Abstract
Osteoclasts are specialized cells that degrade and resorb bone. Bisphosphonates (BPs) are drugs with well-known capacity to inhibit the resorption of mineralized tissues. Nitrogen-containing BPs, like alendronate (ALN) and zoledronic acid (ZA), inactivate osteoclast activity mostly by alterations on the cytoskeleton architecture of the cell. In this study, we used an in vitro model to test the hypothesis that bisphosphonates may have inhibitory effects on the osteoclastogenesis and osteoclast activity after the therapy was discontinued. Primary osteoclasts were generated from mouse bone marrow in media supplemented with 1,25-dihydroxyvitamin D3 and cultivated over bones pre-treated with ALN and ZA. The pre-saturation of the bone slices with bisphosphonates did not affect cell viability. We found, however, that by disrupting the gene expression of RANKL and OPG the osteoclastogenesis and resorption activity of osteoclasts was significantly disturbed. These inhibitory effects were confirmed by scanning electron microscopy resorption assay, assessment of osteoclast ultrastructure, and by gene expression analysis of TRAP and Cathepsin K. In conclusion, ALN and ZA adhered to the bone matrix reduced the osteoclast activity in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the discovery that early bisphosphonates (BPs) had the property of inhibiting hard tissue resorption (Fleisch et al. 1969), they are still the main therapeutic drugs administered for prevention and treatment of osteoporosis (Compston 2020) and other diseases like osteogenesis imperfecta, Paget’s disease and bone-related cancer. There are two classes of BPs: non-nitrogen containing molecule (etidronate, clodronate) and nitrogen-containing type (alendronate, pamidronate, zoledronic acid). The addition of the nitrogen atom (amino group) has made these drugs even more potent and effective in preventing bone resorption (Russell 2011).
Alendronate (ALN) is still one of the most employed BPs during the past years due to its proven efficacy (Unnanuntana et al. 2017; Cummings et al. 2020). Zoledronic acid (ZA) has been widely employed in the management of osteoporosis and other therapies such as the inhibition of progression of bone metastatic tumors (Choi et al. 2017; von Moos et al. 2019; Compston 2020). Both drugs belong to the nitrogen-containing type of BPs, which acts on bone resorption by inhibiting the mevalonate pathway that regulates osteoclast cytoskeleton arrangement, establishment of cell polarized morphology and formation of its ruffled border membrane (Rogers et al. 2011). Hence, both ALN and ZA promote the inhibition of bone resorption by the inactivation of osteoclast activity.
Nitrogen-containing BPs have high affinity for hydroxyapatite due to their avid absorption, low desorption and high re-attachment to the bone (Russell 2011). In vitro studies have indicated that ZA has the greatest binding affinity for hydroxyapatite amongst BPs, followed by ALN (Nancollas et al. 2006; Henneman et al. 2008). This high binding affinity could result in a very long half-life into bone and, consequently, in prolonged effects of these drugs. Early studies have indicated that the persistent anti-resorptive effect lasting up to 10 years after withdrawal of the drug (Khan et al. 1997).
Although new anti-resorptive therapies as inhibitor of osteoclast formation (anti-RANKL) and specific inhibitor of osteoclastic cathepsin K (Sims and Ng 2014) have been introduced and replaced BPs for the treatment of resorptive disorders, a large cohort of patients has received bisphosphonate therapy as part of their medical history. These patients may face a persistent inhibition of bone resorption and may represent a clinical challenge in general conditions like bone fracture repair or in dental procedures such as orthodontic tooth movement, alveolar repair following tooth extractions, and even the risk of developing medication-related osteonecrosis of the jaws (MRONJ) after oral surgeries, in special those performed in mandible (Coskun Benlidayi and Guzel 2013; Kishimoto et al. 2019). Such clinical situations demand intense bone remodeling activity, which includes the recruitment, differentiation and resorption activity by osteoclasts. The mechanisms of the persistent effects of bisphosphonate therapy on osteoclastogenesis and resorption have not yet been studied. Indeed, understanding such mechanisms will allow the discovery of therapeutic approaches for the prevention and management of bone healing complications in patients that require further treatments involving bone remodeling.
