Abstract
Echinacoside is a group of natural compounds extracted from medicinal plants Cistanche and Echinacea, which has neuroprotective, antiaging, immunomodulatory and anticancer effects, but its specific role and mechanism in tumor remains partially unclear. To our knowledge, it was the first time to reported the effect of Echinacoside in ovarian cancer. Colony formation, TUNEL staining, Transwell and tube formation assays were conducted to analyze the proliferation, apoptosis, invasion and tube formation abilities of serous ovarian carcinoma cells (SKOV3 and OVCAR-3), respectively. The expressions of apoptosis-, invasion- and PI3K/AKT pathway-related proteins were measured by western blotting. In addition, PI3K agonist (740Y-P) was used to assess the regulatory effect of Echinacoside on PI3K/AKT signaling in ovarian cancer. Finally, the anti-tumor effect of Echinacoside on SKOV3-xenografted mice was evaluated by xenograft tumor mouse model. Our results demonstrated Echinacoside concentration-dependently reduced the proliferation, migration and angiogenesis of ovarian cancer cells, whereas promoted apoptosis. Moreover, western blotting revealed that Echinacoside suppressed the growth of ovarian cancer cells by downregulating the phosphorylation levels of PI3K, AKT and mTOR, which could be partially reversed by 740Y-P. Further, in vivo results showed that Echinacoside could effectively alleviate the tumor growth of xenograft mice, accompanied by the decrease of PI3K/AKT signaling. In general, our results demonstrate that Echinacoside could reduce the ovarian cancer progression through inhibition of PI3K/AKT pathway, suggesting that Echinacoside may be a new treatment option for ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is one of the most common and lethal gynecological malignancy in the female reproductive system. According to statistics, there were approximately 295,000 new cases and more than 184,000 deaths worldwide in 2018 (Bray et al. 2018; Kannan et al. 2014) Due to the complexity of ovarian tissue and endocrine function, the early symptoms of patients are usually not obvious, resulting in about 70% of patients being in advanced stages by the time of their first diagnosis (Swiatly et al. 2017) Currently, ovarian cancer is mainly treated by surgery resection combined with adjuvant chemotherapy, however, in view of some surgical limitations, chemotherapy resistance and tumor recurrence and metastasis, the 5-year survival rate of patients with advanced ovarian cancer was still less than 30% (Qiu et al. 2014). Therefore, understanding the pathogenesis of ovarian cancer and finding therapeutic approach is of great significance to improve the survival status of patients with ovarian cancer.
In recent years, with the development of molecular biology and traditional Chinese medicine, the application of effective ingredient of traditional Chinese medicine to clear the lesion has become a development trend in oncology field. Echinacoside (Fig. 1A) is a type of natural compounds, which has been widely used in the treatment of common cold and infection in Europe.(Jiang and Tu 2009; Dong et al. 2015) Recent studies have shown that Echinacoside has antioxidant, antiaging and antitumor activities (Wu et al. 2007; Xie et al. 2009; Wang et al. 2015) Especially in terms of antitumor effects, in vitro studies have demonstrated that Echinacoside impaired the growth and induce apoptosis of colon pancreatic cancer cells, while its effect on ovarian cancer remains unknown (Dong et al. 2015; Wang et al. 2016; Deng et al. 2004). Additionally, the activation of PI3K/AKT signaling is widely involved in the regulation of tumor cell proliferation, invasion, metastasis and apoptosis, which is closely associated with the tumor progression. Over recent years, a large number of studies demonstrated PI3K/AKT signaling is overactive by allowing abnormal proliferation and apoptosis in ovarian cancer (Jin et al. 2017; Ma et al. 2018). Therefore, whether Echinacoside plays a protective role in ovarian cancer by regulating PI3K/AKT remains to be further studied.
Echinacoside inhibited the viability of ovarian cancer cells. A Structures of Echinacoside. B Effect of different Echinacoside concentrations (0, 6.25, 12.5, 25, 50, 100, 200 and 400 μM) on the viability of SKOV3 cell viability was detected by CCK-8. C Effect of different Echinacoside concentrations (0, 6.25, 12.5, 25, 50, 100, 200 and 400 μM) on the OVCAR-3 cell viability was detected by CCK-8. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. 0 μM. ECH Echinacoside
In this study, we tried to investigate whether Echinacoside suppressed ovarian cancer cell proliferation, invasion and angiogenesis, and induced apoptosis by downregulating the PI3K/AKT pathway. Meanwhile, the xenograft tumor model was constructed by subcutaneous injection of ovarian cancer cells to explore the effect of Echinacoside on ovarian cancer growth in mice.
