Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a class of drugs that promote urinary glucose excretion in the treatment of diabetes, have provoked large interest of scientific and professional community due to their positive and, somehow, unexpected results in the three major cardiovascular outcome trials (EMPA-REG OUTCOME trial with empagliflozin, CANVAS Program with canagliflozin, and DECLARE-TIMI 58 with dapagliflozin). In fact, along with the reduction of major adverse cardiovascular events, SGLT2 inhibitors reduced significantly hospitalization for heart failure regardless of existing atherosclerotic cardiovascular disease or a history of heart failure. The latter have reminded us of the frequent but neglected entity of diabetic cardiomyopathy which is currently poorly understood despite its great clinical importance. Physiological mechanisms responsible for the benefits of SGLT2 inhibitors are complex and multifactorial and still not well defined. Interestingly, the time frame of their effect excludes a glucose- and antiatherosclerotic-mediated effect. It would be of great importance to better understand SGLT2 inhibitor mechanisms of action since they could have a potential to be used in early stages of diabetes as cardioprotective agents. There are widely available biomarkers as well as echocardiography that are used in everyday clinical practice and could elucidate physiological mechanisms in the heart protection with SGLT2 inhibitors treatment but studies are still lacking. The purpose of this minireview is to summarize the latest concepts about SGLT2 inhibitors and its benefits regarding diabetic cardiomyopathy especially on its early stage development and to discuss controversies and potential future developments in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Great interest of scientific and professional community is provoked when the unexpected beneficial effects of certain drug occur, especially on entities that are epidemiologically very significant as type 2 diabetes.
This is currently emerging with sodium-glucose cotransporter 2 (SGLT2) inhibitors that have also reminded us of the neglected but common entity of diabetic cardiomyopathy, which is probably even more frequent than ischemic events in diabetes [1].
Before the results of the three major outcome trials with SGLT2 inhibitors (EMPA-REG OUTCOME trial with empagliflozin, CANVAS Program with canagliflozin, and DECLARE-TIMI 58 with dapagliflozin), no one could have predicted that SGLT2 inhibitors, that work to promote urinary glucose excretion in the treatment of hyperglycemia, would notably reduce mortality in patients with type 2 diabetes, possibly driven by a reduction in heart failure as opposed to atherothrombotic events [2,3,4]. But how do SGLT2 inhibitors work to reduce hospitalization for heart failure, not only in patients suffering from the disease, but also in those who (still) do not have this disease, as it was shown by DECLARE-TIMI 58 trial is still controversial. Physiological mechanisms responsible for these benefits are not yet well understood. The time frame of the effect precludes a glucose- and antiatherosclerotic-mediated effect and while struggling to understand the potential mechanisms responsible for cardioprotective effects of SGLT2 inhibitors and their clinical implications, some questions have come into focus: can SGLT2 inhibitors be used to prevent heart failure in diabetes, do SGLT2 inhibitors reverse pathological cardiac remodeling in subjects with diabetes, and can SGLT2 inhibitors be a treatment for heart failure in patients without type 2 diabetes [5,6,7,8]? So, in addition to having partly changed the US and European guidelines when it comes to patients with established atherosclerotic disease, these drugs have the potential to further be used in the sphere of prevention and cardioprotection [9].
The purpose of this minireview is to summarize the latest concepts about SGLT2 inhibitors and its benefits regarding diabetic cardiomyopathy especially on its early stage development as well as to discuss controversies and potential future developments in the field.
Discussion and future perspective
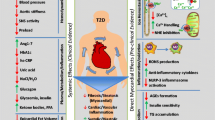
Cardiovascular disease is the primary cause of morbidity and mortality in type 2 diabetes. Diabetic patients are predisposed to a distinct cardiomyopathy (diabetic cardiomyopathy) independent of concomitant macro- and micro-vascular complication which is characterized by the development of myocardial fibrosis, cardiomyocyte hypertrophy, and apoptosis. The pathophysiology underlying diabetic cardiomyopathy is multifactorial, with elevated oxidative stress as a key contributor [10]. This clinical entity is currently poorly understood, but is obviously of great clinical importance, given the robust association of diabetes with heart failure and increased cardiovascular mortality [11]. Despite its great significance, a specific strategy to prevent or treat heart failure associated with diabetes has not been established [12].