With the aim of identifying the maintenance of inhibitory effects of BPs on resorption, we applied an in vitro approach to examine how bone slices previously saturated with ALN and ZA but no longer administered with these BPs may affect key osteoclast signaling molecules.
Materials and methods
Preparation of bone slices
Bone slices were obtained from bovine cortical bone. The bone was sectioned at 200 μm in thickness with a low-speed diamond saw by using an IsoMet 1000 Precision Cutter (Buehler, Rockville, IN, USA) and trimmed into a surface area of 1 cm2. They were dried, de-contaminated in a 1% penicillin–streptomycin (pen-strep) solution and then rinsed twice in distilled water.
Saturation analysis of bone slices with nitrogen-containing bisphosphonates
To establish the concentration of nitrogen-containing BPs to be used in the experiment, tests were performed to detect the minimum amount of ALN (Sigma, St. Louis, MO) needed to saturate bone slices. We used an adaptation of the protocol established by Al Deeb et al. (2004) to detect ALN in a spectrophotometer (Al Deeb et al. 2004). For this, a solution of O-phthalaldehyde and mercaptoethanol (OPA-ME) was prepared, which reacted with ALN in a derivatization reaction, becoming detectable in a spectrophotometer. ALN solutions were prepared at the concentrations of 1 μM, 10 μM, 100 μM, 1 mM and 10 mM, and reacted at the ratio of 1:1 with OPA-ME solution for 60 min in a 24 wells plate. Then, these solutions were submitted to spectrophotometer reading at 333 nm. After that, bovine bone slices were incubated in these solutions to incorporate ALN into bone matrix. This solution reacted overnight, and after that, the bones were removed, and a new reading was performed to detect the minimum ALN concentration needed to saturate the bone slice. The same minimum concentration was used for ZA (Zometa; Novartis Pharmaceuticals) saturation.
Primary culture
Mouse marrow-derived osteoclast primary cultures were generated as previously described (Holliday et al. 1995). Briefly, Balb-c mice were euthanized by cervical dislocation then the femora and tibia were dissected from adherent tissue, and marrow was removed by flushing with α-MEM supplemented with 10% fetal bovine serum, 1% glutamine and 1% pen-strep (α-MEM D10). The marrow cells were washed twice with α-MEM D10 then seeded at 1 × 106/cm2 density on tissue culture plates for 5 days in α-MEM D10 supplemented with 10−8 M 1,25-dihydroxyvitamin D3. The cultured cells were fed after 3 days by replacing half the media per plate and adding fresh 1,25-dihydroxyvitamin D3 (D3). After 5 days in culture, cells were removed from the plate with a cell lifter and seeded onto bone slices pre-treated with ALN, ZA or PBS at 1 × 106/cm2 density in 24-well plates with α-MEM D10 supplemented with fresh D3 that was replaced every 3 days for the following experiments described below.
TRAP histochemistry
After 3 days of culture in the presence of a bone slice coated with bisphosphonate or PBS, osteoclasts appeared on the plastic surrounding the bone. They were detected as giant cells which were submitted to TRAP histochemistry as previously described (Toro et al. 2013). Briefly, after the saturated bone slices were removed, the cells were fixed in 2% formaldehyde (freshly prepared from paraformaldehyde), rinsed in PBS, permeabilized in 1% triton X-100 and stained with the leucocyte acid phosphatase (TRAP) assay (Sigma) following the manufacturer’s instructions. The TRAP-positive cells in a 0.5 cm2 area were counted by an examined blinded to the experimental groups and classified according to the number of nuclei: mononuclear, multinucleated (2–5 nuclei), giant (> 6 nuclei). This experiment was not carried on the cells on the bone due to the impossibility of examination of the stained cells under the light microscope.
TUNEL assay
For the apoptosis assay, after 3 days of culture, the saturated bone slices were removed and the cells remaining on the plate were fixed as described above, rinsed in PBS then stained with the Apoptag kit (Millipore) following the manufacturer’s instructions. The cells with TUNEL-positive nuclei in a 0.5 cm2 area were counted by an examiner blinded to the experimental groups. This experiment was not carried on the cells on the bone due to the impossibility of examination of the stained cells under the light microscope.