Materials and methods
Cell culture
Two serous ovarian carcinoma cells (SKOV3 and OVCAR-3) were purchased from Shanghai Cellular Research Institute (Shanghai, China). The two cells were cultured in RPMI1640 medium containing 10% fetal bovine serum (FBS; HyClone, Pittsburgh, USA), 100 U/ml penicillin and 100 U/ml streptomycin. Cell incubator was set at 95% relative humidity and 37 °C under 5% the CO2 environment. Cells were passaged when the cell fusion reached 80%. Cells at logarithmic phase were used for the follow-up experimental studies. Echinacoside was obtained from Sigma-Aldrich (Sigma, St Louis, MO) and different concentration of Echinacoside dilutions were made using PBS. SKOV3 and OVCAR-3 cells were incubated with the proper concentration of Echinacoside for 24 h at 37 °C. PI3K/AKT signaling agonist 740Y-P was purchased from TOCRIS (Bristol, UK). SKOV3 and OVCAR-3 cells treated with Echinacoside were co-incubated with 740Y-P (100 μg/ml) for 24 h at 37 °C.
CCK-8 assay
Cell Counting Kit-8 (CCK-8) assay kit (Dojindo Laboratories, Japan) was employed to determine cell proliferation. SKOV3 and OVCAR-3 cell suspensions were inoculated into 96-well plates at the density of 2 × 104 cells/ml and coincubated with different concentrations of Echinacoside (0, 6.25, 12.5, 25, 50, 100, 200 and 400 μM) for 24 h. After the end of culture, 10 μl CCK8 solution was added to each hole and then incubated for 4 h. The absorbance of each group at 450 nm was measured by the automatic microplate reader (Syngene, USA).
Colony formation assay
SKOV3 and OVCAR-3 cells in logarithmic phase were digested with 0.25% trypsin and then inoculated into 6-well plates at a density of 500 cells/well. Cells were cultured under conventional condition for 2 weeks. Next, colonies were fixed with 4% paraformaldehyde and then stained with crystal violet. The number of cell colonies was observed and recorded under an inverted microscope (Olympus IX71, Japan).
TUNEL staining
SKOV3 and OVCAR-3 cells stimulated by different concentrations of Echinacoside (0, 25, 50 and 100 μM) were inoculated on sterilized glass cover slips in 6-well plates at the density of 2 × 105 cells/ml and then cultured for 24 h. Next, these cells were fixed with 4% paraformaldehyde for 20 min, immersed in 3% H2O2 and permeabilized with 0.5% Triton X-100. In strict accordance with the instructions, apoptotic cells were stained by TUNEL apoptosis detection kit (Beyotime, Beijing) and were labeled with DAPI for 1 min at 37 °C. The nuclei of normal cells are stained in blue, and the nuclei of apoptotic cells were stained with red. The number of apoptotic cells and total cells were counted under the fluorescence microscope, and the apoptosis rate of each group was calculated.
Western blot
Total proteins were extracted from SKOV3 and OVCAR-3 cells stimulated by different concentrations of Echinacoside (0, 25, 50 and 100 μM) using RIPA lysis solution. The purity and concentration of proteins were determined by the ultraviolet spectrophotometer (Thermo Scientific, Boston, MA) and BCA protein quantitative kit (Biyuntian), respectively. The same amount of protein in each group was separated by 10% SDS-PAGE and transferred to PVDF membranes. These protein membranes were blocked with 5% skimmed milk for 1 h and incubated with the primary antibodies against Ki-67 (1:5000; ab209897, Abcam), Cleaved caspase-3 (1:500; ab32042, Abcam), Bax (1:1000; ab32503, Abcam), Bcl-2 (1:1000; ab32124, Abcam), E-cadherin (1:10,000; ab40772, Abcam), N-cadherin (1:1000; ab245117, Abcam), VEGFA (1:1000; ab214424, Abcam), PI3K (1:2000; ab151549, Abcam), p-PI3K (1:1000; ab138364, Abcam), AKT (1:1000; ab38449, Abcam), p-AKT (S473; 1:5000; ab81283, Abcam), p-AKT (T308; 1:5000; ab38449, Abcam), mTOR (1:2000; ab2732, Abcam), p-mTOR (S2448; 1:2000; ab1109268, Abcam) and β-actin (1:1000; ab8227, Abcam) at 4 °C overnight. Then these membranes were incubated with the corresponding secondary antibody at room temperature for 2 h. After washing the membrane with TBST twice, protein bands were visualized by enhanced ECL kit (GE Healthcare) and the intensity of target protein was assessed using Quantity One software.