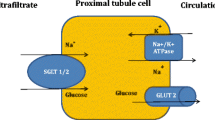
SGLT-2 inhibitors have become the topic of interest due to the benefits in cardiovascular outcome trials beyond other antidiabetic drugs. They are a novel class of antidiabetic drugs which produce glycosuric and natriuretic effects by inhibiting glucose and sodium reabsorption from the proximal tubules in the kidney. The sodium-glucose co-transporters (SGLT) are a family of active glucose transporter proteins with two major isoforms, SGLT-1 and SGLT-2. SGLT-1 expression is found in the heart, liver, small intestine, lung, and kidney, while SGLT-2 is predominantly found in the kidney [13]. According to the authors of a new meta-analysis of the three major cardiovascular outcome trials with these drugs (the EMPA-REG OUTCOME trial, the CANVAS Program, and the DECLARE-TIMI 58 trial), SGLT2 inhibitors have moderate benefits on atherosclerotic major adverse cardiovascular events that seem confined to patients with established atherosclerotic cardiovascular disease and also have robust benefits on reducing hospitalization for heart failure and progression of renal disease regardless of existing atherosclerotic cardiovascular disease or a history of heart failure (31% relative risk reduction of hospitalization for heart failure and 45% relative risk reduction of progression of renal disease) [14]. With these three large clinical outcome studies, it seems likely that the cardiovascular benefit of SGLT2 inhibitors is a class effect. However, there are some limitations when interpreting the results, especially regarding mechanistic investigations and analyses because the exact inclusion criteria and definitions of endpoints varied among the included trials, the presence of established atherosclerotic cardiovascular disease and heart failure was investigator-reported in all trials, and echocardiographic or biomarker studies were not performed to evaluate, for example, specific cardiac phenotypes such as heart failure with a preserved ejection fraction or heart failure with a reduced ejection fraction [8]. Moreover, it is also possible that in those trials a certain number of patients had asymptomatic left ventricular dysfunction that represents either systolic or diastolic abnormalities in the absence of clinically detectable cardiac disease and is highly prevalent in diabetic patients, with estimates ranging from 50 to 70% [15, 16].
The mechanisms underlying cardiovascular and renal protection by SGLT2 inhibitors in diabetes are multifactorial, complex, and not completely understood. The current hypotheses of possible mechanisms of action to explain cardiac protection and effect on cardiac performance regarding heart failure by SGLT2 inhibitors are discussed below:
-
1.
Glucose excretion caused by SGLT2 inhibitors results in loss of calories and subsequently decreased body weight [17]. Weight loss reflects both reduced fat and fluid loss observed with SGLT2 inhibition and partially contributes to the blood pressure reduction noticed with SGLT2 inhibitor therapy [18].
-
2.
A metabolic study in diabetic patients demonstrated that lowering elevated blood glucose levels with empagliflozin reduced glucose toxicity and improved β-cell function and insulin sensitivity [19]. Decreasing chronic hyperglycemia by promoting urinary glucose excretion (opposite to improving glucose uptake caused by insulin, incretins, and thiazolidinediones) may reduce the effects of glucotoxicity on the heart, thus reducing the risk of heart failure in diabetic patients. Decreases in glucose flux associated with SGLT2 inhibitors could also modulate inflammatory processes as inflammatory M1 macrophages preferentially utilize glucose through the glycolysis pathway. In this way, SGLT2 inhibitors may act to dampen the inflammatory response, which is known to contribute to diabetic cardiomyopathy [6].
-
3.