Cell viability assay
For the cell viability assay, after 3 days of culture the cells on the saturated bone slices and surrounding plate were rinsed with PBS and incubated in 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO) at 37 ℃ for 4 h. After the incubation, an equal volume of DMSO was added to each well, developed for 5 min with mild shaking then measured in a spectrophotometer at 540 nm.
Bone resorption assay
After 5 days of culture on the bone slices pre-treated with ALN, ZA or PBS, resorption assays were performed by scanning electron microscopy as previously described (Toro et al. 2013). Briefly, cultured bone slices were rinsed with sodium hypochlorite 2.5% for 15 min, washed with distilled water, dehydrated in crescent ethanol concentrations, treated with hexamethyldisilazane (HMDS) for 10 min, and then air-dried in a hood. Samples received a 25 nm gold-coating then examined in a LEO 430 scanning electron microscope operated at 10–15 kV. The resorbed area was determined by obtaining micrographs at 200× in five random areas, then determined each area on Adobe Photoshop by overlaying a grid and counting grid intersections over pits vs. total grid intersections. Pits were defined as continuous resorbed areas. Area per pit was calculated for each examined slice.
Osteoclast ultrastructure
After 3 days of culture, the saturated bone slices with cultured marrow osteoclasts were processed as previously described (Szewczyk et al. 2013). Briefly, samples were fixed in 4% glutaraldehyde in 0.2% cacodylate buffer for 18 h at 4 ℃. Next, the specimens were rinsed in cacodylate buffer and decalcified in 4.13% EDTA for 7 days at 4 ℃, post-fixed in 1% osmium tetroxide for 1 h, dehydrated in crescent concentrations of ethanol and finally infiltrated and embedded in Spurr epoxy resin (Electron Microscopy Sciences). Ultrathin sections (80 nm) were obtained in a Leica Ultracut R microtome with a diamond knife and collected onto Parlodion-coated 300-mesh nickel grids, stained with uranyl acetate and lead citrate then examined in a JEOL 1010 transmission electron microscope equipped with a Gatan digital imaging system.
Quantitative reverse-transcription polymerase chain reaction
After 3 days of culture, cells were removed from the saturated bone slices by scraping and RNA was isolated with the Charge Switch Total RNA Cell Kit (Thermo Fischer Scientific) and cDNA was obtained with the SuperScript First Strand kit (Thermo Fisher Scientific). The gene expression of osteoclastogenesis regulators OPG and RANKL and differentiation markers TRAP and Cathepsin K were verified with inventoried TaqMan probes in a StepOne Plus instrument (Thermo Fisher Scientific). The housekeeping genes HPRT1 and 18S were used as reference genes. All reactions were performed in triplicate.
Data analysis
The statistical analysis was performed with GraphPad Prism 7 software. All of quantitative data were submitted to the student’s t-test or one-way ANOVA analysis, and Tukey post-hoc tests were performed. p value below 0.05 was considered significant.
Results
Saturation analysis of bone slices with nitrogen-containing bisphosphonates
After spectrophotometry analysis, the minimum concentration of ALN necessary to saturate bone slices was established. At concentrations below 5 mg (1 mM), ALN was fully incorporated into the bone matrix and no ALN was detected in the solution. At concentrations above 1 mM, this bisphosphonate could be detected in the solution (Fig. 1). As the bone slices weighed 0.05295 g on average, the concentration used for incubations was 0.604 mg ALN/g of bone. In consequence, bone slices were incubated in solutions of 1 mM of both nitrogen-containing BPs, ALN or ZA, in PBS overnight; controls were incubated in PBS.
Tartrate-resistant acid phosphatase (TRAP)-positive cells
After 3 days of culture in the presence of a bone slice coated with bisphosphonate or PBS, the bone was removed and the cells remaining on the plate were stained for TRAP histochemistry for osteoclast detection. ALN specimens showed a higher number of mononuclear TRAP-positive cells compared to the other groups, however, the total amounts of multinucleated and giant cells were significantly higher in PBS compared to ALN and ZA (Fig. 2).
