, the present meta-analysis is the first to examine the effects of RAS inhibitors on LV structure and function and exercise capacity in HFpEF patients and to show that RAS inhibitors improved LV systolic function in these patients.
Although the present meta-analysis does not provide the mechanisms for the observed potentially beneficial effect of RAS inhibitors on LV systolic function in HFpEF patients, there are several possible explanations. First, the observed increased EF may be due to the protective effect of RAS inhibitors on LV remodeling. In an animal model of hypertensive heart disease, treatment with RAS inhibitors prevented LV dilatation and retained LV contractility normal [25, 26]. Similarly, animal and human studies reported that RAS inhibitors prevented LV dilatation after myocardial infarction [25, 27]. Furthermore, hypertensive heart disease and coronary artery disease are the two most common underlying cardiac diseases in HFpEF patients [28, 29]. Second, the observed increased EF may be due to the vasodilating effect of RAS inhibitors. In the present meta-analysis, we observed that RAS inhibitors reduced blood pressure. Given the afterload dependence of EF [30], the observed increased EF may partly result from the vasodilating effect of RAS inhibitors.
In the present-meta-analysis, despite the increased EF, RAS inhibitors did not improve exercise capacity in HFpEF patients. There appear to be several possible explanations for the observations. First, LV diastolic abnormalities were reported to contribute to limited exercise capacity greater than LV systolic performance in HFpEF patients [31]. Furthermore, emerging data suggest that a limited increase in heart rate (chronotropic incompetence) as well as impaired oxygen utilization by active muscles during exercise may also play an important role in limiting exercise performance in HFpEF patients [32]. Consistent with this explanation, one meta-analysis reported that aerobic exercise training improved exercise capacity without significant change in LV function in HFpEF patients [33].
Our observed potentially beneficial effect of RAS inhibitors on LV systolic function in HFpEF patients may have an important clinical implication. There is accumulating evidence that a substantial proportion of HFpEF patients develop reduced EF over time and that these patients have a worse prognosis [34,35,36]. Although several RCTs have reported that RAS inhibitors did not improve survival in HFpEF patients [22,23,24], our meta-analysis suggests the potential prognostic benefit of RAS inhibitors for HFpEF patients with declining EF. Further studies are warranted to examine whether RAS inhibitors may improve survival in HFpEF patients with declining EF.
There are several limitations to our study. First, our meta-analysis included trials that were conducted before the definition of HFpEF was developed. Several of these trials defined preserved EF as greater than or equal to 40% or 45% [14,15,16], which is not consistent with a definition of HFpEF in recent guidelines [28, 29]. However, the mean values of baseline EF in these trials were generally higher than those in the trials which used 50% as a cutoff point of EF (Supplement Table 1). Thus, there appeared to be only a very few patients with EF < 50% included in the present meta-analysis. Second, the effects of the doses of RAS inhibitors on outcomes were not assessed. Further studies are warranted to examine whether different doses of RAS inhibitors differently impact on LV function and structure as well as exercise capacity in HFpEF patients. Third, the limited number of studies in our meta-analysis did not allow us to perform pooled analysis by drug class. Studies have reported that ARBs favorably impact exercise capacity in various populations, while the results of the impact of ACE-Is are mixed [37]. Further studies are warranted to examine the comparative effects of ARBs and ACE-Is on exercise capacity in HFpEF patients. Fourth, the number of patients included in our meta-analysis was relatively small and measures of LV diastolic function or structure were not consistently reported in the included trials. In experimental animal models of HFpEF, RAS inhibitors have been reported to exert beneficial effects on LV hypertrophy and fibrosis [38, 39]. Our observed neutral effects of RAS inhibitors on LV diastolic function and structure may be due in part to limited power. Furthermore, there is substantial variation in baseline clinical characteristics including gender, comorbidities such as atrial fibrillation and coronary artery disease, exercise capacity, echocardiographic variables, and drug treatment across the included trials. Further trials with larger sample size as well as more homogeneous baseline clinical characteristics are necessary. Finally, RCTs have strict enrollment criteria and patients with HFpEF are often elderly with many comorbidities [40]. Thus, the patients who participated in the RCTs in our meta-analysis might represent a selected group of patients that was poorly representative of patients treated in routine clinical practice. Consistent with this, the prevalence of comorbidities such as atrial fibrillation and coronary artery disease in our meta-analysis is lower than that in observational studies [40]. Further studies are necessary to examine whether our observed potential benefit of RAS inhibitors could be extended to real-world patients.
In conclusion, our meta-analysis suggests that RAS inhibitors may improve LV systolic function in HFpEF patients. Given the limited number of patients and the substantial variation in baseline clinical characteristics in the included trials, further large trials for HFpEF patients with homogeneous clinical characteristics are necessary not only to confirm our observed potential benefit of RAS inhibitors on LV systolic function but also to determine the effects on LV structure and diastolic function in HFpEF patients.
