Abstract
At the beginning of the twentieth century Haeckel’s biogenetic law was widely questioned. On the one hand, there were those who wanted to dismiss it altogether: ontogeny and phylogeny did not have any systematic or interesting relation. On the other hand, there were those who sought to revise it. They argued that while Haeckel’s recapitulationism might have been erroneous, this should not deter the research over the relation between evolution and development. The British embryologist Walter Garstang was one of the main figures on the “revisionists” side. In this paper, I first situate Garstang’s contribution to embryology and evolution within the extraordinarily creative period of the first three decades of the twentieth century. Then, I review some of Garstang’s specific ideas in detail, especially his most well-known 1922 paper “The Theory of Recapitulation.” Finally, I look at how the demise of the biogenetic law in light of Garstang’s views—as well as from the perspective of contemporary developmental evolution—should be understood. My main concern is not about the dismissal of Haeckel’s law or the sidelining of embryology in the twentieth-century evolutionary biology. I am rather interested in exploring why Garstang’s revised version of biogenetic law—which was entirely consistent with the neo-Darwinian perspective underpinning the Modern synthesis—did not spur a major new agenda in evolutionary biology after the 1930s.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“It has long been thought that the relations between embryology and evolution were governed by the theory of recapitulation, that the discrediting of this theory has resulted in an impression that those who reject it also reject any relation between evolution and embryology. This is far from being true” (de Beer 1958, p. 15). With these words, the British embryologist Gavin de Beer introduced his short essay on Darwin’s views on embryology and evolution. The paper emphasized how the demise of Haeckel’s biogenetic law did not imply the repudiation of any relationship between ontogeny and phylogeny. De Beer noted that Darwin had already “…imagined that evolutionary novelties arise during development in the ancestor and tend to appear at the same stage of development in the descendant, which is the point of view adopted today” (de Beer 1958, p. 17). Yet, de Beer also recognized that Darwin was not the first to point this out. The theoretical relation between embryology and evolution predated Darwin and outlived him. It was hinted at by Étienne Geoffroy Saint-Hilaire (1772–1844), developed by Johann Friedrich Meckel (1781–1833) and Étienne Serres (1786–1868), re-emphasized by Fritz Müller (1821–1897) in Für Darwin (1864), and widely popularized by Ernst Haeckel (1834–1919) (see Breidbach and Ghiselin 2007). Then, in the twentieth century, it was updated and reformulated by Alexi Sewertzoff, Adolf Naef, and Walter Garstang, among others (Ridley 1986; Hall 2000; Churchill 2007; Holland 2011; Olsson et al. 2017). When Stephen Jay Gould reopened the Pandora’s box of recapitulationism in 1977, he claimed that, although the idea of parallelism had been so significant for the history of biology, it was shunned or ignored by most evolutionary biologists after the Second World War. This, he believed, was related to some extent to the spectacular collapse of Haeckel’s version of biogenetic law, the encompassing theory that had dominated biology for half a century.
With the wisdom of historical hindsight, we can argue that the demise of Haeckel’s biogenetic law since the late nineteenth century produced two opposite reactions. On the one hand, there were those who wanted to dismiss it altogether: ontogeny and phylogeny did not have any systematic or interesting relation. Accordingly, embryology and evolutionary biology were two fields with different goals and methods. On the other hand, there were those who did not want to throw the baby out with the bathwater and their argument was straightforward: while Haeckel's law might have been erroneous, this should not deter the research over the relation between evolution and development. The biogenetic law should not be eliminated but amended. It had to evolve along the trajectory first suggested by Karl Ernst von Baer’s insights, namely, that the similarity we observe among the early ontogenetic stages of embryos of different species may not reveal phylogenetic unfolding but rather some kind of common forms shared by all Metazoa.
Of course, von Baer’s profound insights were highly valued and frequently invoked through both the nineteenth and the early twentieth centuries. Yet, many of von Baer’s competent readers believed that, while he was right in denying recapitulation, he was entirely wrong in dismissing evolution. His antievolutionary inclinations needed to be updated in line with new post-Haeckelian sensibilities. And this was precisely the strategy followed by many of those who tried to rescue embryology from Haeckelism and, therefore, from oblivion. In fact, rescuers like de Beer entirely retooled the relation between evolution and development, inverting their causal link. The early embryonic stage common to many different species could be reinterpreted as ancestral developmental processes frozen in time, not as adult forms fixed and condensed in earlier ontogenetic cycles. If that were the case, embryologists could explore the possibility that small changes in early developmental paths might have induced great changes in adult morphological forms. Small ontogenetic mutations could be the cause of evolutionary novelties, while phylogeny itself had to be seen as a collection of developmental deviations, not as a set of terminal additions. This was indeed the conceptual path taken by the British embryologist Walter Garstang, who is the subject of this paper.
Walter Garstang was born in Blackburn, England in 1868. In 1884, he went up to Oxford to study medicine but graduated in zoology under Henry Nottidge Moseley’s supervision. From 1888, he worked at the Plymouth Marine Laboratory as assistant of the Oxford zoologist Gilbert Bourne. In Plymouth, Garstang met Ray Lancaster: “…the man who was largely to rule my destinies for the next 20 years,” he remembered in a fragment of his autobiography (Hardy 1951, p. 561). In the following years, Garstang became one of the leading marine biologists in the UK and, in 1907, he was appointed chair of zoology at the University of Leeds. In 1912, in cooperation with Alfred Denny, a zoologist at the University of Sheffield, he established a marine laboratory for the use of their students at Robin Hood’s Bay, near Whitby on the North Yorkshire coast (Eastham 1949, p. 519). Garstang spent the rest of his academic life in Leeds. Alister C. Hardy (1896–1985), the marine biologist who was deeply inspired by Garstang’s later views on evolution, divided Garstang’s life into three periods. The first entirely focused on marine biology; the second dedicated to fishery; and the third, while at Leeds, devoted to addressing more general problems in biology, in particular the relation between ontogeny and phylogeny (Hardy 1962). In this paper, I will mainly be concerned with the last period.