Cell viability and apoptosis
The cell viability was tested after 3 days of culture in the presence of a bone slice coated with bisphosphonate or PBS. Discrete but significant increase of cell viability was observed only when compared the ZA treated cells to PBS. No cytotoxic effect was found at the concentration of BPs used (Fig. 3a).
After 3 days of culture in the presence of a bone treated with ALN, ZA or PBS, the TUNEL assay was employed to confirm the presence of apoptotic cells. The total amount of apoptotic nuclei was significantly higher in the ALN group, compared to PBS and ZA (Fig. 3b).
Bone resorption
After 5 days of culture in the presence of a bone treated with ALN, ZA or PBS, bone slices were analyzed using scanning electron microscopy (SEM). Concavities of several sizes representing Howship’s lacunae were found on the surface of the bone in all groups (Fig. 4). However, PBS group had significantly larger resorbed area (p < 0.02) than the ALN and ZA treated groups (Fig. 5).
Osteoclast ultrastructure
The PBS-coated bone slices showed multinucleated osteoclasts with exuberant podosomes and ruffled border attached to the bone surface. On the bone previously treated with ALN or ZA, the multinucleated cells detected did not present attachment and resorption apparatus; they were observed without clear zone or ruffled border and unattached to the bone (Fig. 6).
Transmission electron micrographs of bone marrow cells cultivated for 3 days on bone slices show, in a, an active osteoclast with podosomes attached to the bone, in b, osteoclasts near to the bone surface but not adhered, and in c, fusiform mesenchymal cells on the bone surface, while rounded cells with numerous but short processes are close but not attached to the bone
Expression of osteoclast signaling, differentiation, and activity markers
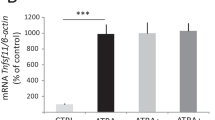
After 3 days of culture, the gene expression of osteoclastogenesis regulators RANKL and OPG were highly expressed in ZA group, with significantly higher expression of OPG. In ALN group, although these genes expression were lower than in the ZA group, OPG expression was also higher than RANKL. The expression of osteoclast activity markers, TRAP and Cathepsin K, were significantly higher in the PBS group, compared to ALN and ZA (Fig. 7).
Discussion
The results of the present study showed that ALN and ZA have inhibitory effects on osteoclastogenesis when incorporated into bone slice, even without addition of the drugs into the culture media. The bone slices saturated with both BPs affected cultured osteoclasts, as shown by TRAP histochemistry, viability and apoptosis assays, scanning and transmission electron microscopy and gene expression analyses.
This in vitro approach attempts to mimic what happens in vivo, simulating the presence of remaining BPs attached to bone surface after stopping treatment. Its prolonged effect is related to the hydroxyapatite binding affinity of BPs (Henneman et al. 2008; Russell 2011). It has been previously demonstrated that ALN binding to bone in vitro is saturable and reversible at the same concentration employed in the present study (1 mM), which suggests that BP binds to the resorption surfaces and is later released during acidification (Sato et al. 1991).
The saturation analysis of bone slices proved to be effective to test this condition. Although some early studies have used pre-treated bone slices (van der Pluijm et al. 1996; Breuil et al. 1998) they did not quantify the amount of BP that remained adhered to the bone. In addition, many studies using the osteoclast culture model often treated the cells directly with BPs; moreover, they often do not use any bone substrate (Martins et al. 2015; Gao et al. 2017).
The remaining BPs attached to the mineralized bone matrix yield no cytotoxic effect to the cultured cells. However, high level of apoptosis took place in the ALN group, as revealed by TUNEL. Although it is believed that the mechanism of action of nitrogen-BPs, such as ALN and ZA, do not promote osteoclast apoptosis, Tai et al. observed that ZA triggers in vitro reactive oxygen species (ROS) and mitochondrial damage, resulting in apoptosis of precursors and mature osteoclasts (Tai et al. 2017). Numerous apoptotic cells after treatment with ALN were also observed in other in vivo studies after TUNEL staining (Bradaschia-Correa et al. 2013), and in vitro by flow cytometry (Ding et al. 2018).
