Abstract
MicroRNAs act as regulators in ovarian tumorigenesis and progression by involving different molecular pathways. Here, we examined the role of miR-135b on growth, chemotherapy resistance in OVCAR3 and SKOV3 ovarian cancer cells. MTT assay was performed to examine proliferation. Transwell migration and matrigel invasion assays were used to assess migration and invasion. Caspase-Glo3/7 assay was carried out to evaluate apoptosis. The dual-luciferase reporter assay was performed to validate the putative binding site. Meanwhile, the miR-135b levels in human ovarian cancer tissue were detected by qPCR assay. Overexpression of miR-135b increased growth, and improved migration and invasion in ovarian cancer cells. Meanwhile, overexpression of miR-135b decreased the cisplatin treatment sensitivity in OVCAR3 and SKOV3 cells. The cisplatin-induced apoptosis was decreased by miR-135b. Furthermore, miR-135b could alter epithelial to mesenchymal transition (EMT) associated proteins expression including E-cadherin, N-cadherin, snail and Vimentin in ovarian cancer cells. Further study demonstrated aberrant expression of miR-135b regulated PTEN and p-AKT expression in ovarian cancer cells. The expression level of miR-135b was increased in human ovarian cancer tissue, compared with normal ovary tissue. MiR-135b involves in tumorigenesis and progression in ovarian cancer cells, and might serve as a promising biomarker to predict chemotherapy sensitivity and prognosis in ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer remains the leading cause of cancer death due to the difficulty of early detection (Torre et al. 2017). The current screening methods for ovarian cancer included ultrasound and CA-125 (Rauh-Hain et al. 2011). However, only 15% of all ovarian cancers are detected at early stage (Smith and Smith 2017; Torre et al. 2018). Chemotherapy plays important roles in ovarian cancer therapy (Cristea et al. 2010). Cisplatin is a platinum-based chemotherapy drug and is widely used for a variety of cancers including ovarian cancer (Dasari et al. 2014). Cisplatin inhibits proliferation of cancer cells by interfering with the process of cell division. Recently, the development of cisplatin resistance in human cancer cells has been reported (Shen et al.2012). Reduced accumulation of cisplatin in cancer cells is one of the most important mechanisms of cellular resistance to cisplatin. The median survival time of patients with resistant ovarian cancer is significantly short (Soyama et al. 2017). Therefore, understanding the chemoresistance molecular mechanisms will help find out more effective ways in ovarian cancer treatment.
MicroRNAs are non-coding small RNA molecules of about 22 nucleotides in length. MiRNAs can bind to the 3′-untranslated region (3′-UTR) of target mRNAs and influence multiple critical cellular processes including proliferation, apoptosis and differentiation by inhibiting the translation process, or initiate the process of mRNA degradation (Macfarlane and Murphy 2010). Aberrant miRNA expression has been reported in a variety of human neoplasms, including ovarian cancer tissues (Mihailescu and Mihailescu 2015). The miRNA profiling studies have demonstrated that the expression patterns of numerous miRNAs in ovarian cancer tissue are different from that in normal ovarian tissue (Li et al. 2010). The aberrant expressions of these miRNAs are associated with the ovarian tumorigenesis, invasion, metastasis and response to therapy by interacting with different signaling pathways and transcription factors in ovarian cancer (Di Leva and Croce 2013). These findings indicate that miRNAs play important roles in early diagnosis and may be an attractive target for treatment.
Recently, studies have shown that aberrant expression of miR-135b is associated with carcinogenesis in variety of organs. However, the effects of miR-135b on ovarian cancer have not been well studied. In this study, we found that miR-135b increased growth and improved migration and invasion in ovarian cancer cells. Meanwhile, miR-135b decreased cisplatin treatment sensitivity in ovarian cancer cells by regulating PTEN and p-AKT expression. These findings indicated that miR-135b might serve as a promising biomarker to predict chemotherapy sensitivity and prognosis in ovarian cancer.
Materials and methods
Cell lines
Two ovarian cancer cell lines, OVCAR3 and SKOV3, were obtained from the American Type Culture Collection (ATCC, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, USA). The medium was mixed with 10% fetal bovine serum (Gibco, USA), 100 units of penicillin/ml and 100 mg of streptomycin/ml (Invitrogen, USA). When ovarian cancer cell confluence reached 70%, cells were used for experiments.