Garstang has little in common with the traditional historical heroes of science. Unlike Darwin or Haeckel, or even de Beer himself, he never published an ambitious synthesis in the form of a seminal monograph that could disseminate his views, even among a lay public. His ideas are scattered in published addresses and in very technical (and sometimes quite dry and descriptive) reports. He did not leave any substantial legacy and his life was not enlivened by extraordinary events. To the historian approaching his work and his biography, he might appear as a somewhat modest figure. Nonetheless, there are at least three reasons why Garstang’s work should be reconsidered. First, it is interesting in itself and biologists can still learn from it. Not surprisingly, in the last few decades, and especially with the rise of evolutionary developmental biology, there has been a revival of interest among embryologists in his work (see Hall 2000; Holland 2011). Second, it complicates the historiography in an interesting way and forces us to update received narratives. It is often assumed that during the first decades of the twentieth century, most embryologists rejected Darwinism and Mendelian genetics, which many regard as a major reason why embryology itself was sidelined by the architects of modern evolutionary biology (Provine 1980; Hamburger 1980). However, Garstang, like de Beer, was not anti-Darwinian and had no problem accepting, and even using, the findings of genetics. And, third, and most importantly, Garstang’s work says something very interesting about the scientific and social environment that determined the success or failure of different theoretical alternatives. His story reveals some of the epistemic values that shaped evolutionary biology (and biology in general) after the First World War.
In this paper, I will first situate Garstang’s contribution to embryology and evolution within the extraordinarily creative period of the first three decades of the twentieth century. Then, I will review some of Garstang’s specific ideas in detail, especially his most well-known 1922 paper “The Theory of Recapitulation.” Finally, I will look at how the demise of the biogenetic law in light of Garstang’s views—as well as from the perspective of contemporary developmental evolution—should be understood. My main concern in this paper is not with the dismissal of Haeckel’s law or the sidelining of embryology in the twentieth-century evolutionary biology (although both themes are part of my narrative); I am rather interested in exploring why Garstang’s revised version of biogenetic law—which was entirely consistent with the neo-Darwinian perspective underpinning the Modern synthesis—did not spur a major new agenda in evolutionary biology after the 1930s.Footnote 1 In 1977, for example, Stephen Jay Gould made an interesting prediction. He observed that the biology of the future would be a synthesis between molecular and evolutionary biology via the intermediary of development. If evolution is mostly about regulatory changes in development, future evolutionary biologists could not overlook embryology (Gould 1977, pp. 408–409). Yet in 1922, Garstang reached a similar conclusion: future evolutionary biologists could not dismiss development if they wanted to understand how organisms evolve. Garstang’s view of evolution was not entirely dissimilar to Gould’s: both conceived of evolution as the synthesis of development, heredity (genetics), and adaptation. However, while the emergence of evo-devo after the 1980s vindicated Gould’s prophecy, Garstang’s earlier expectations were fruitless. We can therefore reasonably ask, why did Garstang’s expectations fail whereas Gould’s succeeded? In what follows, I aim to provide a framework for answering this question.
Garstang’s Weird Wonder
The first three decades of the twentieth century were extraordinary times for biology. Many trends that had originated in the previous century reached their peak in the heated controversies affecting an increasingly transnational community of zoologists. The eclipse of Darwinism was underway, giving rise to multiple alternative perspectives. Lamarckism was alive and kicking in various versions. Experimental embryology, from Wilhelm Roux to Hans Driesch, had introduced perplexing new views on the nature of early development, feeding interminable philosophical debates. Mendel’s laws had been “rediscovered,” Theodor Bovery and Walter Sutton had laid the foundation for the chromosome theory of heredity, and Thomas Hunt Morgan was about to write one of the most exciting chapters in the history of experimental biology. Alexej Nikolajevich Sewertzoff’s evolutionary morphology prompted a host of new concepts illustrating how speciation might happen (Levit et al. 2004; Olsson et al. 2010). The Scottish zoologist D’Arcy Thompson published the first edition of On Growth and Form (1917), which inspired Julian Huxley a decade later in his studies on allometry (Esposito 2014). In the 1920s, Hans Spemann and Hilde Mangold identified what they called the organizer, and through the 1920s and 1930s, many scholars, including Joseph Needham and Conrad Hal Waddington, struggled to identify its chemical nature, fostering, at the same time, the development of biochemical embryology in England (Horder 2008). Finally, from the late 1920s, J. B. S. Haldane, Ronald Fisher, and Sewall Wright laid the foundations of the new Synthetic Theory of evolution, linking Neo-Darwinism with population genetics (Allen 1975).
This is only a short and very partial list of developments illustrating how many schools, doctrines, theories, and approaches emerged during the first decades of the twentieth century. Indeed, biologists quarreled more than ever about what it meant to do good biology, and therefore about how to test theories, perform experiments, observations and understand heredity, development and evolution. When we attempt to map the history of biology of the early twentieth century, we realize that it encompasses an extraordinarily complex landscape composed of many alternative views. It is within this context that I will situate Garstang’s own speculations.
In order to tease out the theoretical and experimental richness of this creative period, it might be helpful to revisit the conceptual experiment that Gould proposed in Wonderful Life (1989). Gould imagined that if the tape of life could be rewound back to the Cambrian period in Canada’s Burgess Shale, we would observe many different “weird wonders”—strange organisms—which, although well adapted to their environment, did not survive. We would never imagine that, in the midst of such biodiversity, the little primitive chordate paleontologists called Pikaia would be so successful in the following geological periods. Other weird wonders could have survived, and, today, fictitious descendants of Onychophorans could be asking perplexingly why chordates did not make the grade. If the evolutionary history of life is contingent, as Gould emphasized with his rewind experiment, then we could use the same thought experiment on human history and, in particular, on the history of science. We might explore, for example, the possibility that something like a Cambrian explosion in evolutionary biology happened between the late nineteenth and early twentieth-centuries. Then, the vast and chaotic proliferation of theories and perspectives that had characterized the first decades of the twentieth-century life sciences was increasingly streamlined and reduced after the Second World War. Garstang’s stance was one of those that barely survived (others simply became extinct) along with other perspectives. Following Gould’s conceptual experiment, we could also imagine that if a contemporary evo-devo biologist could travel back to the 1920s, she would probably bet on Garstang’s success, although we know, with hindsight, that such a gamble would fail.Footnote 2 Yet, before examining why a hypothetical time traveling biologist would eventually bet on the wrong horse, we need to contextualize Garstang’s work as well as pinpoint his real aims, allies, and critical targets.