Transwell assay
Cell invasion ability were detected by Transwell assay. Prior to the start of the experiment, a proliferation inhibitor mitomycin C (Sigma, USA) added and cells were incubated for a total of 2 h to reduce cell proliferation as described elsewhere (Li et al. 2007). Next, these cells were inoculated into the upper layer of Transewell chamber (BD Biosciences) coated with Matrigel (BD) at the density of 1 × 105 cells/ml, and the upper culture medium was not supplemented with FBS. RPMI1640 culture medium containing 10% FBS was added to the lower chamber. After 24 h, sterile cotton was used to wiped off the Matrigel and upper chamber cells, then these cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. These invasion cells were visualized by optical microscope and photographed.
Tube formation assay
Human umbilical vein endothelial cells (HUVEC) were used to analyze the tube formation ability of SKOV3 and OVCAR-3 cells. In order to avoid the influence of HUVEC cell proliferation on the results of angiogenesis assay, mitomycin C (5 µg/ml) was added to the cell culture medium to inhibit cell replication as described in previous study (Ma et al. 1496). And these cells were then cultured in RPMI1640 medium without FBS for 24 h under the conventional conditions. Subsequently, 50 μl of Matrigel was added to each well of 96-well plates and allowed to polymerize. HUVEC cells at 2 × 104 cells/well density were inoculated on 96-well plates and co-cultured with conditioned medium derived from SKOV3 and OVCAR-3 cells stimulated with different Echinacoside concentrations (0, 25, 50 and 100 μM) for 12 h. The tube formation ability of HUVEC cell was measured by Image J software.
Animal study
A total of ten male BALB/C nude mice (5 weeks) were obtained from the Animal Centre of Xi’an Jiaotong University (Shaanxi, China). Mice were free to diet and drink water prior to study. All the animal experiments have been approved by the Animal Ethics Committee of Maternal and Child Healthcare Hospital of Shaanxi Province. SKOV3 cell concentration was adjusted to 5 × 107 cells/ml. Each nude mouse was subcutaneously injected with 0.2 ml SKOV3 cell suspension (approximately, 1 × 107 cells). After the injection was completed, the liquid exudation at the injection-site was observed. Until the subcutaneous tumor volume was about 100 mm3, these mice were divided into control group (n = 5) and Echinacoside group (n = 5). Mice in the Echinacoside group were injected intraperitoneally with Echinacoside (10 mg/kg), while the mice in the control group were injected with PBS of the same volume. The body weight and tumor volume of mice were measured every day after injection. After 30 days post injection, mice were euthanized with a lethal dose of pentobarbital sodium. These subcutaneous tumor tissues were stripped and stored for next study. Tumor volume (mm3) was measured by three-dimensional image reconstruction using Living Image software version 4.3.1.
Immunohistochemical (IHC) analysis
The expression of Ki-67, Cleaved caspase-3, Bcl-2, E-cadherin, N-cadherin and VEGFA in tumor tissues from mice were detected by Immunohistochemistry. Briefly, following fixation, the tumors tissues embedded in paraffin were cut into 4-μm thick sections and deparaffinated in dimethylbenzene for 5 min. Then these sections were blocked and cooled to incubate with the primary antibodies against Ki-67 (1:5000; ab209897, Abcam), Cleaved caspase-3 (1:500; ab32042, Abcam), Bcl-2 (1:1000; ab32124, Abcam), E-cadherin (1:10,000; ab40772, Abcam), N-cadherin (1:1000; ab245117, Abcam) and VEGFA (1:1000; ab214424, Abcam) at 4 °C overnight. These slides were then incubated with peroxidase–conjugated secondary antibody (1:2000; goat anti-rat cat. no. A0192, Beyotime, China) at room temperature for 20 min and detection was performed by using diaminobenzidine for 3 min. The staining section were recorded by the light microscope (Micromed, Shenzhen, China) at ×400 magnification.
Statistical analysis
SPSS 22.0 software (SPSS, Chicago, IL, USA) was employed to analyzed all experimental data, and these results were all expressed as the mean ± standard deviation. Students t test was used for comparison between two groups, and One-way analysis of variance (ANOVA) followed by Tukey post hoc test was used for the comparison among groups. P < 0.05 indicates the difference was significant.