Therapy with SGLT2 inhibitors is associated with reductions in plasma uric acid concentrations and there are evidences suggesting that uric acid levels can predict an adverse prognosis in heart failure [20, 21]. There are also some data showing that uric acid may play a causative role in hypertension, metabolic syndrome, endothelial dysfunction, and renal damage [22]. Interestingly, the hypertension associated with hyperuricemia in rats is linked to reduced expression of macula densa neuronal nitric oxide synthase which normally affects cardiac function through modulating cardiac excitation-contraction coupling by facilitating sarcoplasmic reticulum calcium release [23]. Decreasing uric acid could work through these mechanisms in cardiovascular protection. Nevertheless, the potential benefits of uric acid reduction with SGLT2 inhibitors need further investigations.
-
4.
The heart can use different energy sources. Diabetic cardiomyopathy is characterized by increased fatty acid uptake and oxidation causing alterations in cardiac mitochondrial energy metabolism thus contributing to contractile dysfunction and reduced cardiac efficiency [24]. In the setting of diabetes with heart failure, ketone bodies can act as a “super fuel” by releasing energy more efficiently than glucose or fatty acids and it has been shown that SGLT2 inhibitors can switch metabolism from glucose to fatty acid oxidation with increased synthesis of ketones [25, 26]. This could contribute to increased heart function during SGLT2 inhibitor therapy. Furthermore, ketone bodies have a significant role in epigenetic and cellular signaling and can work as antioxidants and anti-inflammatory molecules reducing oxidative stress and inflammation, which are key contributors to the development of diabetic cardiomyopathy [27,28,29,30]. At present, whether SGLT2 inhibitors might influence cardiac function through modulating ketone bodies needs more studies [31].
-
5.
Hemodynamic effects of SGLT2 inhibitors via natriuresis and osmotic diuresis cause a reduction in blood pressure and intravascular volume that decrease cardiac load (simultaneously reduce both preload and afterload). Blood pressure could also be altered through reduced arterial stiffness and, as already mentioned, through weight loss [32]. Reducing the load of the heart could give the rapid results observed with SGLT2 inhibition. However, how could this affect patients without heart failure at baseline remains to be clarified. Furthermore, from previous studies, it is known that commonly used diuretics were not associated with reduced cardiovascular death [6].
-
6.
Based on the observation that the reduction in blood pressure seen with SGLT2 inhibitors was not associated with increased heart rate, it has been proposed that the diuretic effect of SGLT2 inhibitors does not activate neurohumoral factors and in this way is beneficial for heart failure [33].
-
7.
An increase in glucagon level noticed with SGLT2 inhibitor therapy has been proposed as a possible contributor to the reduced cardiovascular risk considering its inotropic effect and consequently better cardiac performance. This effect is not abolished by autonomic blockade, which implies a specific action of the hormone on the heart independent of the catecholamine release induced by glucagon [34,35,36]. Furthermore, this inotropic action in human heart is well represented in the non-failing heart, but declines in the failing heart. Therefore, the plasma levels of glucagon could contribute to the maintenance of the heart function when the heart failure is in its early stages. Glucagon has known antiarrhythmogenic effect as well [37]. Paradoxically, this hormone has been always considered deleterious in diabetes so consequences of changes in glucagon levels remain to be more clearly defined.
-
8.
There are some discrepancies in the renin-angiotensin-aldosterone system (RAAS) activity and SGLT2 inhibition. Studies in type 1 and type 2 diabetes found that RAAS activity was increased maybe due to a compensatory response to decreased blood pressure, volume contraction, and natriuresis. However, SGLT2 inhibition leads to an effect consistent with afferent vasoconstriction as opposed to efferent vasodilation associated with RAAS inhibition [38, 39]. SGLT2 inhibition-induced volume contraction is accompanied by an increase in circulating RAAS mediators which could be significant for patients concomitantly treated with RAAS inhibitors because combined use of RAAS and SGLT2 inhibitors may lead to synergistic beneficial effects. Future studies are needed to evaluate the combined strategy of RAAS and SGLT2 inhibitors [40].
-
9.
Elevation of hematocrit and erythropoietin has been reported during treatment with SGLT2 inhibitors and that may be connected to increased oxygen delivery from SGLT2 inhibitor-associated hemoconcentration which could increase cardiac efficiency [6, 39]. Moreover, the increase in hematocrit in diabetic patients receiving SGLT2 inhibitors could be regarded as a surrogate marker of renal recovery from tubulointerstitial injury [33].