First, Garstang believed in the force of natural selection as well as the explicative soundness of genetics; therefore, his targets were neither Darwinians or geneticists. Furthermore, unlike many of his peers, he was not fascinated by sweeping generalizations and overarching theories explaining the origin and evolution of the cosmos and life on earth. He was never involved in the metaphysical discussions praising or disparaging vitalism, mechanism, organicism or what William Emerson Ritter called elementalism (Esposito 2015). Unlike many of his colleagues, Garstang was simply unconcerned by many philosophical debates surrounding the more technical issues he was exploring. And although his explicit adversaries were Haeckel and Haeckelians (for instance, Ernest MacBride), Garstang’s criticisms of them should not be overemphasized. In fact, as Mark Ridley aptly observed, “The biogenetic law was completely dead in Oxford by 1905. The embryologists, the experimentalists [J. W.] Jenkinson, the phylogeneticist [E. S.] Goodrich, and [G. E.] Smith who did both, unanimously pronounced recapitulation an error” (Ridley 1986, p. 61). As an Oxonian, Garstang did not aim to attack straw men: it is unlikely that a sophisticated embryologist such as he was involved in a crusade against the few surviving mavericks such as MacBride, who still stuck with outworn science. Rather, Garstang’s anti-Haeckelianism was much subtler and more strategic: it sought to rescue embryology from the pernicious influences of the earlier century, while advancing and strengthening its credentials as an experimental science that could still say something about the causes of evolution. In short, Garstang stood for a renewed kind of von Baerianism adapted to Darwinian biology. He aimed for an effective synthesis between the new experimental embryology (which had already dismissed recapitulation at the end of the nineteenth century) and the new science of heredity, believing that both could shed light on the causes of evolution.
Hence, while Garstang’s apparent enemies were Haeckel and Haeckelians, I defend the view that his real enemies were younger biologists who tended to dismiss entirely the parallelism between ontogeny and phylogeny on the assumption that the whole Haeckelian agenda was an irremediable fraud. Although Garstang did not explicitly mention any scholar in particular, I argue that Thomas Hunt Morgan and his group are the most likely candidates. Garstang not only considered Morgan´s Drosophila experiments as good examples that could be turned against outdated forms of recapitulationism, but he also deemed those experiments as evidence showing why development still mattered for understanding evolution. To Garstang, Morgan´s theory of the gene was not in contradiction with an updated form of embryology and it was an essential complement that could provide a better understanding of evolution. Accordingly, Garstang’s strategy for redeeming embryology from its troubles was both subtle and ingenious. First, he reviewed the original version of Haeckel’s biogenetic law in order to show that there was a grain of truth in it, arguing that abandoning Haeckel’s recapitulationism did not imply the rejection of all kind of parallelism between ontogeny and phylogeny. The relation between embryology and evolution still stood, but it was embryology that caused, and therefore explained, evolution. Second, de-Haeckelized recapitulationist embryology, updated by Darwinism and Mendelism (and Morgan’s genetics), was presented as one possible answer to the origins of species. Small, inherited mutations, he claimed, could produce important changes in the earliest stages of development that, under the pressure of natural selection, could foster great leaps in evolution.
Garstang’s proposal was ambitious and promising, but it did underestimate the force of the scientific trend that had first sidelined Haeckel’s laws from mainstream embryology and then split it from evolutionary biology. Saving embryology from Haeckel’s biogenetic jaws, I argue, was not enough. Many elements conspired against developmental biology as the science of the causes of evolution, and Garstang’s perspective fell in the category of weird wonders that were marginalized (or in other cases forgotten) within the history of twentieth-century evolutionary biology. But before the question can be posed of why Garstang’s perspective was sidelined, his weird wonder must be presented in more detail.
Repetition and Creation
Garstang had been speculating on the nature of the relation between ontogeny and phylogeny since the late nineteenth century. Well before his address at the Linnaean Society of London in 1921, he had assessed the limitations of the Haeckelians’ biogenetic law as well as the relevance of embryology for understanding the origin of evolutionary novelties. As a young man, Garstang tested his ideas on one of the most controversial debates in the history of evolutionary biology: the origin of vertebrates. While Darwin and Haeckel had sided for the ascidian option (Amphioxus, Balanoglossus), Anton Dohrn had strenuously defended the idea that vertebrates had evolved from annelid worms. Others advanced the possibility that chordates were instead related to arthropods (Bowler 1996). The discussion lost steam after 1900, but in his 1894 paper on the phylogeny of chordates, Garstang defended a quite orthodox view siding with a version of the ascidian theory: chordates derived from echinoderms. The basic view was that echinoderms preceded Balanoglossus-Amphioxus which ultimately led to modern chordates (Bowler 1996). In particular, he argued that vertebrates were derived from echinoderm pelagic sedentary larvae. He speculated that small changes in the development of those larvae could provide the material for important evolutionary leaps (Garstang 1894; see also Holland 2011). He plainly rejected the Haeckelian idea that the larvae corresponded to ancestral adult forms of extinct organisms because larval forms were the result of both heredity and local adaptation. For Garstang, in fact, those larvae were full-blown, fit organisms, not surviving fossils imprisoned in the early stages of echinoderms´ development. Yet, it was only after 1900 that Garstang began to play with the idea of what he later called paedomorphosis, namely, the retention of larval characteristics in the adult stages. He hypothesized that chordates could have been the outcome of paedomorphic marine invertebrates (echinoderm larvae) while supposing that specific adaptations of larval forms, fixed by natural selection, could outweigh the selective pressures on adult forms (Hardy 1962).
As de Beer observed in the 1930s, commenting Garstang’s hypothesis, whereas nobody could seriously defend the existence of morphological relations between adult echinoderms and vertebrates, possible links between echinoderm larva and proto-chordates could be seriously considered (Fig. 1). Essentially, Garstang (and de Beer) believed that if the larval form of echinoderm persisted and became sexually mature, then it could supply all the morphological conditions for the evolution of chordates.
In that case, a small “deviation” in the first stages of the ontogenic process could produce very significant changes in the evolution of the species.
De Beer’s depiction of Garstang’s theory. (de Beer 1954, p. 53)
Now, while Garstang had developed most of his fundamental ideas on the relation between ontogeny and phylogeny in the early twentieth century, it was only in the 1920s that his views found a consistent formulation (Hardy 1962). His 1922 paper “The Theory of Recapitulation: A Critical Re-Statement of the Biogenetic Law,” based on his 1921 address at the Linnaean Society of London, is one of his most systematic discussions of the topic, although he continued to explore various aspects and instances of the same position in ensuing papers. As the title of the 1922’s paper indicates, Garstang’s intentions were clear from the beginning. He wanted to provide a re-statement of the biogenetic law, not a general refutation of it. This is an important point that can easily be missed. No doubt, Garstang’s critical target was Haeckel’s specific version of the biogenetic law, but he did not pretend to put the last nail in the coffin of the recapitulationist creed. Rather, he wanted to reassess “in accord to modern knowledge, the theoretical relations of ontogeny to phylogeny, and then to subject the alternative theories to verification by test-case” (1922, p. 81).