Transfection
The miR-135b mimic and miR-135b inhibitor were purchased from Invitrogen. The scramble miRNA was used as negative control (Invitrogen, USA). Lipofectamine 2000 (Invitrogen, USA) was used to transfect miRNA mimic and inhibitor according to the manufacturer’s instructions. Ovarian cancer cells were seeded in 6 well plates and grown for 24 h. And then the medium was replaced with transfection medium containing either miR-135b mimic or miR-135b inhibitor. OVCAR3 and SKOV3 cells were cultured for no more than 6 h. Then these cells were grown in complete medium for further analyses.
Quantitative real-time polymerase chain reaction (qPCR)
Quantitative Real-Time PCR (qPCR) was carried out to analyze miR-135b expression level in OVCAR3 and SKOV3 cells after transfections. TRIzol reagent (Invitrogen, USA) was used to extract total RNA from transfected OVCAR3 and SKOV3 cells. Reverse transcription was performed using High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, USA). Briefly, 1 µg total RNA was added to reverse transcription master mix and centrifuge to spin down the contents and eliminate air bubbles. The RT-PCR were performed as follows: 95 °C for 2 min, 38 cycles at 95 °C for 20 s, 60 °C for 30 s, and a dissociation stage. The primers were synthesized by Sigma (USA) and the sequences were as follow: miR-135b forward: 5’- GTAAATGTTTGTATATGTG-3’ and reverse 5’- TCAAATAAATACCTAA-3’. Each reaction was performed in triplicates. U6 was used as the endogenous control.
Cell proliferation assay
MTT assay was performed to examine the ovarian cancer cell proliferation. Briefly, transfected OVCAR3 and SKOV3 cells were placed at 5 × 103 cells/well in 96-well plates in complete medium and grew overnight. Then the medium was replaced with medium containing different concentration of cisplatin (0, 2, 4 and 6 µM). Every 48 h, 20 µl of MTT solution (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) was added into the medium, and incubated with the cells for 4 h at 37 °C in a humidified incubator. Then the culture medium was removed, and DMSO was added and incubated for 30 min with gentle agitation. The signal was detected on a microplate reader (BioTek, Germany) at 570 nm.
Caspase 3/7 activity-based apoptosis assay
The transfected ovarian cancer cells were seeded in 96-well plates and cultured overnight at 37 °C in a humidified incubator. Then the supernatant was removed and complete medium with different concentrations of cisplatin (0, 2, 4 and 6 µM) were added to each well. OVCAR3 and SKOV3 cells were cultured for 48 h. Then the caspase-3/7 activity was examined using Caspase-Glo assay kit (Promega, USA). Briefly, Caspase-Glo reagent was incubated with the ovarian cancer cells for 2 h at room temperature. Then, the luminescence was detected with parameters of 1 min lag time and 0.5 s/well read time in a plate-reading luminometer (ThermoFisher, USA).
Transwell assay
Transwell assays were conducted to examine ovarian cancer cell invasion and migration. The invasion assay was performed using the upper chambers coated with Matrigel (BD Bioscience, USA). The migration assay was conducted using the top chambers without Matrigel. In brief, 5 × 104 ovarian cancer cells were seeded in the upper chambers in serum free DMEM medium, and the DMEM medium supplemented with 10% FBS was added into the lower chambers. After 18 h incubation in a humidified incubator at 37 °C, the invaded cells on the bottom of membrane were stained for 30 min. The invaded cells were counted at randomly fields and the mean value was calculated.
Western blotting
The transfected ovarian cancer cells were incubated with cold RIPA lysis buffer (1 × 106 of cells per mL) (Abcam, USA) to extract the total protein (Abcam, USA). Then 30 µg of protein was separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (10%). The protein was transferred to PVDF membranes (Sigma, USA). 5% nonfat dried milk in PBS was used to mount the membranes at room temperature for one hour with gentle agitation. Next, the membranes were probed with different primary antibodies (Table 1) overnight at 4 °C with gentle agitation. After incubation with secondary antibodies (1:1000) at room temperature for 1 h, the signal on the membrane was detected using enhanced chemiluminescence reagent (Pierce, USA). The results were normalized to GAPDH (loading control) and expressed as relative density.
Luciferase assay
The possible binding site of miR-135b was identified using miRDB online database. The 3’-UTR of PTEN sequence containing the potential miR-135b binding site was amplified from human normal cells genomic DNA. Using the In-Fusion Dry-Down PCR Cloning Kit (Clontech, USA), the amplified sequence was cloned into the Xbal site of the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, USA). MiR-135b binding sites seed region was mutated and was used as control. OVCAR3 cells were co-transfected with Luc-PTEN and miR-135b mimic using Lipofectamine RNAiMAX. Luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega, USA).