Two main stages of osteoclast biology have been evaluated herein, the differentiation stage and the activation/resorption stage. Inhibition of osteoclastogenesis occurred in both AN and ZA treatment groups though different mechanisms. When ALN was present in the bone surface, the osteoclastogenesis inhibition occurred via decrease in RANKL expression. The same mechanism of osteoclast inhibition was found in a previous study where ALN was administered to young rats and the inactivation of osteoclasts through reduced RANKL expression resulted in the impairment of tooth eruption (Bradaschia-Correa et al. 2013) The ZA-treated group, in contrast, highly expressed OPG as mechanism of inhibition of osteoclastogenesis. Indeed, the gene expression of RANKL and OPG could indicate whether there is stimulation or inhibition of osteoclastogenesis; they were in apparent balance in the PBS group, as occurs in physiological situations (Arana-Chavez and Bradaschia-Correa 2009). The inhibition of osteoclastogenesis by both BPs was furthered demonstrated by TRAP histochemistry. PBS-treated cultures showed a high number of multinucleated and giant TRAP-positive cells, which are considered real osteoclasts. Contrarily, both BP-treated cultures showed a higher population of mononuclear TRAP-positive cells, which are likely non-activated osteoclast precursors. These findings show that the effect of both BPs avoided the early steps of osteoclastogenesis, in which the fusion/activation of osteoclasts take place.
At later stages, when osteoclast activation/resorption occurs, the gene expression of the bone resorption markers TRAP and Cathepsin-K, as well as transmission and scanning electron microscopy evaluation also showed decreased resorption activity by both BPs. When the bone slices were pre-treated with ALN and ZA, the gene expression of TRAP and Cathepsin-K was reduced in comparison to the PBS group, indicating the reduction of the resorption activity. This inhibition was confirmed by TEM ultrastructural analysis, which detected osteoclasts with full resorption apparatus (prominent podosomes and ruffled border) in the PBS-treated group while ALN and ZA groups showed multinucleated latent osteoclasts non-attached to the bone surface. Consequently, the amount of resorption pits observed by SEM on the bone surfaces was lower in ALN and ZA groups compared to the PBS group. Previous in vivo studies showed that ALN-treated rats exhibit large amounts of latent osteoclasts, which do not attach to the bone matrix and lack polarization and resorption apparatus (Bradaschia-Correa et al. 2013; Rezende et al. 2017).
The ultrastructural analyses were important tools for evaluating the BP effect on the resorptive activity of cultured osteoclasts. Whereas some studies only show the gene expression and/or the differentiation stages, transmission electron microscopy revealed the ultrastructural characteristics of osteoclasts adhered to the saturated bone slices. Moreover, examination of bone surfaces by scanning electron microscopy precisely revealed whether bone resorption occurred, which cannot be demonstrated as clearly by conventional light microscopy (Koek et al. 2017; Manrique et al. 2017).
Although nitrogen-containing BPs have a similar mechanism of action, the potency of these drugs is quite different. Indeed, the higher potency of ZA in comparison to that of ALN is well known. However, given the limitations of an in vitro approach and the lifetime of mouse marrow-derived osteoclasts, this difference between ALN and ZA was not observed in this study. Nevertheless, the remaining inhibition observed in the current study may be also expected in vivo, thus impacting on physiological bone turnover or even oversuppressing its remodeling events (Ma et al. 2017). Considering this BP inhibition effect, all clinical procedures that involve bone remodeling on patients that previously received nitrogen-containing BP therapy such as orthodontic tooth movement, tooth extractions (Jabbour et al. 2014), or dental implants must be carefully evaluated, postponed, or even suspended in front of the risk of severe complications as MRONJ, osteomyelitis and necrosis (Bertoldo et al. 2007).
It is concluded that the nitrogen-containing BPs ALN and ZA previously incorporated into bone slices but no longer administered to cell cultures have effects that affect the differentiation and activity of osteoclasts.
Data availability
The work described in this manuscript has not been published previously and it is not under consideration for publication elsewhere. The manuscript has been approved by all authors.