The paper rests on two fundamental premises and a daring conclusion: (1) No one can deny the relation between evolution and embryology, and (2) Haeckel’s recapitulationism presumes an erroneous causal link between ontogeny and phylogeny. As a consequence, phylogeny had not to be conceived as a chronological sequence of adult forms, but as the historical record of diverging ontogenies. As he explained: “The ontogeny of a given animal is an epitome of its phylogeny, and may be said, in the true sense of the word, to recapitulate phylogeny, i.e. to sum it up, recall the main phases of it. This is the parallelism observed by Meckel, von Baer, and many others, expressed in evolutionary terms. It exists and is undeniable” (1922, p. 84). In other words, if the evidence persistently showed that some kind of parallelism existed, then the conclusion followed that the real issue with Haeckel was not that he was entirely wrong, but that, in confusing the causal direction connecting ontogeny and phylogeny, he had missed the real link between the two. In fact, while Haeckel had argued that phylogeny was the mechanical cause of ontogeny, the opposite was true because: “the real phylogeny of Metazoa has never been a direct succession of adult forms, but a succession of ontogenies or life cycles” (1922, p. 82).
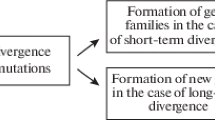
The point against Haeckel is theoretically subtle and powerful: to see evolution as a sequential display of adult forms means to engage in a pernicious abstraction.Footnote 3 Organisms are born, grow, and die, and their first interaction with the world is as a zygote. Heredity does not produce full-grown adults; it triggers the complex reproduction of characters throughout whole lifecycles, which are constantly open, at whatever stage considered, to specific adaptations. Moreover, the zygote itself, just like any following stage of organic development, cannot be deemed an imperfect form waiting to reach perfect adaptation in adulthood. Each stage is a combination of adaptation and heredity. The adult form is only a phase within the complex life cycle of each individual. For example, the ontogeny of a frog does not recapitulate its ancestors, but is itself a variation on the ontogeny of a previous evolutionary form: “the life cycle of the Frog is a modification of the life cycle of an ancestral freshwater Fish” (1922, p. 85). That elucidated why parallels can be recognized between ontogeny and phylogeny, while also explaining why Haeckel’s biogenetic mechanism could not work. Caenogenetic interpolations should not be seen as new, derailed adaptive ontogenetic trends hiding the normal and real process of evolution (palingenesis); they were themselves the basis of evolution, inaugurating new ontogenies and, thus, new life cycles (Fig. 2).
Garstang’s representation of the relation between ontogeny and phylogeny. On the right side are the succession of ontogenies (zygotes), while on the left the phyletic succession of adult forms. The two lines converge toward a common ancestor. The arrows in the middle represent the relation across different ontogenetic cycles (i.e., parallelism). Common ancestors share common ontogenetic cycles while phylogenetic divergence is explained as the result of mutations occurring in the early stages of ontogenetic cycles. (Garstang 1922, p. 83)
As previously mentioned, Garstang believed that Morgan’s Drosophila experiments demonstrated the point very well. When a wingless female is produced through artificial crossings, the novelty is not interpreted as a new terminal addition to the normal Drosophila development; neither is it considered as an ancestral element emerging from the depth of its phylogenetic past. It is a different kind of Drosophila because it has a different kind of ontogenetic process. Small inherited mutations needed to be understood as changes producing new forms within a whole developmental cycle. In Garstang’s words:
Zygotic mutations have caused the changes; natural selection has controlled the breeding of successive generations; and heredity has perpetuated the results of the selection. Certain ancestral adult characters are disappearing from the ontogeny; and the condition of a flea, ontogenetically, as well as finally, without a trace of wings at any stage, is likely to be the end result. (1922, p. 88)
Drosophila experiments did not only expose the weakness of traditional recapitulationism. Crucially, for Garstang, those experiments provided a small glimpse of how evolution might work through the gradual modification of ontogeny. Of course, this was not obvious if one severed heredity from embryology and embryology from evolution. Morgan, in fact, had strategically separated heredity from developmental problems. He conceived genetics as a science focused on the transmission of characters and not their expression (Allen 1978; Amundson 2005). But from Garstang’s perspective, the split between genetics and embryology precluded the possibility to understand how the interaction between genes and development fueled evolutionary changes. This was, I argue, the real polemic target of Garstang behind his restatement of the biogenetic law. Indeed, Garstang saw evolution as an adaptive change of developmental paths over time, and one of the most interesting corollaries of his view was that the biologist needed to look at all kinds of variations. Variations occur among adults, but they also occur among embryos and even among the different stages of the embryo’s development. This means that natural selection could work upon larvae or upon any stage of ontogenetic development. From a general perspective, Garstang supposed that development proposes (ontogenetic novelties), selection disposes (filtering non-adaptive forms), and evolution is the historical mirror of such process. In a lyrical passage, Garstang expresses this view:
That “little twist of brain”, which distinguishes one philosopher from another, is not more striking in its effects than are those trifling touches to the structure of the heart which transformed the cold-blooded Reptile and Stegochephalan into the warm-blooded bird and Mammal respectively. Yet these are changes which, however graduated through successive generations at the outset, were not of a character to have been completed, or even initiated, in any adult stage of ontogeny. They must have been first manifested as a series of embryonic mutations, subjected continuously to selective tests of their relative physiological efficiency. Age bears the buffets of the world, but youth regenerates it. (1922, p. 92)
At the end of the paper, Garstang offered a Decalogue of ten points to keep in mind for the good recapitulationist of the twentieth century. The third one, after a redefinition of the concepts of ontogeny and phylogeny, commanded emphatically that recapitulation should not be seen in terms of phylogenetic repetition, but rather in terms of creation: “Ontogeny does not recapitulate phylogeny: it creates it” (1922, p. 98). According to the new and revised recapitulationism, development provided the real engine of evolutionary novelties, and, not surprisingly, Garstang saw a shining future for embryology: it was the main entry point for explaining and understanding the causes of evolution. While embryologists could not extrapolate from the developing embryo the organic history of the species, they could certainly speculate about how small mutations could have brought about drastic changes in phylum and subphylum. As Garstang ironically concluded: “Ontogeny is not an animated cinema show of ancestral portraits; but zygotes may be likened to conjurers playing the old tricks for the most part, and occasionally opening a surprise packet—nor do they always keep their novelties back until the end of the performance” (1922, p. 100).