-
10.
Anti-oxidative, anti-inflammatory, antifibrotic, and anti-apoptotic effects of SGLT2 inhibitors have been shown in experimental diabetic cardiomyopathy models. They all play an important role to the development of diabetic cardiomyopathy [13].
-
11.
SGLT2 inhibitors may directly inhibit sodium-hydrogen exchange, which may lead to a reduction in cardiac remodeling, injury, hypertrophy, fibrosis, and systolic dysfunction [41]. Baartscheer et al. showed that the SGLT2 inhibitor empagliflozin inhibited cardiomyocyte sodium-hydrogen exchanger and, through this mechanism, reduced cytoplasmic sodium and calcium levels, while increasing mitochondrial calcium levels [42]. It is an important finding since heart failure is associated with intracellular cardiomyocyte sodium and calcium loading [43, 44].
-
12.
It has been proposed that SGLT2 inhibitors may reestablish the balance between pro- and anti-inflammatory adipokines [8]. Adipose tissue is namely an active paracrine and endocrine organ that releases several active mediators that influence not only body weight homeostasis but also atherosclerosis, insulin resistance, diabetes, inflammation, coagulation, and fibrinolysis [45]. Perivascular and epicardial fat has also been implicated in the origin of heart failure, through altered paracrine regulation of adipokines on the myocardium [46]. It has been shown that canagliflozin reduces serum leptin levels and increases the levels of the anti-inflammatory adipokine adiponectin when compared with the sulfonylurea glimepiride. Additionally, other studies have demonstrated that dapagliflozin reduces epicardial adipose tissue volume [47, 48].
-
13.
Renoprotection of SGLT2 inhibitors together with the natriuresis they induce could explain the reduction in hospitalization for heart failure [49]. Furthermore, reductions in both the hospitalization for heart failure and progression of kidney disease and their downstream complications may reduce the risk of cardiovascular death [14].
-
14.
There are interesting electrophysiological findings with SGLT2 inhibitors which may be linked to the reduction of fatal arrhythmias and thus reduced cardiovascular events in diabetic patients. Namely, in a retrospective study by Sato et al. it was found that treatment with SGLT2 inhibitors reverses ventricular repolarization heterogeneity in people with type 2 diabetes, independent of their effect on glycemic control [50]. One should also consider glucagon anti-arrhythmogenic effects as mentioned already.
Better understanding of SGLT2 inhibitor mechanisms of action as well as a better knowledge of risk factors and evolution of diabetic cardiomyopathy would allow the design of effective prevention strategies [51]. In order to provide information on the mechanisms potentially involved in the diabetic cardiac dysfunctions and possible pharmacological interventions in subjects at risk, larger studies with pathway-specific biomarkers that are validated against hard endpoints and echocardiographic evaluation are needed. Biomarkers and echocardiography are broadly available so changes in myocardial structure and function could be detected before the appearance of heart failure symptoms, as well as being of prognostic value [52]. When planning cardiovascular studies in type 2 diabetes, selection of biomarkers is a complex issue; the selected biomarkers should cover all aspects of the known pathophysiology of the cardiovascular disease and their levels should also be modifiable by therapy since heart failure is not the result of a single pathophysiologic process. Indeed, it is a syndrome initiated by cardiac pressure or/and volume overload characterized by a broad range of pathophysiological presentation, properties, and potential outcomes [53, 54].