References
Al Deeb SK, Hamdan II, Al Najjar SM (2004) Spectroscopic and HPLC methods for the determination of alendronate in tablets and urine. Talanta 64:695–702. https://doi.org/10.1016/j.talanta.2004.03.044
Arana-Chavez VE, Bradaschia-Correa V (2009) Clastic cells: mineralized tissue resorption in health and disease. Int J Biochem Cell Biol 41:446–450. https://doi.org/10.1016/j.biocel.2008.09.007
Bertoldo F, Santini D, Lo Cascio V (2007) Bisphosphonates and osteomyelitis of the jaw: a pathogenic puzzle. Nat Clin Pract Oncol 4(12):711–721. https://doi.org/10.1038/ncponc1000
Bradaschia-Correa V, Moreira MM, Arana-Chavez VE (2013) Reduced RANKL expression impedes osteoclast activation and tooth eruption in alendronate-treated rats. Cell Tissue Res 353:79–86. https://doi.org/10.1007/s00441-013-1623-9
Breuil V, Cosman F, Stein L et al (1998) Human osteoclast formation and activity in vitro: effects of alendronate. J Bone Miner Res 13:1721–1729. https://doi.org/10.1359/jbmr.1998.13.11.1721
Choi NK, Solomon DH, Tsacogianis TN et al (2017) Comparative safety and effectiveness of denosumab versus zoledronic acid in patients with osteoporosis: a cohort study. J Bone Miner Res 32:611–617. https://doi.org/10.1002/jbmr.3019
Compston J (2020) Practical guidance for the use of bisphosphonates in osteoporosis. Bone 136:115330. https://doi.org/10.1016/j.bone.2020.115330
Coskun Benlidayi I, Guzel R (2013) Oral bisphosphonate related osteonecrosis of the jaw: a challenging adverse effect. ISRN Rheumatol 2013:215034. https://doi.org/10.1155/2013/215034
Cummings SR, Santora AC, Black DM, Russell RGG (2020) History of alendronate. Bone 137:115411. https://doi.org/10.1016/j.bone.2020.115411
Ding N, Liu C, Yao L et al (2018) Alendronate induces osteoclast precursor apoptosis via peroxisomal dysfunction mediated ER stress. J Cell Physiol 233:7415–7423. https://doi.org/10.1002/jcp.26587
Fleisch H, Russell RGG, Francis MD (1969) Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 165:1262–1264. https://doi.org/10.1126/science.165.3899.1262
Gao SY, Zheng G, Wang L et al (2017) Zoledronate suppressed angiogenesis and osteogenesis by inhibiting osteoclasts formation and secretion of PDGF-BB. PLoS ONE 12:e0179248. https://doi.org/10.1371/journal.pone.0179248
Henneman ZJ, Nancollas GH, Ebetino FH et al (2008) Bisphosphonate binding affinity as assessed by inhibition of carbonated apatite dissolution in vitro. J Biomed Mater Res A 85:993–1000. https://doi.org/10.1002/jbm.a.31599
Holliday LS, Dean AD, Greenwald JE, Gluck SL (1995) C-type natriuretic peptide increases bone resorption in 1,25-dihydroxyvitamin D3-stimulated mouse bone marrow cultures. J Biol Chem 270:18983–18989. https://doi.org/10.1074/jbc.270.32.18983
Jabbour Z, El-Hakim M, Henderson JE et al (2014) Bisphosphonates inhibit bone remodeling in the jaw bones of rats and delay healing following tooth extractions. Oral Oncol 50(5):485–490. https://doi.org/10.1016/j.oraloncology.2014.02.013
Khan SA, Kanis JA, Vasikaran S et al (1997) Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res 12:1700–1707. https://doi.org/10.1359/jbmr.1997.12.10.1700
Kishimoto H, Noguchi K, Takaoka K (2019) Novel insight into the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Jpn Dent Sci Rev 55(95):102. https://doi.org/10.1016/j.jdsr.2018.09.002
Koek WNH, van der Eerden BCJ, Alves RDAM et al (2017) Osteoclastogenic capacity of peripheral blood mononuclear cells is not different between women with and without osteoporosis. Bone 95:108–114. https://doi.org/10.1016/j.bone.2016.11.010
Ma S, Goh EL, Jin A et al (2017) Long-term effects of bisphosphonate therapy: perforations, microcracks and mechanical properties. Sci Rep 7:43399. https://doi.org/10.1038/srep43399