Of course, we know today that Garstang’s reassessment of the biogenetic law was not entirely original (Olsson et al. 2009; Holland 2011; Esposito 2017a, b). The idea that development could be a source of evolutionary novelties was hinted at, imagined, proposed, and finally assumed by different evolutionists, embryologists, or physiologists ever since Darwin or Fritz Müller speculated about the important relation between embryology and evolution. De Beer himself mentioned no less than eight of his contemporaries (aside from Garstang) who upheld the view (1954, p. 7). The idea was in the air from the second half of the nineteenth century onwards and continued to inform many biological syntheses during the first decades of the twentieth century (Esposito 2017a, b). Garstang was part of this general and international trend. What he added to this trend was his profound knowledge of marine biology, which, as de Beer promptly recognized, allowed Garstang to become the first zoologist to focus on the early developmental stage of marine invertebrates (echinoderms) as possible candidates for understanding the evolution of vertebrates: “Garstang was the first to look for the trace of the ancestors of the vertebrates in early instead of adult stages of invertebrates” (de Beer 1954, p. 52).
However, after the 1930s, this trend started to change. In reviewing the history of embryology during the first decades of the twentieth century, Victor Hamburger observed that, while the major architects of the modern synthesis “hardly mention embryonic development” (1980, p. 97), embryologists were not really interested in evolution. He noted that “Many prominent embryologists actively minimized the importance of natural selection, even as late as the 1940s and 1950s” (1980, p. 96). As a consequence, embryology and evolutionary biology started to take separate paths. In addition, Hamburger contended that the alienation of embryology from evolutionary biology was also due to a lack of factual knowledge: only with the articulation of developmental and physiological genetics could the gap between embryology and evolution really be bridged. However, what about the attempts by Garstang and many other embryologists to update Haeckel’s law? After all, Garstang accepted Mendelian genetics and used Morgan’s works with approval. He was far from being an anti-Darwinist and was deeply fascinated by evolutionary issues. Garstang, as de Beer recognized many years later, worked in the shadow of Darwin, who had acknowledged the fundamental relation between embryology and evolution, especially in The Variation of Animals and Plants under Domestication (Darwin 1868; see Breidbach and Ghiselin 2007). Garstang was thus not an eccentric Lamarckian holist upholding the uselessness of genetics for embryology. He connected these two, via evolution, well before physiological geneticists revealed the complexity of genetic regulation (and, of course, he was not alone in doing that). Thus, the question is not whether, why, and how experimental embryology came to be sidelined by evolutionary biology’s mainstream, but why the new updated version of biogenetic law (such as that proposed by Garstang) did not receive wider attention among evolutionary biologists.
Marginalization and Revival
Garstang did not leave behind any school, but his idea that ontogeny creates phylogeny did influence important embryologists. One of these was Gavin de Beer, who published Embryology and Evolution in 1930 and later revised and republished it as Embryos and Ancestors (1954). Then, Garstang’s agenda continued through the work of Alister C. Hardy, Norman J. Berril, and Donald T. Anderson, and in Gould’s 1977 vindication of the whole research program (in the Anglo-American world). Not surprisingly, Gould quoted Garstang several times in his book, recognizing that something new was happening when Garstang restated the biogenetic law. Haeckel’s recapitulationism was certainly dead by the 1920s, but Garstang’s principle and de Beer’s follow up might well have inspired an entire branch of evolutionary studies. After all, both embryologists presented a new, updated version of the biogenetic law that could have been incorporated within mainstream evolutionary biology. However, this was not the case. Why, then, did Garstang’s principle meet only a modest reception? Why was Garstang’s updated embryology not integrated into the Modern Synthetic theory of evolution? What kind of theoretical, institutional, or social obstacles prevented its incorporation into mainstream evolutionary biology?
Hardy identified three reasons behind the modesty of Garstang’s success. First, his over-speculative inclinations: “Speculation and still more speculation! … which prevented more of his contemporaries from taking a serious interest in his views,” Hardy observed, adding, “had he lived a generation earlier, he would indeed have been a leader of zoological thought” (Hardy 1962, pp. 14–15). Second, Garstang’s idiosyncratic communication of his findings through poetic verses rather than in somber scientific prose (Garstang 1951; Hardy 1962, p. 2); and, third, the contrast of his ideas with Haeckel’s biogenetic law, which was still dominating the minds of most 1920s biologists. Altogether, these reasons could explain why Garstang’s ideas were marginalized from mainstream biological discussion. Hardy’s interpretation, however, is largely flawed. Garstang was not more speculative than many other, more successful biologists. After all, the history of evolutionary biology is filled with wild speculation: Darwin himself was often (and rhetorically) accused of such. The second reason might be partly valid, not because Garstang wrote in verses (of course, he did, but his poems were published posthumously), but rather because he never published a major and accessible monograph summarizing his views (as did de Beer, for instance). On the contrary, Garstang’s scientific papers are generally technical and very descriptive, leaving his most speculative conclusions to further analyses and assessments. Finally, the clash with Haeckel’s law needs to be qualified, given that recapitulationism had been under attack ever since the late nineteenth century. After the First World War, Haeckel’s law was largely discredited, and nonconformity to it could not have been the cause of Garstang’s neglect.
In order to frame a plausible reason for Garstang’s relative oblivion, I think that it is necessary to reconsider the refutation of Haeckel’s biogenetic law. This because the rejection of the Haeckel’s law did not only consist in the rejection of a scientific hypothesis. Instead, it consisted in the repudiation of a whole research program in which embryology and evolutionary biology were conceptually intertwined. Garstang, in fact, had understood too well that the refutation of Haeckel’s biogenetic law could easily trigger a lasting and unnecessary split between the two fields. It is precisely for this reason that he tried to re-assess—and not discard—the law. In short, the fate of Garstang’s view was intimately linked to the reasons and events that had led to the collapse of Haeckelian recapitulationism.