MiR-135b expression on human ovarian cancer tissue
Formalin-fixed paraffin-embedded (FFPE) tissue was obtained from 38 ovarian cancer patients between 2015 and 2019. The average age of the patients was 63.5 years. All four stages (I to IV) were included in this study. Non-neoplastic tissue was used as control. This study was approved by the ethical committee of Jilin University. All patients signed the informed consent that the tissue can be used for research and molecular studies. The diagnosis was confirmed on hematoxylin and eosin (H&E) staining. The tissue was macrodissected from unstained FFPE sections. For microdissection, tumor areas were marked by a pathologist on H&E stained slides under microscope. The interested areas were scraped from three serial FFPE sections. Total RNA extraction was performed using PureLink™ FFPE Total RNA Isolation Kit (ThermoFisher Scientific, USA). Briefly, FFPE sections were placed in paraffin melting buffer, and incubated with proteinase K for 3 h at 60 °C. The tissue lysate was collected in RNase-free pipette tip and mixed with binding buffer and ethanol. Then the samples were transfer to a Spin Cartridge and centrifuge for 1 min. After wash with washing buffer, RNA was eluted with RNase-free water. QPCR were performed as the above mentioned methods.
Statistical analysis
SPSS 12.0 statistical software (IBM Corp, USA) was used for statistical analysis by using two-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
MiR-135b improved proliferation of ovarian cancer cells and induced cisplatin resistance in dose dependent manner.
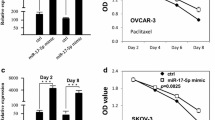
MTT assay was performed to evaluate the role of miR-135b on proliferation of ovarian cancer cells with or without cisplatin treatment. The scrambled negative control RNAs were used as negative control. MiR-135b expression level was significantly increased in both OVCAR3 (Fig. 1A) and SKOV3 (Fig. 2A) cells after transfection of miR-135b mimic. MiR-135b overexpression increased growth of OVCAR3 (Fig. 1B) and SKOV3 cells (Fig. 2B) (p < 0.05). Additionally, the survival rate of OVCAR3 (Fig. 1C–E) and SKOV3 cells (Fig. 2C–E) was improved after miR-135b overexpression at presence of cisplatin (p < 0.05).
Upregulation of miR-135b improved the growth and survival of OVCAR3 cells. A The miR-135b expression in OVCAR3 ovarian cancer cells transfected with miR-135b mimic. B The growth of OVCAR3 cells after miR-135b upregulation. C–E The growth of OVCAR3 cells transfected with miR-135b mimic in presence of different doses of cisplatin (2, 4 and 6µM)
Downregulation of miR-135b decreased the growth and survival of OVCAR3 cells. A The miR-135b expression in OVCAR3 ovarian cancer cells transfected with miR-135b inhibitor. B The growth of OVCAR3 cells after downregulation of miR-135b. C–E The growth of OVCAR3 cells transfected with miR-135b inhibitor in presence of different doses of cisplatin (2, 4 and 6µM)
MiR-135b level was dramatically inhibited in both OVCAR3 (Fig. 3A) and SKOV3 (Fig. 4A) cells after transfection of miR-135b inhibitor. Down-regulation of miR-135b inhibited growth of OVCAR3 (Fig. 3B) and SKOV3 (Fig. 4B). Furthermore, the survival rate of OVCAR3 (Fig. 3C–E) and SKOV3 (Fig. 4C–E) was decreased after down-regulation of miR-135b at the presence of cisplatin (P < 0.05).
Upregulation of miR-135b improved the growth and survival of SKOV3 cells. A The miR-135b expression in SKOV3 ovarian cancer cells transfected with miR-135b mimic. B The growth of SKOV3 cells after miR-135b upregulation. C–E The growth of SKOV3 cells transfected with miR-135b mimic in presence of different doses of cisplatin (2, 4 and 6µM)
Downregulation of miR-135b decreased the growth and survival of SKOV3 cells. A The miR-135b expression in SKOV3 ovarian cancer cells transfected with miR-135b inhibitor. B The growth of SKOV3 cells after downregulation of miR-135b. C–E The growth of SKOV3 cells transfected with miR-135b inhibitor in presence of different doses of cisplatin (2, 4 and 6µM)
MiR-135b inhibited cisplatin-induced apoptosis of ovarian cancer cells
To examine the role of miR-135b on cisplatin-induced apoptosis, ovarian cancer cells were cultured with different doses of cisplatin. The caspase 3/7 activity was determined to assess the cisplatin-induced apoptosis. Up-regulation of miR-135b inhibited cisplatin-induced apoptosis in OVCAR3 cells (Fig. 5A) and SKOV3 cells (Fig. 5B). MiR-135b overexpression inhibited BAX expression, while the Bcl-2 expression was enhanced in OVCAR3 and SKOV3 ovarian cancer cells at presence of cisplatin (Fig. 5C and D).