Results
Echinacoside inhibited the viability of ovarian cancer cells
First, to evaluate the effect of Echinacoside on the activity of ovarian cancer cells and determine the most suitable concentration of Echinacoside. Ovarian cancer cells (SKOV3 and OVCAR-3) were first treated with different Echinacoside concentrations (0, 6.25, 12.5, 25, 50, 100, 200 and 400 μM) for 24 h. As shown in Fig. 1B, as the Echinacoside concentration increased, the SKOV3 cell viability showed decreased in a concentration-dependent manner. IC50 value for Echinacoside was 41.35 μM, and the decrease in the activity of SKOV3 cells was the most obvious as the concentration of Echinacoside greater than 200 μM. As shown in Fig. 1C, similar results were seen in OVCAR-3 cells. Echinacoside suppressed OVCAR-3 cell viability in a concentration-dependent manner. IC50 value for Echinacoside was 58.86 μM, and when the concentration of Echinacoside was more than 200 μM, the activity of OVCAR-3 cells decreased significantly. Therefore, we determined the most suitable concentration gradient of Echinacoside (0, 25, 50 and 100 μM) for the follow-up study.
Echinacoside suppressed the proliferation and induced apoptosis in ovarian cancer cells
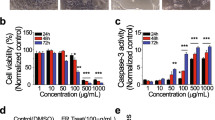
To study the specific effect of Echinacoside on ovarian cancer cells, SKOV3 and OVACR-3 cells were incubated with different Echinacoside concentrations (0, 25, 50 and 100 μM) for 24 h. Effects of different concentrations of Echinacoside on the proliferation and apoptosis of SKOV3 and OVCAR-3 cells were detected by colony formation and TUNEL staining assays. As described in Fig. 2A, Echinacoside could gradually reduce the number of colonies in a concentration-dependent manner. The inhibition of ability reached its maximum at 100 μM. As shown in Fig. 2B, with the increase of Echinacoside concentration, the apoptosis rate of SKOV3 and OVCAR-3 cells was significantly increased. Additionally, the expression of proliferation-related and apoptosis-related proteins were measured by western blotting. As summarized in Fig. 2C, Echinacoside could gradually downregulate the expression of proliferating protein Ki-67 and Bcl-2 as the Echinacoside increased, while upregulate the expression of Bax and Cleaved caspase-3. These above results demonstrated that different Echinacoside concentrations (25, 50 and 100 μM) effectively reduced ovarian cancer cell proliferation and induced apoptosis.
Echinacoside suppressed the proliferation and induced apoptosis in ovarian cancer cells. A Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on the colony formation ability of SKOV3 and OVCAR-3 cells was detected by colony formation assay. B Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on apoptosis levels of SKOV3 and OVCAR-3 cells was determined by TUNEL assay. C Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on the expression of Ki-67, Cleaved caspase-3, Bcl-2 and Bax of SKOV3 and OVCAR-3 cells was evaluated by western blotting. *p < 0.05 and **p < 0.01 vs. ECH 0 μM. ECH Echinacoside
Echinacoside reduced the invasion and tumor angiogenesis in ovarian cancer cells
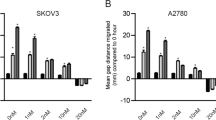
Subsequently, we further tested the inhibitory effect of Echinacoside on the invasion and angiogenesis of ovarian cancer cells. Cell invasion and angiogenesis were detected by Transwell and tube formation assays, respectively. As described in Fig. 3A, different Echinacoside concentrations (25, 50 and 100 μM) gradually inhibited the invasive ability of SKOV3 and OVCAR-3 cells as the Echinacoside concentration increased. As shown in Fig. 3B, with the increase of Echinacoside concentration, Echinacoside could gradually reduce the tube-formation capacity of HUVEC. In addition, the expression of invasion-related proteins and angiogenic factor VEGFA was measured using western blotting assay. As demonstrated in Fig. 3C, with the increase of Echinacoside concentration, the protein level of E-cadherin was gradually increased, whereas the protein levels of N-cadherin and VEGFA were decreased gradually. The above results showed that Echinacoside had significant inhibitory effects on ovarian cancer cell invasion and angiogenesis, and the inhibitory effect was positively associated with Echinacoside concentration.