For example, a well-validated and widely used biomarker N-terminal pro-brain natriuretic peptide (NT-proBNP) that is released into the bloodstream in direct proportion to the mechanical stress of the myocardium has been proved useful in screening asymptomatic subjects at risk of developing heart failure, such as the elderly and those with diabetes, asymptomatic coronary artery disease, and hypertension [55]. Despite this, in experimental studies and studies on humans, the effects of SGLT2 inhibitors on plasma NT-proBNP have been inconsistent. In fact, in some studies, therapy with SGLT2 inhibitors has reduced the value of NT-proBNP, but in the others, NT-proBNP values were increased or unchanged during SGLT2 inhibitor treatment [39, 56,57,58]. Considering its significance, especially as a marker of risk, it would be appropriate to determine the effect of SGLT2 inhibitors on NT-proBNP and other natriuretic peptides in asymptomatic individuals and in those with developed heart failure symptoms. Interestingly, atrial natriuretic peptide (ANP) and endothelin-3 are endogenous vasoactive factors that exert potent diuretic and natriuretic actions, and it has been shown that they inhibit SGLT2 activity in the kidney [59]. ANP release is impaired in asymptomatic patients with cardiomyopathy. If SGLT2 transporters are already inhibited with SGLT2 inhibitors, although speculative, ANP could exert and enhance its other functions like renin secretion rate and aldosterone production inhibition or modulate arterial and cardiac baroreflex mechanism, and SGLT2 inhibition treatment could possibly restore the ANP secretagogue response in heart failure as it was shown with RAAS inhibition [60]. To the best of our knowledge, this hypothesis has not been discussed yet.
There are other widely available biomarkers that could also elucidate physiological mechanisms in the heart protection with SGLT inhibitor treatment. Such an example is C-reactive protein (CRP). Multivariate analysis indicated that increased CRP level is an independent predictor of adverse outcomes in patients with acute or chronic heart failure and, for example, in the Framingham Heart Study, CRP was noted to identify asymptomatic older subjects who were at high risk of the future development of heart failure [55]. Furthermore, there are emerging data on adipose tissue as a source of plasma CRP so it has been addressed as a novel proatherogenic plasma adipokine [45]. Despite this, data on CRP and other inflammatory markers are relatively scarce in humans and further studies in diabetes patients with specific assessment of inflammatory markers are still necessary to determine the contribution of the anti-inflammatory action of SGLT2 inhibitors to the reduction of cardiovascular complications [61]. Together with inflammation, oxidative stress also plays an important role in diabetic myocardial damage. Since it is difficult to measure reactive oxygen species directly in humans, indirect markers of oxidative stress have been sought like myeloperoxidase (MPO). The levels of plasma MPO correlate with the severity of heart failure and are an independent predictor of death from heart failure [55]. The results of prospective studies (showing for example that high MPO levels were able to predict increased risk of developing coronary artery disease in healthy individuals), as well as the ready availability of commercial assays, make MPO one of the most promising biomarkers of oxidative stress for cardiologists, but was not yet evaluated in studies with SGLT2 inhibitors [62].
SGLT-2 inhibitors have also shown improvement in cardiac function in diabetic cardiomyopathy models and myocardial ischemic models of mice and rats but information with respect to SGLT2 inhibitors effect on myocardial function is very limited in humans [13, 51]. To prevent the progression of heart failure in diabetic patients, a sensitive method of diagnosing the presence of diabetic cardiomyopathy is important. Still, clinical diagnostic methods to monitor myocardial disease progression in diabetic patients are not well established [63]. A recent sensitive and non-invasive method for the evaluation of myocardial systolic and diastolic function—2D-speckle-tracking echocardiography (STE)—has been developed and validated (against tissue Doppler, sonomicrometry and cardiac magnetic resonance) in large number of patients, including subjects with type 2 diabetes and has been shown that among type 2 diabetes patients with a normal 2D ejection fraction, an abnormal global longitudinal strain measured by STE has been found in 30–50% of the subjects. That means that STE could be used to detect subclinical cardiac dysfunction in diabetic patients [51]. More studies on cardiac function and SGLT2 inhibitors, especially using STE are needed.
In addition to diabetic cardiomyopathy, there are several ongoing studies at the moment addressing the issue of SGLT2 inhibitors in individuals with chronic heart failure and chronic kidney disease without established diabetes [64]. Lastly, we would also encourage more studies on mechanisms of arrhythmias and SGLT2 inhibition as well as the effects of SGLT2 inhibitors on the sympathetic nervous system and neurohumoral activation.