MiR-135b reduced cisplatin-induced apoptosis in ovarians cancer cells. A The caspase 3/7 activity in OVCAR3 ovarian cancer cells transfected with miR-135b mimic in the presence of different doses of cisplatin (2, 4 and 6µM). B The caspase 3/7 activity in SKOV3 ovarian cancer cells transfected with miR-135b in the presence of different doses of cisplatin (2, 4 and 6µM). C The expression of apoptotic proteins in OVCAR3 cells and SKOV3 cells after cisplatin treatment. D The apoptotic proteins expression level in OVCAR3 cells and SKOV3 cells after transfection with miR-135b mimic
MiR-135b regulated invasion and migration in ovarian cancer cells
The invasion and migration were evaluated using transwell invasion and migration assay. MiR-135b overexpression increased invasion and migration in OVCAR3 cells (Fig. 6A, B) and SKOV3 cells (Fig. 6E, F). In contrast, down-regulation of miR-135b inhibited invasion and migration in OVCAR3 cells (Fig. 6C, D) and SKOV3 cells (Fig. 6G, H).
The role of miR-135b on invasion and migration of OVCAR3 cells and SKOV3 ovarian cancer cells. A Upreguation of miR-135b increased migration of OVCAR3 ovarian cancer cells. B Upreguation of miR-135b increased invasion of OVCAR3 cells. C Downregulation of miR-135b decreased migration of OVCAR3 ovarian cancer cells. D Downregulation of miR-135b decreased invasion of OVCAR3 cells
E Upreguation of miR-135b increased migration of SKOV3 ovarian cancer cells. B Upreguation of miR-135b increased invasion of SKOV3 cells. C Downregulation of miR-135b decreased migration of SKOV3 7 ovarian cancer cells. D Downregulation of miR-135b decreased invasion of SKOV3 cells
EMT proteins and PTEN expression were changed by miR-135b
Western blot was performed to investigate the potential signaling pathways miR-135b involved in cell growth, invasion and migration in ovarian cancer cells. E-cadherin expression was inhibited and N-cadherin, snail and Vimentin were significantly increased in OVCAR3 cells and SKOV3 cells after miR-135b overexpression (Fig. 7A–C). However, E-cadherin expression was increased and N-cadherin, snail and Vimentin were inhibited in OVCAR3 cells and SKOV3 cells after miR-135b down-regulation(Fig. 8A–C). Interestingly, miR-135b overexpression decreased PTEN level, but increased p-AKT level in ovarian cancer cells (Fig. 7D–F). Meanwhile, the down-regulation of miR-135b increased PTEN level, but decreased p-AKT level (Fig. 8D–F).
EMT protein levels were changed by overexpression of miR-135b and increased AKT level in OVCAR3 cells and SKOV3 cells. A The EMT protein expression after transfected with miR-135b mimic. The graph indicated the relative level of EMT proteins in OVCAR3 cells B and SKOV3 cells (C). D PTEN, p-AKT and AKT levels. The graph indicated that the relative level of proteins in OVCAR3 cells (E) and SKOV3 cells (F)
(B) E-cadherin: p = 0.026; N-cadherin: p = 0.021; SNAIL: p = 0.024; vimentin: p = 0.019; (C) E-cadherin: p = 0.018; N-cadherin: p = 0.019; SNAIL: p = 0.038; vimentin: p = 0.025
EMT protein levels were changed by downregulation of MiR-135b and decreased AKT level in OVCAR3 cells and SKOV3 cells. A The EMT protein levels after transfection with miR-135b inhibitor. The graph indicated the relative level of EMT proteins in OVCAR3 cells (B) and SKOV3 cells (C). D PTEN, p-AKT and AKT levels. The graph indicated the relative level of proteins in OVCAR3 cells (E) and SKOV3 cells (F)
(B) E-cadherin: p = 0.036; N-cadherin: p = 0.031; SNAIL: p = 0.034; vimentin: p = 0.027; (C) E-cadherin: p = 0.038; N-cadherin: p = 0.029; SNAIL: p = 0.0324; vimentin: p = 0.031
MiR-135b directly targeted 3’-UTR of PTEN
Using miRNA target prediction tools, 3’-UTR of PTEN was identified as the possible binding site of miR-135b (Fig. 9A). To demonstrate the potential binding site, dual-luciferase reporter assay was carried out. The scrambled control and mutated binding sites were used as control. Luciferase activity kept similar when mu-PTEN-3’-UTR-pGL3 was co-transfected with miR-135b mimic (Fig. 9B). Luciferase activity was significantly decreased when wt- PTEN-3’-UTR-pGL3 was co-transfected with miR-135b mimic (p < 0.05) (Fig. 9C). These results demonstrated that PTEN was directly regulated by miR-135b.