Echinacoside reduced the invasion and tumor angiogenesis in ovarian cancer cells. A Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on the invasion ability of SKOV3 and OVCAR-3 cells was detected by Transwell assay. B Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on angiogenesis of SKOV3 and OVCAR-3 cells was assessed by in vitro tube formation assay. C Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on the expression of VEGFA, N-cadherin and E-cadherin of SKOV3 and OVCAR-3 cells was evaluated by western blotting. *p < 0.05 and **p < 0.01 vs. ECH 0 μM. ECH Echinacoside
Echinacoside inhibited the growth of ovarian cancer cells through downregulating PI3K/AKT signaling
Having identified the tumor-suppressive role of Echinacoside in ovarian cancer cells, we next studied the specific mechanism. The effect of different concentrations of Echinacoside (0, 25, 50 and 100 μM) on the phosphorylation of PI3K/AKT pathway was first measured by western blotting. As described in Fig. 4A, the protein levels of p-PI3K, p-AKT (S473), p-AKT (T308) and p-mTOR (S2448) in SKOV3 and OVCAR-3 cells were gradually decreased as the concentration increased. These results indicated that Echinacoside may reduce ovarian cancer progression through downregulating PI3K/AKT phosphorylation. Later, the PI3K activator 740 Y–P was used to verify the above conjecture. As summarized in Fig. 4B, 740 Y–P significantly alleviated the inhibitory effect of high concentration of Echinacoside (100 μM) on the protein expression of the levels of phosphorylated PI3K, AKT (S473), AKT (T308) and mTOR (S2448). Then, colony formation, TUNEL staining, Transwell and tube formation assays were used to verify whether Echinacoside played a vital role in ovarian cancer cell SKOV3 by regulating PI3K/AKT. As demonstrated in Fig. 4C–F, high concentration of Echinacoside (100 μM) significantly impaired the proliferation, invasion and angiogenesis of SKOV3 cells whereas induced apoptosis. These above changes could be reversed by 740 Y–P. Therefore, Echinacoside could alleviate the ovarian cancer progression and play a tumor suppressor role by inhibiting PI3K/AKT phosphorylation.
Echinacoside inhibited the growth of ovarian cancer cells through downregulating PI3K/AKT signaling. A Effect of different concentrations Echinacoside (0, 25, 50 and 100 μM) on the phosphorylation of signaling proteins (PI3K, AKT-S473, AKT-T308 and mTOR-S2448) involved in PI3K/AKT signaling in SKOV3 and OVCAR-3 cells was measured by western blotting. *p < 0.05 and **p < 0.01 vs. ECH 0 μM. B Effect of 740 Y–P, a PI3K activator, on the phosphorylation of signaling proteins (PI3K, AKT-S473, AKT-T308 and mTOR-S2448) involved in PI3K/AKT signaling in SKOV3 cells stimulated with Echinacoside (100 μM) was evaluated by western blotting. **p < 0.01 vs. control. C Effect of 740 Y–P on the colony formation ability of SKOV3 cells stimulated with Echinacoside (100 μM) was detected by colony formation assay. **p < 0.01 vs. control. D Effect of 740 Y–P on apoptosis level of SKOV3 cells stimulated with Echinacoside (100 μM) was detected by TUNEL assay. **p < 0.01 vs. control. E Effect of 740 Y–P on the invasion ability of SKOV3 cells stimulated with Echinacoside (100 μM) was evaluated by Transwell assay. **p < 0.01 vs. control. F Effect of 740 Y–P on angiogenesis of SKOV3 cells stimulated with Echinacoside (100 μM) was assessed by in vitro tube formation assay. **p < 0.01 vs. control. ECH Echinacoside, con., ECM 0 μM
Echinacoside inhibited ovarian cancer cell growth in vivo
Finally, the anti-tumor effect of Echinacoside was measured using xenograft model in nude mice. As demonstrated in Fig. 5A and B, the subcutaneous tumor volumes of mice treated with Echinacoside were significantly decreased compared to control, but the weight of mice were not altered. Moreover, as described in Fig. 5C, the volume of subcutaneous tumor in the Echinacoside group was significantly decreased compared to control. In addition, the expression of proliferation-, apoptosis- and invasion-related proteins in tumor tissues was evaluated using immunohistochemistry. Legend as in Fig. 5D and E the expressions of Ki-67, Bcl-2, N-cadherin and VEGFA in Echinacoside group were significantly decreased, while the expressions of Cleaved caspase-3 and E-cadherin increased significantly. Then, as shown in Fig. 5F, western blot assay showed that Echinacoside significantly restrained the levels of phosphorylated PI3K, AKT (S473), AKT (T308) and mTOR (S2448). Based on these above experimental results, Echinacoside could effectively inhibit the ovarian cancer progression in vivo and in vitro, and its mechanism may be associated with the inhibition of PI3K/AKT/mTOR signaling.