Manrique E, Castillo LM, Lazala O et al (2017) Bone resorptive activity of human peripheral blood mononuclear cells after fusion with polyethylene glycol. J Bone Miner Metab 35:127–141. https://doi.org/10.1007/s00774-016-0744-0
Martins CA, Leyhausen G, Volk J, Geurtsen W (2015) Effects of alendronate on osteoclast formation and activity in vitro. J Endod 41:45–49. https://doi.org/10.1016/j.joen.2014.07.010
Nancollas GH, Tang R, Phipps RJ et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627. https://doi.org/10.1016/j.bone.2005.05.003
Rezende E, Bradaschia-Correa V, Siviero F et al (2017) Effects of bisphosphonates on osteogenesis and osteoclastogenesis signaling during the endochondral ossification of growing rats. Cell Tissue Res 368:287–300. https://doi.org/10.1007/s00441-017-2574-3
Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J (2011) Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49:34–41. https://doi.org/10.1016/j.bone.2010.11.008
Russell RGG (2011) Bisphosphonates: the first 40 years. Bone 49:2–19. https://doi.org/10.1016/j.bone.2011.04.022
Sato M, Grasser W, Endo N et al (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Investig 88(6):2095–2105. https://doi.org/10.1172/JCI115539
Sims NA, Ng KW (2014) Implications of osteoblast-osteoclast interactions in the management of osteoporosis by antiresorptive agents denosumab and odanacatib. Curr Osteoporos Rep 12(1):98–106. https://doi.org/10.1007/s11914-014-0196-1
Szewczyk KA, Fuller K, Chambers TJ (2013) Distinctive subdomains in the resorbing surface of osteoclasts. PLoS ONE 8:e60285. https://doi.org/10.1371/journal.pone.0060285
Tai TW, Chen CY, Su FC et al (2017) Reactive oxygen species are required for zoledronic acid-induced apoptosis in osteoclast precursors and mature osteoclast-like cells. Sci Rep 7:44245. https://doi.org/10.1038/srep44245
Toro EJ, Zuo J, Guiterrez A et al (2013) Bis-enoxacin inhibits bone resorption and orthodontic tooth movement. J Dent Res 92:925–931. https://doi.org/10.1177/0022034513501876
Unnanuntana A, Jarusriwanna A, Songcharoen P (2017) Randomized clinical trial comparing efficacy and safety of brand versus generic alendronate (Bonmax®) for osteoporosis treatment. PLoS ONE 12:e0180325. https://doi.org/10.1371/journal.pone.0180325
van der Pluijm G, Vloedgraven H, van Beek E et al (1996) Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Invest 98:698–705. https://doi.org/10.1172/JCI118841
von Moos R, Costa L, Gonzalez-Suarez E et al (2019) Management of bone health in solid tumours: from bisphosphonates to a monoclonal antibody. Cancer Treat Rev 76:57–67. https://doi.org/10.1016/j.ctrv.2019.05.003
Acknowledgements
The authors thank Prof. Glaucia Machado-Santelli for her advice and Mrs. Elisangela Chinen for her technical assistance. This work was supported by FAPESP (Brazil)—Grant #13/02240-9.
Author information
Authors and Affiliations
Contributions
VBC: conceptualization, formal analysis, investigation, writing – original draft preparation, review & editing, funding acquisition. GCRS: formal analysis, investigation. LPF: formal analysis, investigation, writing—review & editing, PRT: formal analysis, investigation. VEAC: conceptualization, resources, writing—review and editing, supervision, funding acquisition. Each author has read and approved the manuscript prior to submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Ethical approval
Animal experiments in this study were approved by the Ethics Committee for Animal Research of the University of São Paulo School of Dentistry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bradaschia-Correa, V., Ribeiro-Santos, G.C., de Faria, L.P. et al. Inhibition of osteoclastogenesis after bisphosphonate therapy discontinuation: an in vitro approach. J Mol Histol 53, 669–677 (2022). https://doi.org/10.1007/s10735-022-10083-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10083-9