It would be of great importance to better understand SGLT2 inhibitor mechanisms of action since they could have a potential to be used in early stages of diabetes as cardioprotective agents. Biomarkers and echocardiographic parameters (especially STE) could be used to monitor different stages of diabetic cardiomyopathy and the detection of the relative changes in these parameters from baseline rather than an absolute cut-off value as in cardio-oncology might be helpful.
References
Greene SJ, Vaduganathan M, Khan MS, Bakris GL, Weir MR, Seltzer JH, Sattar N, McGuire DK, Januzzi JL, Stockbridge N, Butler J (2018) Prevalent and incident heart failure in cardiovascular outcome trials of patients with type 2 diabetes. J Am Coll Cardiol 71:1379–1390
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380(4):347–357
Verma S, McMurray JJV, Cherney DZI (2017) The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol 2(9):939–940
Staels B (2017) Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Med 130(6S):S30–S39
Byrne NJ, Parajuli N, Levasseur JL et al (2017) Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC: Basic Translational Sci 2(4):347–354
Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61(10):2108–2117
American Diabetes Association (2018) Standards of medical care in diabetes-2018. Diabetes Care 41(Suppl.1):S1–S2
Huynh K, Bernardo BC, McMullen JR, Ritchie RH (2014) Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacology Ther 42(3):375–415
Levelt E, Gulsin G, Neubauer S, McCann GP (2018) Mechanisms in endocrinology: diabetic cardiomyopathy: pathophysiology and potential metabolic interventions state of the art review. Eur J Endocrinol 178(4):R127–R139
Takayuki M, Satoshi Y, Hidemichi K, Tetsuji M (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18(2):149–166
Lahnwong S, Chattipakorn SC, Chattipakorn N (2018) Potential mechanisms responsible for cardioprotective efects of sodium–glucose co-transporter 2 inhibitors. Cardiovasc Diabetol 17(1):101
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166):31–39
Ciof G, Giorda CB, Chinali M et al (2012) Analysis of midwall shortening reveals high prevalence of left ventricular myocardial dysfunction in patients with diabetes mellitus: the DYDA study. Eur J Prev Cardiol 19(5):935–943
Faden G, Faganello G, De Feo S et al (2013) The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: data from the SHORTWAVE study. Diabetes Res Clin Pract 101(3):309–316
Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S (2014) Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 16(2):159–169
DeFronzo RA (2016) The EMPA-REG study: what has it told to us? J Diabetes Complicat 30(1):1–2
Kern M, Kloting N, Mark M, Mayoux E, Klein T, Bluher M (2016) The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism 65(2):114–123
Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J (2013) Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med 125:181–189
Kittleson MM, St. John ME, Bead V et al (2007) Increased levels of uric acid predict haemodynamic compromise in patients with heart failure independentely of B-type natriuretic peptide levels. Heart 93:365–367
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359(17):1811–1821
Hare JM, Johnson RJ (2003) Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 107:1951–1953
Fillmore N, Mori J, Lopaschuk GD (2014) Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171:2080–2090
Mudaliar S, Alloju S, Henry RR (2016) Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 39:1115–1122
Daniele G, Xiong J, Solis-Herrera C, Merovci A, Eldor R, Tripathy D, DeFronzo RA, Norton L, Abdul-Ghani M (2016) Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care 39:2036–2041
Newman JC, Verdin E (2014) Ketone bodies as signaling metabolites. Trends Endocrinol Metab 25:42–52
Newman JC, Verdin E (2014) Beta-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract 106(2):173–181
Haces ML, Hernandez-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L (2008) Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol 211(1):85–96
Prattichizzo F, De Nigris V, Micheloni S, La Sala L, Ceriello A (2018) Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: is low-grade inflammation the neglected component? Diabetes Obes Metab 20(11):2515–2522
Kutoh E, Hayashi J (2019) Effect of canagliflozin on heart function involving ketone bodies in patients with type 2 diabetes. Drug Res (Stuttg) 69(05):297–300
Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA (2016) SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPAREG OUTCOME study. Diabetes Care 39:717–725