Echinacoside inhibited ovarian cancer cell growth in vivo. A Tumor volume changes in SKOV3-xenografted mice injected with Echinacoside (10 mg/kg). B Body weight changes of SKOV3-xenografted mice injected with Echinacoside (10 mg/kg). C Nude mice were sacrificed on day 30 and tumor weight in SKOV3-xenografted mice injected with Echinacoside (10 mg/kg) was tested. D Effect of Echinacoside (10 mg/kg) on the expression of Ki-67, E-cadherin, N-cadherin, Bcl-2, Cleaved caspase-3 and VEGFA in SKOV3-xenografted mice was measured using HE staining assay. E Immunohistochemistry was conducted to determine the effect of Echinacoside (10 mg/kg) on the expression of Ki-67, E-cadherin, N-cadherin, Bcl-2, Cleaved caspase-3 and VEGFA in SKOV3-xenografted mice. F Effect of Echinacoside (10 mg/kg) on the phosphorylation of signaling proteins (PI3K, AKT-S473, AKT-T308 and mTOR-S2448) involved in the PI3K/AKT pathway in SKOV3-xenografted mice was evaluated by western blotting. **p < 0.01 vs. control. ECH Echinacoside; con., ECM 0 μM
Discussion
To our knowledge, it is the first time to explore the specific role of Echinacoside in ovarian cancer. In the present study, we provided a novel strategy for serous ovarian carcinoma. Here, SKOV3 cells were performed to construct subcutaneous transplanted tumor model by referring to the previous studies (Huang et al. 2020; Omar et al. 2020) Echinacoside could effectively inhibit ovarian cancer cell proliferation, invasion, angiogenesis while promote apoptosis in a concentration-dependent manner. Meanwhile, Echinacoside strongly reduced tumor growth in vivo. Additionally, its mechanism may be achieved by modulating the PI3K/AKT signaling. These data provide a theoretical basis for confirming the anti-tumor effect of Echinacoside in ovarian cancer.
Chinese traditional medicine possesses many advantages including multiple targets, lower adverse reactions and flexible administration, which makes its role in anti-tumor increasingly eviednt. Echinacoside, a natural phenylethanoid glycosides, has the effects of antioxidant, anti-inflammatory and anti-tumor (Wu et al. 2007; Xie et al. 2009; Wang et al. 2015). Previous studies reported that Echinacoside inhibited breast cancer cell growth by regulating Wnt/β-catenin pathway (Tang et al. 2020). In addition, Echinacoside could promote apoptosis of hepatocellular carcinoma cells through miR-503-3p shock TGF-β1/Smad pathway (Li et al. 2021). In colon cancer, Echinacoside acts as a tumor suppressor in contributing to cell apoptosis and cell-cycle block (Dong et al. 2015). These above results suggest that Echinacoside may exhibited remarkably tumor suppression efficacy in most of tumors. Therefore, we speculate that Echinacoside may also be playing the tumor-suppressive role in ovarian cancer. In this study, Echinacoside concentration-dependently inhibited the viability and invasion, while induce apoptosis of ovarian cancer cells. Moreover, Echinacoside (10 mg/kg) showed obvious anti-tumor effect on tumor xenograft mice. This observation was consistent with the previous anti-tumor effect of Echinacoside in other tumors, indicating that Echinacoside has broad application prospect in treating ovarian cancer.
Tumor angiogenesis refers to the neovascularization in solid tumors to supply nutrient and oxygen, thus promoting tumour spreading. This process occurs at any time in the development of tumors, which is pivotal in tumor metastasis (Lugano et al. 2020; Carmeliet and Jain 2011). Increasing evidences have demonstrated that VEGFA, an angiogenic/vasculogenic factor, plays a vital role as the key regulator of normal and abnormal angiogenesis (Huang et al. 2017). One study showed that VEGFA could strongly stimulate endothelial cell migration, proliferation and angiogenesis. Meanwhile, in various different physiological and pathophysiological situations, the inactivation of VEGFA can lead to the degeneration of vascular system and aggravate the progression of cancer (Ferrara 2001) In ovarian cancer, VEGFA inhibition promoted the sensitivity of ovarian cancer cells to chemotherapy by decreasing autophagy (Li et al. 2020). Furthermore, overexpression of VEGFA facilitated angiogenesis, invasion and metastasis of ovarian cancer cells (Wang et al. 2008). In the present work, Echinacoside suppressed angiogenesis and VEGFA protein expression in ovarian cancer cells. These above results revealed that Echinacoside could impair ovarian cancer cell growth by downregulating the expression of VEGFA to inhibit angiogenesis.