References
Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D (1999) Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 33:1948–1955
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259
Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H (2009) Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry Of Heart Failure in Cardiology (JCARE-CARD). Circ J. 73:1893–1900
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362:777–781
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359:2456–2467
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27:2338–2345
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM (2014) Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370:1383–1392
Yamamoto K, Origasa H, Hori M (2013) Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 15:110–118
Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP (2002) Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288:2144–2150
Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD (2011) Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 13:1296–1304
Hogg K, McMurray J (2005) Neurohumoral pathways in heart failure with preserved systolic function. Prog Cardiovasc Dis. 47:357–366
Wright JW, Mizutani S, Harding JW (2008) Pathways involved in the transition from hypertension to hypertrophy to heart failure. Treatment strategies. Heart Fail Rev 13:367–375
Aronow WS, Kronzon I (1993) Effect of enalapril on congestive heart failure treated with diuretics in elderly patients with prior myocardial infarction and normal left ventricular ejection fraction. Am J Cardiol. 71:602–604
Zi M, Carmichael N, Lye M (2003) The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther 17:133–139
Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE (2008) The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 94:573–580
Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM (2009) A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail 11:980–989
Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, Little WC (2010) A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail 3:477–485
Upadhya B, Brubaker PH, Morgan TM, Eggebeen JD, Jao GT, Stewart KP, Kitzman DW (2018) The effect of Aliskiren on exercise capacity in older patients with heart failure and preserved ejection fraction: a randomized, placebo-controlled, double-blind trial. Am Heart J 201:164–167
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 151:264–269 W64
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Follmann D, Elliott P, Suh I, Cutler J (1992) Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 45:769–773
Shah RV, Desai AS, Givertz MM (2010) The effect of renin-angiotensin system inhibitors on mortality and heart failure hospitalization in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis. J Card Fail 16:260–267
Khan MS, Fonarow GC, Khan H, Greene SJ, Anker SD, Gheorghiade M, Butler J (2017) Renin-angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Fail 4:402–408
Martin N, Manoharan K, Thomas J, Davies C, Lumbers RT (2018) Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 6:CD012721
Pfeffer JM, Pfeffer MA (1988) Angiotensin converting enzyme inhibition and ventricular remodeling in heart failure. Am J Med. 84:37–44
Boluyt MO, Bing OH, Lakatta EG (1995) The ageing spontaneously hypertensive rat as a model of the transition from stable compensated hypertrophy to heart failure. Eur Heart J 16(Suppl N):19–30
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101:2981–2988
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013(62):e147–e239
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016(37):2129–2200
Little WC, Braunwald E (1997) Assessment of cardiac function. In: Braunwald (ed) Heart disease: A text book of cardiovascular medicine, 5th edn. W.B.Saunders Company, Philadelphia, pp 421–444
Zile MR, Kjellstrom B, Bennett T, Cho Y, Baicu CF, Aaron MF, Abraham WT, Bourge RC, Kueffer FJ (2013) Effects of exercise on left ventricular systolic and diastolic properties in patients with heart failure and a preserved ejection fraction versus heart failure and a reduced ejection fraction. Circ Heart Fail. 6:508–516
Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW (2015) Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 119:739–744
Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD (2015) Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8:33–40
Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM (2012) Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 5:720–726
Kalogeropoulos AP, Kim S, Rawal S, Jadonath A, Tangutoori R, Georgiopoulou V (2019) Serial changes in left ventricular ejection fraction and outcomes in outpatients with heart failure and preserved ejection fraction. Am J Cardiol 124:729–735
Park JJ, Park CS, Mebazaa A, Oh IY, Park HA, Cho HJ, Lee HY, Kim KH, Yoo BS, Kang SM, Baek SH, Jeon ES, Kim JJ, Cho MC, Chae SC, Oh BH, Choi DJ (2020) Characteristics and outcomes of HFpEF with declining ejection fraction. Clin Res Cardiol 109:225–234
Simon CB, Lee-McMullen B, Phelan D, Gilkes J, Carter CS, Buford TW (2015) The renin-angiotensin system and prevention of age-related functional decline: where are we now? Age (Dordr) 37:9753
Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, Nakayama H, Otsu K, Suzuki K, Tada M, Hori M, Miwa T, Masuyama T (2003) Angiotensin II type 1 receptor blockade prevents diastolic heart failure through modulation of ca(2+) regulatory proteins and extracellular matrix. J Hypertens 21:1737–1745
Yamamoto K, Mano T, Yoshida J, Sakata Y, Nishikawa N, Nishio M, Ohtani T, Hori M, Miwa T, Masuyama T (2005) ACE inhibitor and angiotensin II type 1 receptor blocker differently regulate ventricular fibrosis in hypertensive diastolic heart failure. J Hypertens 23:393–400
Vaduganathan M, Michel A, Hall K, Mulligan C, Nodari S, Shah SJ, Senni M, Triggiani M, Butler J, Gheorghiade M (2016) Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail 18:54–65
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ohte has received lecture fees from Daiichi Sankyo Co. and grant support from Takeda Pharmaceutical Co. Ltd., Daiichi Sankyo Co., Ltd., and Otsuka Pharmaceutical Co., Ltd. Dr. Kamiya has received lecture fees from Astellas Pharma Inc. and Mochida Pharmaceutical Co., Ltd.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Table 1
(DOCX 69 kb).
Supplemental Table 2
(DOCX 67 kb).
Rights and permissions
About this article
Cite this article
Fukuta, H., Goto, T., Wakami, K. et al. Effect of renin-angiotensin system inhibition on cardiac structure and function and exercise capacity in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev 26, 1477–1484 (2021). https://doi.org/10.1007/s10741-020-09969-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09969-1