miR-135b directly targeted 3’-UTR of PTEN. A. The possible binding site of miR-135b on 3’-UTR of PTEN; B. Luciferase activity after mut-PTEN-3’-UTR-pGL3 co-transfected with miR-135b mimic; C. Luciferase activity after wt-PTEN-3’-UTR-pGL3 co-transfected with miR-135b mimic. B p = 0.151; C p = 0.012
Note: Pairwise Turkey-Kramer analysis after one-way ANOVA was performed and showed the difference in means between wt- PTEN-3’-UTR-pGL3 co-transfected with miR-135b mimic and mu-PTEN-3’-UTR-pGL3 co-transfected with miR-135b mimic is statistically significant
MiR-135b expression on human ovarian cancer tissue
To examine miR-135b level in human ovarian cancer tissue, formalin-fixed paraffin-embedded (FFPE) tissue was collected and qRT-PCR was performed. As shown in Fig. 10A, miR-135b levels was higher in ovarian cancer tissue, compared with normal ovarian tissue. In ovarian cancer expressing high-level miR-135b (Fig. 10B and supplemental figure), Ki-67 proliferation index is increased. However, PTEN expression was decreased in ovarian cancer expressing high-level miR-135b.
Discussion
Emerging evidence has demonstrated that miRNAs play critical roles in tumorigenesis and progression by involving different signaling pathways (Nana-Sinkam et al. 2014).
In our study, up-regulation of miR-135b enhanced the proliferation of ovarian cancer cells, and down-regulation of miR-135b suppressed proliferation of ovarian cancer cells. These results indicated that miR-135b plays an essential role in proliferation of ovarian cancer cells. Cisplatin is one of the most effective chemotherapy agents and has been used to treat various cancers including ovarian cancer, testicle cancer and solid tumor of head and neck. In the past several decades, cisplatin has significantly improved survival rate in ovarian cancer patients (Dasari et al. 2014). However, development of resistance remains a major problem. Many signaling pathways involve in resistance development, such as cell growth-promoting pathways, DNA damage repair, apoptotic pathways and copper metabolisms (Shen et al. 2012). Our study showed miR-135b overexpression improved survival of ovarian cancer cells after cisplatin treatment. However, down-regulation of miR-135b decreased survival of ovarian cancer cells. These findings indicated that miR-135b mediated cisplatin resistance in ovarian cancer cells. Further study indicated miR-135b expression was associated with the cisplatin-induced apoptosis in ovarian cancer cells. These results suggested miR-135b might affect chemosensitivity in ovarian cancer cells to cisplatin therapy.
Epithelial–mesenchymal transition (EMT) is a biological process that epithelial cells gain mesenchymal features by multiple signaling pathways involvement. It has been shown that EMT plays important roles in the progression, invasion and metastasis of cancer cells including ovarian cancer. Recent studies have demonstrated that miRNAs contribute the progression of ovarian cancer by regulation of EMT. In the present study, we found that miR-35b played significant roles in invasion and migration of ovarian cancer cells by regulation of EMT. These findings indicated that the expression level of miR-135b was associated with ovarian cancer progression. Further study suggested that miR-135b changed the expression level of Phosphatase and Tensin homolog (PTEN) and p-AKT in ovarian cancer cells. PTEN is a well-known tumor suppressor and negatively regulates PI2K/AKT signaling. Inactivation of PTEN is associated with increased cancer cell survival, proliferation and decreased apoptosis in different cancers. Downregulation of PTEN contributes increased expression of WNT4, which is an important regulator of Müllerian duct development. WNT4 is also associated with increased migration and colonization of the ovary in a beta-catenin independent manner (Russo et al. 2018; Cai et al. 2014). The recent meta-analysis showed that aberrant expression of PI3K and pAKT could predict poor outcomes in ovarian cancer patients (Cai et al. 2014). Recent studies have provided evidences that miRNAs can regulate the expression of PTEN in ovarian cancer. Luo et al showed that miR-20a was associated with the EMT phenotypical transformation in ovarian cancer cells by regulation of PTEN (Luo et al. 2013). Another study by Wang et al. found that miR-19a promoted the growth of ovarian cancer cells by directly binding to the 3’-UTR of PTEN (Wang et al. 2018). Our results suggested that miR-135b affected proliferation by regulation of PTEN signaling pathway.