After determining the anti-tumor effect of Echinacoside in ovarian cancer, we further investigated the specific mechanism of Echinacoside. Currently, it has been found that there are many signal pathways regulating tumor cell proliferation and angiogenesis, among which PI3K/AKT/mTOR is one of commonly extensively studied and important pathways. It is generally known that PI3K/AKT/mTOR signaling is the central regulator in tumor cell proliferation and metastasis. PI3K kinase is activated after cells stimulated, and then activate many downstream targets including mTOR through phosphorylating AKT and thus exert the corresponding biological effects (Isonishi et al. 2007; Yang et al. 2008) Through comparative genomic hybridization (CGH) analysis for ovarian cancer, PI3K/AKT/mTOR signaling is the most frequently altered signal transduction pathways (Hay and Sonenberg 2004; Huang et al. 2011). Meanwhile, the PI3K/AKT/mTOR pathway has been confirmed to be activated by intricate signaling cascades in about 70% of ovarian cancer cases, which could enhance proliferation, migration, invasion and chemoresistance (Dobbin and Landen 2013). For instance, Imam et al. found that the low survival rate of ovarian cancer is correlated closely with the expression of p-AKT and PI3K, and the activation of PI3K/AKT/mTOR signaling was considerated to be an independent negative prognostic factor in ovarian cancer (Imam et al. 2012; Cheaib et al. 2015). Li et al. reported that lncRNA-JPX promoted ovarian cancer cell transfer procedures via facilitating PI3K/AKT/mTOR signaling (Li et al. 2018). Therefore, we speculate that Echinacoside may suppress the progression of ovarian cancer through PI3K/AKT/mTOR pathway. In this study, high concentration of Echinacoside (100 μM) significantly downregulated PI3K, AKT and mTOR phosphorylation levels in ovarian cancer in vivo and in vitro. PI3K agonist 740Y-P could alleviate these inhibitory effects of Echinacoside on the ovarian cancer cell growth by activating PI3K/AKT/mTOR pathway.
In conclusion, our results confirmed the feasibility of Echinacoside in treating ovarian cancer, and revealed that the anti-tumor effect of Echinacoside may be implemented at least in part through the downregulation of PI3K/AKT pathway. However, our study of Echinacoside in ovarian cancer is limited to experimental cells and animals, some problems in clinical studies, especially the therapeutic dose of Echinacoside and valuable treatment modality require a thorough study in follow-up researches. Overall, our findings demonstrate for the first time that Echinacoside has been shown to effectively repress the growth of serous ovarian cancer cells. These above results provide a new therapeutic strategy for the clinical treatment of ovarian cancer.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre JA (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discovery 10(6):417–427
Cheaib B, Auguste A, Leary A (2015) The PI3K/Akt/mTOR pathway in ovarian cancer: therapeutic opportunities and challenges. Chin J Cancer 34(1):4–16
Deng M, Zhao JY, Tu PF, Jiang Y, Li ZB, Wang YH (2004) Echinacoside rescues the SHSY5Y neuronal cells from TNFα-induced apoptosis. Eur J Pharmacol 505(1–3):11–18
Dobbin ZC, Landen CN (2013) The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int J Mol Sci 14(4):8213–8227
Dong L, Yu D, Wu N, Wang H, Niu J, Wang Y et al (2015) Echinacoside induces apoptosis in human SW480 colorectal cancer cells by induction of oxidative DNA damages. Int J Mol Sci 16(7):14655–14668
Ferrara N (2001) Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280(6):1358–1366
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
Huang J, Zhang L, Greshock J, Colligon TA, Wang Y, Ward R et al (2011) Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosom Cancer 50(8):606–618
Huang F, Yao Y, Wu J, Liu Q, Zhang J, Pu X et al (2017) Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-κB/VEGF signaling. American Journal of Translational Research 9(12):5538
Huang C, Li H, Feng Y, Li XL et al (2020) Combination therapy with B7H3-redirected bispecific antibody and Sorafenib elicits enhanced synergistic antitumor efficacy. Theranostics 10(23):10498
Imam JS, Plyler JR, Bansal H, Prajapati S, Bansal S, Rebeles J et al (2012) Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS ONE 7(12):52397