Many scholars have tried to identify the rationales behind the rejection of Haeckel’s law. In addition to Gould, Rasmussen (1991), Bowler (1996), Churchill (2007), and Nyhart (1995) advanced a host of compelling accounts (and E. S. Russell did before them). The reasons can be divided between external ones, originating from cultural or disciplinary factors, and internal ones, founded on empirical or theoretical considerations. As in many other cases in science, it can be expected that both factors mirror one another. Here I present only a short list of the most important elements arrayed against Haeckel’s law. First, the empirical evidence that ontogeny actually does not recapitulate phylogeny. As Russell observed in his pioneering Form and Function, ever since the 1890s observations had accumulated leading to the definitive dismissal of Haeckel’s recapitulationism (1916, pp. 349–365). Gould postponed the demise of Haeckel’s law for a couple of decades. He argued that the law could not be empirically disproved because it could be always saved by the strategic addition of more Caenogenetic interpolations. No number of contrary instances could lead naturalists to discard the law. For Gould, only the general acceptance of Mendelian genetics after the 1920s made Haeckel’s law untenable. Genetics undermined two essential principles of recapitulationism: terminal addition and ontogenic condensation. However, Gould accepted Russell’s earlier insight that from the late nineteenth-century, embryology became increasingly more experimental, mechanistic, and therefore wary of historical speculations over presumed phylogenetic trees. Gould saw a kind of paradigmatic shift from the recapitulationist to the experimental gaze: while the former regarded embryonic stages as ancestral forms repeating evolutionary history, the latter looked at the same phenomena as proximate stages in mechanical development (Gould 1977, p. 187). With this shift, development could be easily severed from evolutionary concerns.
Of course, both paradigms could coexist in principle, but in the real world, coexistence came up against the limits of material resources and opportunity: “Both schools [recapitulationists and experimentalists] had to compete for a limited number of academic positions and the status they entailed. To establish themselves, experimental embryologists had to displace a generation of Haeckelian morphologists” (Gould 1977, p. 196). Rasmussen develops this insight, dismissing Gould’s first argument that flagged genetics as having caused the fall of Haeckel’s recapitulationism: “Mendelian genetics contributed nothing toward rendering the biogenetic law theoretically untenable” (1991, p. 69). The adverb theoretically here is crucial for understanding Rasmussen’s view. He argues that while genetics did not challenge recapitulationism theoretically, it did condemn it practically and institutionally. Genetics “depended for its very existence on the overthrowing not only of recapitulation itself, but also on the older ideas of heredity implicit in the biogenetic law” (Rasmussen 1991, p. 71). For Rasmussen, the reason for the dismissal of Haeckel’s law was a disciplinary struggle pitting the old guard—the comparative and descriptive embryologists—against the up-and-coming experimental physiologists and geneticists. The struggle for academic authority overshadowed arguments and observations, while disciplinary interests sidelined embryology as a discipline with the potential to account for evolutionary novelties. Accordingly, throughout the twentieth century, the historical unfolding of a very sophisticated and successful syllogism condemned Garstang’s agenda: heredity (genetics) and evolutionary biology separated from embryology. Embryology was dismissed for its supposed lack of experimental and quantitative rigor. Therefore, evolutionary biology was reshaped in terms of transmission genetics, while genetics itself, as the exemplar of a quantitative and experimental discipline, could not tolerate other gods beyond the gene for explaining heredity and development. Rasmussen identified Morgan as the main éminence grise behind the implementation of such an argument: “Morgan managed deftly to reshuffle interests and groups within biology to his own advantage, by a combination of displacing goals, inventing new goals, and inventing new groups” (1991, p. 88).
Rasmussen also observed how Garstang, and then de Beer, could do nothing to save embryology from the disruptive force of Morgan’s Machiavellian strategies. They were unable to dissociate development from the anachronistic clutches of its past. The re-statement of the biogenetic law was not enough because Morgan’s demand for a quantitative and experimental approach was too restrictive for a discipline that was mainly qualitative and, to a certain extent, perceived as too speculative. Even though Garstang and de Beer were not averse to Darwinism and were quite perceptive on genetics, their perspective received only a small fraction of the attention received by Morgan’s agenda. While I deem Rasmussen’s view as generally correct, he might have overstated Morgan’s political and rhetorical powers. After all, there were many biologists in France, Germany, and Russia who were not so overwhelmingly affected by Morgan (and then, of course, by Sewall Wright, R. A. Fisher, and Theodosius Dobzhansky’s powerful synthesis of genetics and evolution). And even in the Anglo-American context, the interest over developmental and physiological genetics was well represented, especially if the persistent attention over homeotic mutants is considered (Davis et al. 2009). After the 1930s, serious attempts to integrate experimental embryology, evolutionary biology, and genetics were not lacking, both within and outside the Anglo-American world. Thus, Rasmussen’s account may need to be complemented and we might explore the possibility that Morgan’s tactics were also successful because embryology itself was experiencing a deep crisis after 1930. In fact, as Horder aptly observed:
during the 1930s a number of trends converged powerfully within biology that would marginalize embryology.… These trends in general biology were emerging at precisely the time when a sense of deep pessimism descended on the experimental embryologists. This was largely the result of the complexities emerging in the understanding of the organizer, particularly damaging being the dashing of the high reductionist hopes of explaining it in chemical terms.… Despite the brilliance of the work of the Spemann school, embryology as a subject had, by the time of the Second War, descended into a barren “dark age” from which it only slowly recovered. (Horder 2008, pp. 123–124)
The crisis was mainly due to the unmet expectation that embryology should explain development in a mechanical and reductionist way. Not surprisingly, during the first decades of the twentieth century, many first-rate embryologists, including Morgan, Julian Huxley, and Joseph Needham, had gradually turned their attention to more productive topics. The future was not bright for disciplines that celebrated complexity and plasticity, but it was promising for quantitative, determinist, and thus predictive fields that could be easily commercialized for concrete applications (for example, agriculture, medicine, etc.).Footnote 4 Even those phenomena that could have flagged the sophistication of experimental embryology for grasping the causal intricacies of speciation (that is, homeotic mutants) were reduced to a particular kind of genetic change among others (Davis et al. 2009). Thus, embryology could not entirely free itself from its Haeckelian past: it was brought down by its failed scientific aspirations and fell prey to a hostile institutional environment, while brilliant former embryologists such as Morgan prepared its deathbed.
With all this in mind, it would not take too much historical imagination to grasp why Garstang’s attempts to save embryology from timeworn recapitulationism (as well as the efforts of other scholars in other countries) did not bear the fruits he hoped. Embryologists did not disappear, of course, but most evolutionary biologists no longer regarded development as a major source of inspiration for a large part of the twentieth century. Evolutionary biology followed its own course, while embryology took a separate route until comparative evolutionary embryology and developmental genetics converged after the 1970s (Love and Raff 2003). In short, while Garstang’s weird wonder was well equipped to thrive and be incorporated into mainstream twentieth-century evolutionary biology, it did not. There were a host of different reasons conspiring against Garstang’s option: institutional reasons (the growing authority of geneticists and biochemists in the life sciences); changes in epistemic values (preference for predictive and quantitative fields); and disciplinary disenchantment (internal crisis of embryology). All together, these reasons thwarted the further development of Garstang’s promising view and, at the same time, explain why an eventual time traveling biologist would probably bet on the wrong theory.