In summary, our findings demonstrated miR-135b could regulate growth and chemosensitivy in ovarian cancer cells by directly binding to 3’-URT of PTEN. But, ovarian cancer is a heterogeneous disease and many different signaling pathways involve in tumor development in ovarian cancer. Therefore, further studies are required to figure out how miR-135b correlates with other signaling pathway and may help find other ways in ovarian cancer treatment.
Conclusions
miR-135b involves in tumorigenesis and progression in ovarian cancer cells, and might serve as a promising biomarker to predict chemotherapy sensitivity and prognosis in ovarian cancer.
Data Availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Cai J, Xu L, Tang H et al (2014) The role of the PTEN/PI3K/Akt pathway on prognosis in epithelial ovarian cancer: a meta-analysis. Oncologist 19(5):528–535
Cristea M, Han E, Salmon L, Morgan RJ (2010) Practical considerations in ovarian cancer chemotherapy. Therapeutic Adv Med Oncol 2(3):175–187
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Di Leva G, Croce CM (2013) The Role of microRNAs in the Tumorigenesis of Ovarian Cancer. Front Oncol 3:153
Li SD, Zhang JR, Wang YQ, Wan XP (2010) The role of microRNAs in ovarian cancer initiation and progression. J Cell Mol Med 14(9):2240–2249
Luo X, Dong Z, Chen Y, Yang L, Lai D (2013) Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif 46(4):436–446
Macfarlane LA, Murphy PR (2010) MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genom 11(7):537–561
Mihailescu R (2015) Gene expression regulation: lessons from noncoding RNAs. RNA 21(4):695–696
Nana-Sinkam SP, Croce CM (2014) MicroRNA regulation of tumorigenesis, cancer progression and interpatient heterogeneity: towards clinical use. Genome Biol 15(9):445
Rauh-Hain JA, Krivak TC, Del Carmen MG, Olawaiye AB (2011) Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol 4(1):15–21
Russo A, Czarnecki AA, Dean M et al (2018) PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 37(15):1976–1990
Shen DW, Pouliot LM, Hall MD, Gottesman MM (2012) Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 64(3):706–721
Soyama H, Takano M, Miyamoto M et al (2017) Factors favouring long-term survival following recurrence in ovarian cancer. Mol Clin Oncol 7(1):42–46
Torre LA, Islami F, Siegel RL, Ward EM, Jemal A (2017) Global Cancer in Women: Burden and Trends. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research. cosponsored by the American Society of Preventive Oncology 26(4):444–457
Wang Y, Zhao S, Zhu L, Zhang Q, Ren Y (2018) MiR-19a negatively regulated the expression of PTEN and promoted the growth of ovarian cancer cells. Gene 670:166–173
Smith CG (2017) A Resident’s Perspective of Ovarian Cancer. Diagnostics 7(2)
Torre LA, Trabert B, DeSantis CE, et al (2018) Ovarian cancer statistics. CA a cancer journal for clinicians 68(4): 284 – 96
Author information
Authors and Affiliations
Contributions
JW and RZ: performed experiments, write manuscript. BZ, JL and LZ: performed experiments, prepare figures and table. WJ and XL: Collected patient tissue, performed experiments. XD: design the project, write manuscript and statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Ethical approval
Ethical committee of Jilin University approved this study. The informed consents were signed by all patients. All methods were performed in accordance with the relevant guidelines and regulations by the Ethical committee of Jilin University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10735_2022_10080_MOESM1_ESM.tiff
Supplementary file1 The graph indicated Ki67 positive cells (A) and PTEN positive cells (B) in normalovarian tissue, ovarian cancer tissue with low-level miR-135b and ovarian cancer tissue with high-level miR-135b (TIFF 24.7 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, R., Zhang, B. et al. MiR-135b improves proliferation and regulates chemotherapy resistance in ovarian cancer. J Mol Histol 53, 699–712 (2022). https://doi.org/10.1007/s10735-022-10080-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10080-y