Isonishi S, Saitou M, Saitou M, Yasuda M, Tanaka T (2007) Differential regulation of the cytotoxicity activity of paclitaxel by orobol and platelet derived growth factor in human ovarian carcinoma cells. Oncol Rep 18(1):195–201
Jiang Y, Tu PF (2009) Analysis of chemical constituents in Cistanche species. J Chromatogr A 1216(11):1970–1979
Jin Y, Feng SJ, Qiu S, Shao N, Zheng JH (2017) LncRNA MALAT1 promotes proliferation and metastasis in epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med Pharmacol Sci 21(14):3176–3184
Kannan K, Coarfa C, Rajapakshe K, Hawkins SM, Matzuk MM, Milosavljevic A et al (2014) CDKN2D-WDFY2 is a cancer-specific fusion gene recurrent in high-grade serous ovarian carcinoma. PLoS Genet 10(3):1004216
Li W, Li Y, Guan S, Fan J, Chen CF, Bright AM et al (2007) Extracellular heat shock protein-90α: linking hypoxia to skin cell motility and wound healing. EMBO J 26(5):1221–1233
Li J, Feng L, Tian C, Tang YL, Tang Y, Hu FQ (2018) Long noncoding RNA-JPX predicts the poor prognosis of ovarian cancer patients and promotes tumor cell proliferation, invasion and migration by the PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci 22(23):8135–8144
Li X, Hu Z, Shi H, Wang C, Lei J, Cheng Y (2020) Inhibition of VEGFA increases the sensitivity of ovarian cancer cells to chemotherapy by suppressing VEGFA-mediated autophagy. Onco Targets Ther 13(2):8161
Li W, Zhou J, Zhang Y, Zhang J, Li X, Yan Q et al (2021) Echinacoside exerts anti-tumor activity via the miR-503-3p/TGF-β1/Smad aixs in liver cancer. Cancer Cell Int 21(1):1–9
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77(9):1745–1770
Ma Y, Zhou G, Li M, Hu DF, Zhang LQ, Liu P et al (2018) Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochem Int 118(12):233–241
Ma Q, Zhang J, Zhang M, Lan H et al (2020) MicroRNA-29b targeting of cell division cycle 7-related protein kinase (CDC7) regulated vascular smooth muscle cell (VSMC) proliferation and migration. Ann Transl Med 8(22):1496
Omar A, Kalra RS, Putri J, Elwakeel A, Kaul SC, Wadhwa R (2020) Soyasapogenol-A targets CARF and results in suppression of tumor growth and metastasis in p53 compromised cancer cells. Sci Rep 10(1):1–13
Qiu J, Lin Y, Ye L, Ding J, Feng W, Jin H et al (2014) Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol 134(1):121–128
Swiatly A, Horala A, Hajduk J, Matysiak J, Markwitz EN, Kokot ZJ (2017) MALDI-TOF-MS analysis in discovery and identification of serum proteomic patterns of ovarian cancer. BMC Cancer 17(1):1–9
Tang C, Gong L, Xu L, Qiu K, Zhang Z, Wan L (2020) Echinacoside inhibits breast cancer cells by suppressing the Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun 526(1):170–175
Wang J, Sun T, Zhao X, Zhang S, Gu Q, Wang X et al (2008) Functional significance of VEGF-a in human ovarian carcinoma: role in vasculogenic mimicry. Cancer Biol Ther 7(5):758–766
Wang S, Zheng G, Tian S, Zhang Y, Shen L, Pak Y et al (2015) Echinacoside improves hematopoietic function in 5-FU-induced myelosuppression mice. Life Sci 123(1):86–92
Wang W, Luo J, Liang Y, Li X (2016) Echinacoside suppresses pancreatic adenocarcinoma cell growth by inducing apoptosis via the mitogen-activated protein kinase pathway. Mol Med Rep 13(3):2613–2618
Wu Y, Li L, Wen T, Li QY (2007) Protective effects of echinacoside on carbon tetrachloride-induced hepatotoxicity in rats. Toxicology 232(12):50–56
Xie H, Zhu H, Cheng C et al (2009) Echinacoside retards cellular senescence of human fibroblastic cells MRC-5. Die Pharm Int J Pharmac Sci 64(11):752–754
Yang X, Fraser M, Abedini MR, Bai T, Tsang BK (2008) Regulation of apoptosis-inducing factor-mediated, cisplatin-induced apoptosis by Akt. Br J Cancer 98(4):803–808
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This present study was performed based on the principles expressed in the Declaration of Helsinki. All animal experiments were approved by the Ethics Committee of the Maternal and Child Healthcare Hospital of Shaanxi Province and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Tang, N., Liu, N. et al. Echinacoside inhibits the proliferation, migration, invasion and angiogenesis of ovarian cancer cells through PI3K/AKT pathway. J Mol Histol 53, 493–502 (2022). https://doi.org/10.1007/s10735-022-10073-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10073-x