When Gould readdressed the relation between ontogeny and phylogeny in 1977 from both a historical and a scientific perspective, he was aware that he was not resurrecting the undead. He was, rather, shedding light on a neglected agenda that had survived the technocratic era of the Cold War and that emphasized the centrality of embryonic regulation for evolutionary change. After long decades of over-enthusiastic gene-centricity, he celebrated the fact that the embryo was once again in the spotlight. Along the lines of Gould, other scholars started to wipe the dust from old books and articles, realizing that, long ago, Haeckel’s biogenetic law had undergone sophisticated adjustments that could have stimulated a major evo-devo agenda well before the 1980s: they started to reveal the evolutionary biology that might have been.
Conclusion
Revisiting Garstang’s work, together with the rich intellectual landscape in which he moved, we can better appreciate how evolutionary biology changed after the First World War. The explosion of creativity that we find during the first decades of the twentieth century was streamlined into a triumphantly molecular view of life, which marginalized or extinguished many other valid epistemological possibilities (see Kay 1993). In hindsight, there are good reasons for arguing that Garstang’s (and de Beer’s) alternative vision could have fostered a major trend in evolutionary biology, and we may presume this because such a trend has been nascent since the 1980s (with the due differences). If we could rewind the history of biology back to the 1920s, we would have no particular problem in wagering on the success of Garstang’s revised recapitulationist view. After all, it made sense to Gould in 1977, and, as mentioned previously, it makes total sense for most evo-devo biologists today. Nonetheless, apart from de Beer, Hardy, and few other followers, it failed to create a broad consensus within the context of evolutionary biology after the Second World War, even when that relation between embryology and evolution could be reframed within a neo-Darwinian perspective. I have suggested that the motive why that happened can be condensed in three main reasons: institutional factors, a shift in epistemic values, and disciplinary disenchantment.
From a broader viewpoint, we could argue that the progressive institutionalization of science from the nineteenth century onwards had fostered an irreversible fragmentation of biology into many different disciplines and sub-disciplines. Bowler, for instance, mentioned the case of evolutionary morphology, which was first parceled out by paleontology and biogeography and then colonized by genetics and biochemistry, until evolutionary biology was radically restructured after the 1930s (Bowler 1996). Although it would be impossible (or too simplistic) to identify one unique and general trend explaining the path and divergences of all biological disciplines, we can certainly find a recurrent refrain informing the development of the life sciences in the last century, namely, the growing hiatus between those disciplines perceived as experimental and quantitative and those perceived as descriptive and qualitative. Retrospectively, it seems that the disciplinary fragmentation following the logic of institutionalization (and therefore specialization) has benefited the experimental disciplines more than the descriptive ones. Embryology and evolutionary biology (and their controversial relation) did not escape such a tendency. Indeed, following the insights of Gould, Rasmussen, and Horder, I have argued that Garstang’s weird wonder was eclipsed by the internal crisis of embryology, which meant the field could not compete with the predictive and experimental success of younger disciplines. The attempt to save embryology from Haeckel’s recapitulationism could not work because biology itself was heading elsewhere. Former embryologists such as Morgan, Huxley, and many other biologists understood very well how biology was changing: it entered into what Rasmussen dubs the “new order,” where epistemic constraints were too tight for embryology to fit.
Even de Beer recognized that a dramatic split was underway. In the conclusion of his Embryos and Ancestors, he detected the growing divergence between increasingly experimental embryology and the new evolutionary biology, more and more dependent on paleontology:
The analytical and experimental study of embryology is providing an increasing body of information concerning the chain of linked causes the result of which is ontogenetic development. On the other hand, paleontological studies are providing an increasingly precise description of the results of phylogenetic evolution. We may now ask ourselves the question, what is the nature of the assistance which each study can bring to the other? (de Beer 1954, p. 98)
Embryology has radically changed and evolutionary biology has too, de Beer observed. In this new context, the nature of their relation needed to be entirely retooled. But perhaps de Beer’s precious intuition hid something even deeper. The new context demanded, of course, the avoidance of uncontrolled speculation, but it also demanded that the life sciences become more quantitative, like physics and chemistry. It demanded that biology become more experimental and predictive and, especially, well focused and strategically positioned for the attraction of funding. The halcyon times when naturalists investigated the mysteries of the natural world, removed from the pressures of social and institutional accountability, were at an end. Disciplines that could ally themselves to prospective aspirations were much better situated to survive the Second World War and the following Cold War (Esposito 2017b). The century of all-powerful genes, life’s codes, and biological information left little space for alternative views of biological organization. In this context, Garstang’s view had to wait half a century before having its second chance.
Notes
In his introduction to Garstang’s posthumously published book of biological verses (1951), Alister Hardy wondered why so few biologists recognized the significance of Garstang’s ideas in the 1930s, asking: “Why even now, among the younger generation, are there not more who acknowledge the importance of the contribution he has made to the theory of evolution?” (Hardy 1962, p. 1).
De Beer credited Garstang, together with S. G. Kryzanowsky and H. H. Swinnerton, as those who first argued that phylogeny should not be seen as a chronological set of adult forms (1951, p. 9).
On the relation between genetics and medicine, see Comfort (2012).
References
Allen, Garland. 1975. Life Science in the Twentieth Century. New York: Wiley.
Allen, Garland. 1978. Thomas Hunt Morgan: The Man and His Science. Princeton, NJ: Princeton University Press.
Amundson, Ronald. 2005. The Changing Role of the Embryo in Evolutionary Thought: Roots of Evo-Devo. Cambridge: Cambridge University Press.
de Beer, Gavin. 1930. Embryology and Evolution. Oxford: Clarendon Press.
de Beer, Gavin. 1954. Embryos and Ancestors, Revised ed. Oxford: Clarendon Press.
de Beer, Gavin. 1958. Darwin’s Views on the Relations Between Embryology and Evolution. Botanical Journal of the Linnean Society 56 (365): 15–23.
Bowler, Peter. 1996. Life’s Splendid Drama. Chicago, IL: University of Chicago Press.
Breidbach, Olaf, and Michael Ghiselin. 2007. Evolution and Development: Past, Present and Future. Theory in Biosciences 125: 157–171.
Churchill, Frederick. 1980. The Modern Evolutionary Synthesis and the Biogenetic Law. In The Evolutionary Synthesis, Perspectives on the Unification of Biology, ed. E. Mayr and W. Provine, 112–122. Cambridge: Harvard University Press.