Sano M (2018) A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol 71(5):471–476
Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124:499–508
Jones BJ, Tan T, Bloom SR (2012) Minireview: glucagon in stress and energy homeostasis. Endocrinology 153:1049–1054
Regan TJ, Lehan PH, Henneman DH, Behar A, Hellems HK (1964) Myocardial, metabolic and contractile response to glucagon and epinephrine. J Lab Clin Med 63:638–664
Ceriello A, Genovese S, Mannucci E, Gronda E (2016) Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol 15:123
Cherney DZ, Perkins BA, Soleymanlou N et al (2014) Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int 86:1057–1058
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J (2013) Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15:853–862
Cherney DZ, Perkins BA, Soleymanlou N et al (2014) Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 129:587–597
Packer M, Anker SD, Butler J, Filippatos G, Zannad F (2017) Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2:1025–1029
Baartscheer A, Schumacher CA, Wust RC et al (2017) Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60:568–573
Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O'Rourke B, Maack C (2010) Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 121:1606–1613
Baartscheer A, Schumacher CA, Borren MM, Belterman CN, Coronel R, Fiolet JW (2003) Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res 57:1015–1024
Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S (2005) Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol 288(5):H2031–H2041
Patel VB, Shah S, Verma S, Oudit GY (2017) Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev 22:889–902
Timothy Garvey W, Van Gaal L, Leiter LA et al (2018) Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 85:32–37
Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M (2018) The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 17(1):6
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ (2016) Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134:752–772
Sato T, Miki T, Ohnishi H, Yamashita T, Takada A, Yano T, Tanno M, Tsuchida A, Miura T (2017) Effect of sodium–glucose co-transporter-2 inhibitors on impaired ventricular repolarization in people with type 2 diabetes. Diabet Med 34(10):1367–1371
Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V (2017) Impact of empagliflozin on subclinical left ventricular dysfunction and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovasc Dabet 16(1):130
Marwick TH, Ritchie R, Shaw JE, Kaye D (2018) Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol 71(3):339–351
Baldassarre MPA, Andersen A, Consoli A, Knop FK, Vilsboll T (2018) Cardiovascular biomarkers in clinical studies of type 2 diabetes. Diabetes Obes Metab 20(6):1350–1360
van Kimmenade RR, Januzzi JL Jr (2012) Emerging biomarkers in heart failure. Clin Chem 58(1):127–138
Braunwald E (2008) Biomarkers in heart failure. N Engl J Med 358:2148–2159
Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, Arakawa K, Togo M, Inagaki N, Kaku K (2017) Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther 34(2):436–451
Eickhoff MK, Dekkers CCJ, Kramers BJ, Laverman GD, Frimodt-Moller M, Jorgensen NR, Faber J et al (2019) Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med 8(6):779
Januzzi JL, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, Davies MJ (2017) Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol 70(6):704–712
Majowicz MP, Gonzalez Bosc LV, Albertoni Borghese MF, Delgado MF, Ortiz MC, Sterin Speziale N, Vidal NA (2003) Atrial natriuretic peptide and endothelin-3 target renal sodium-glucose cotransporter. Peptides 24(12):1971–1976
Volpe M, Carnovali M, Mastromarino V (2016) The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci 130(2):57–77
Bonnet F, Scheen AJ (2018) Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential diabetes complications and cardiovascular disease. Diabetes Metab 44(6):457–464
Ho E, Galougahi KK, Liu CC, Bhindi R, Figtree GA (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 1(1):483–491
Enomoto M, Ishizu T, Seo Y, Yamamoto M, Suzuki H, Shimano H, Kawakami Y, Aonuma K (2015) Subendocardial systolic dysfunction in asymptomatic normotensive diabetic patients. Circ J 79:1749–1755
https://clinicaltrials.gov/ Accessed 5 July 2019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grubić Rotkvić, P., Cigrovski Berković, M., Bulj, N. et al. Minireview: are SGLT2 inhibitors heart savers in diabetes?. Heart Fail Rev 25, 899–905 (2020). https://doi.org/10.1007/s10741-019-09849-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09849-3