Churchill, Frederick. 2007. Living with the Biogenetic Law: A Reappraisal. In From Embryology to Evo-Devo: A History of Developmental Evolution, ed. M. Laubichler and J. Maienschein, 37–81. Cambridge: MIT Press.
Comfort, Nathaniel. 2012. The Science of Human Perfection: How Genes Became the Heart of American Medicine. New Haven, CT: Yale University Press.
Darwin, Charles. 1868. The Variation of Animals and Plants under Domestication. London: John Murray.
Davis, Gregory K., Michael Dietrich, and David K. Jacobs. 2009. Homeotic Mutants and the Assimilation of Developmental Genetics into the Evolutionary Synthesis, 1915–1952. Transactions of the American Philosophical Society New Series 99 (1): 133–154.
Eastham, L. 1949. Prof. Walter Garstang. Nature 163: 518–519.
Esposito, Maurizio. 2014. Problematic “Idiosyncrasies”: Rediscovering the Historical Context of D’Arcy Wentworth Thompson’s Science of Form. Science in Context 27 (1): 79–107.
Esposito, Maurizio. 2015. More than the Parts: W. E. Ritter, the Scripps Marine Association, and the Organismal Conception of Life. Historical Studies in the Natural Sciences 45 (2): 273–302.
Esposito, Maurizio. 2017a. The Organismal Synthesis: Holistic Science and Developmental Evolution in the English-speaking World, 1915–1954. In The Darwinian Tradition in Context: Research Programs in Evolutionary Biology, ed. R.G. Delisle, 219–241. Cham: Springer.
Esposito, Maurizio. 2017b. Expectation and Futurity: The Remarkable Success of Genetic Determinism. Studies in History and Philosophy of Biology and Biomedical Sciences 62: 1–9.
Garstang, Walter. 1894. Preliminary Note on a New Theory of the Phylogeny of the Chordata. Zoologischer Anzeiger 17: 122–125.
Garstang, Walter. 1922. The Theory of Recapitulation: A Critical Re-statement of the Biogenetic Law. Zoological Journal of the Linnaean Society 35 (232): 81–101.
Garstang, Walter. 1929. The Origin and Evolution of Larval Forms. Report of the British Association for the Advancement of Science (Section D) 96: 77–89.
Garstang, Walter. 1951. Larval Forms and Other Zoological Verses. Oxford: Blackwell.
Gould, Stephen Jay. 1977. Ontogeny and Phylogeny. Cambridge, MA: Belknap Press of Harvard University.
Gould, Stephen Jay. 1989. Wonderful Life: The Burgess Shale and the Nature of History. New York: W. W. Norton & Co.
Hall, Brian Keith. 2000. Balfour, Garstang and de Beer: The First Century of Evolutionary Embryology. American Zoologist 40: 718–728.
Hamburger, Viktor. 1980. Embryology and the Modern Synthesis in Evolutionary Biology. In The Evolutionary Synthesis, Perspectives on the Unification of Biology, ed. E. Mayr and W.B. Provine, 97–112. Cambridge, MA: Harvard University Press.
Hardy, Alister C. 1951. Walter Garstang, 1868–1949. Journal of the Marine Biological Association of the United Kingdom 29 (3): 561–567.
Hardy, Alister C. 1962. Introduction. In Larval Forms and Other Zoological Verses, ed. Walter Garstang. Oxford: Blackwell.
Holland, Nicholas. 2011. Walter Garstang: A Retrospective. Theory on Biosciences 130: 247–258.
Horder, Tim. 2008. A History of Evo-Devo in Britain: Theoretical Ideals Confront Biological Complexity. Annals of the History and Philosophy of Biology 13: 101–174.
Kay, Lily. 1993. The Molecular Vision of Life: Caltech, the Rockfeller Foundation, and the Rise of the New Biology. Oxford: Oxford University Press.
Levit, Georgy, Uwe Hossfeld, and Lennart Olsson. 2004. The Integration of Darwinism and Evolutionary Morphology: Alexej Nikolajevich Sewertzoff (1866–1936) and the Developmental Basis of Evolutionary Change. Journal of Experimental Zoology B 302 (4): 343–354.
Love, Alan, and Rudolf Raff. 2003. Knowing your Ancestors: Themes in the History of Evo-Devo. Evoloutionary Development 5: 327–330.
Nyhart, Lynn. 1995. Biology Takes Form: Animal Morphology and the German Universities, 1800–1900. Chicago, IL: University of Chicago Press.
Olsson, Lennart, Uwe Hossfeld, and Olaf Breidbach. 2009. Preface. Between Ernst Haeckel and the Homeobox: The Role of Developmental Biology in Explaining Evolution. Theory in Biosciences 128: 1–5.
Olsson, Lennart, Georgy Levit, and Uwe Hossfeld. 2010. Evolutionary Developmental Biology, Its Concepts and History with Focus on Russian and German Contributions. Naturwissenschaften 97 (11): 951–969.
Olsson, Lennart, Georgy Levit, and Uwe Hossfeld. 2017. The “Biogenetic Law” in Zoology: From Ernst Haeckel’s Formulation to Current Approaches. Theory in Biosciences 136: 19–29.
Provine, William. 1980. Introduction. In The Evolutionary Synthesis: Perspective on the Unification of Biology, ed. E. Mayr and W. Provine, 96–97. Cambridge: Harvard University Press.
Radick, Gregory. 2008. Why What If? Isis 99: 547–551.
Radick, Gregory. 2016. Experimenting with the Scientific Past. British Journal for the History of Science 49: 153–172.
Rasmussen, Nicholas. 1991. The Decline of Recapitulationism in Early Twentieth-century Biology: Disciplinary Conflict and Consensus on the Battleground of Theory. Journal of the History of Biology 24: 51–89.
Ridley, Mark. 1986. Embryology and Classical Zoology in Britain. In A History of Embryology, ed. T.J. Horder, J.A. Witkowski, and C.C. Wylie, 35–67. Cambridge: Cambridge University Press.
Russell, Eduard Stuart. 1916. Form and Function: A Contribution to the History of Animal Morphology. London: John Murray.
Thompson, D’Arcy Wentworth. 1917. On Growth and Form. Cambridge: Cambridge University Press.
Funding
Funding was provided by Fondecyt Chile (Grant No. 1171017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esposito, M. Beyond Haeckel’s Law: Walter Garstang and the Evolutionary Biology that Might Have Been. J Hist Biol 53, 249–268 (2020). https://doi.org/10.1007/s10739-020-09602-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10739-020-09602-9