Abstract
The striatum is an essential component of the basal ganglia that regulatessensory processing, motor, cognition, and behavior. Depending on the species, the striatum shows a unique structure called caudate–putamen as in mice, or its separation into two regions called caudate and lenticular nuclei, the latter formed by putamen and globus pallidus areas, as in primates. These structures have two compartments, striosome and matrix. We investigated the structural organization, GABAergic and tyrosine hydroxylase (TH) expression in the striatum and globus pallidus of the South American plains vizcacha, Lagostomus maximus. Its striatum showed regionalization arising from the presence of an internal capsule, and a similar organization to a striosome–matrix compartmentalization. GABAergic neurons in the matrix of caudate exhibited parvalbumin, calretinin, calbindin, GAD65, and NADPH-d-immunoreactivity. These were also expressed in cells of the putamen with the exception of calretinin showing neurofibers localization. Globus pallidus showed parvalbumin- and GAD65-immunoreactive cells, and calretinin- and calbindin-immunoreactive neuropil, plus GABA-A-immunoreactive neurofibers. NADPH-d-, GAD65- and GABA-A-immunoreactive neurons were larger than parvalbumin-, calretinin-, and calbindin-immunoreactive cells, whereas calbindin-immunoreactive cells were the most abundant. In addition, TH-immunoreactive neuropil was observed in the matrix of the striatum. A significant larger TH-immunoreactive area and neuron number was found in females compared to males. The presence of an internal capsule suggests an adaptive advantage concerning motor and cognitive abilities favoring reaction time in response to predators. In an anatomy-evolutive perspective, the striatum of vizcacha seems to be closer to that of humans than to that of laboratory traditional models such as mouse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The basal ganglia are composed of the striatum, globus pallidus, subthalamic nucleus and substantia nigra (Smith et al. 1998; Shipp 2017). Within these structures, the striatum (caudate nucleus and putamen) present two distinguishable types of compartment, striosomes (or patches) that form a labyrinthine and interconnected structure, and matrix that keeps and surrounds the striosomes (Graybiel and Ragsdale 1978; Herkenham and Pert 1981; Gerfen 1984). Both compartments have specific properties with GABAergic, dopaminergic and nitrergic neurons (Graybie land Ragsdale 1978; Sandell et al. 1986; Graybiel 1983, 1990; Kubota and Kawaguchi 1993; Holt et al. 1997). The striatum is involved in motor and cognitive functions (DeLong and Georgopoulos 1981; DeLong et al. 1984; Middleton and Strick 1994). In order to build motor patterns based on the environment information as well as on past experience, the striatum integrates the incoming information from all regions of the cerebral cortex (Graybiel 2008). This structure receives inputs from the dopaminergic, glutamatergic and serotonergic systems coming different areas of the brain, including the cortex, mesencephalon, and thalamus (Kubota et al. 1986; Lavoie and Parent 1990; Fujiyama et al. 2006; Kawaguchi et al. 1995). On the other hand, its GABAergic efferents show stimulating or inhibitory effects on movement according to their projection through direct or indirect pathways. In those pathways, the internal and external globus pallidus are involved, respectively (Gerfenand and Wilson 1996; Smith et al. 1998; Gillies et al. 2002; Micheli and Luquin-Piudo 2012; Kita and Jaeger 2016).
The structural organization of the striatum differs among species. In rodents such as mice and rats, the striatum is a unique structure formed by the caudate and putamen areas called caudate-putamen, without internal defined limits (Paxinos and Franklin 2004). However, in primates, the presence of a tract of white matter crossing the striatum, called internal capsule, separates the caudate–putamen into two regions: caudate and lenticular nuclei, the latter formed by putamen and globus pallidus areas (Carpenter 1994; Snell 2007).
The South American plains vizcacha, Lagostomus maximus, is a basal hystricognathe rodent belonging to the family Chinchillidae (Fig. 1), distributed in the Pampas region of Argentina extending up to the South of Paraguay and Bolivia (Jackson et al. 1996). This species is a large herbivore fossorial rodent living in communal burrow systems with nocturnal foraging outings (Llanos and Crespo 1952). Night-time outputs expose them to predation, especially by puma (Puma concolor) and, eventually, by smaller felids like the Geoffroy’s cat (Oncifelis geoffroyi). However, the attack success of predators on vizcacha is low, around 10% (Branch 1995). Vizcachas convert dense grass around burrows to low-growing forbs which together with loud warning vocalization prevent predators to get close enough for a successful ambush (Branch 1993a, 1995). Although these behaviors are supposed to be the main strategy against predation, in situ observation of predator–vizcacha interaction has shown that they have sensorial and motor skills that guarantee their quick escape (Branch 1995). Much of the processing information on those skills involves the striatum, acting together with the cortex and the cerebellum.
The South American plains vizcacha, Lagostomus maximus, and the phylogenetic tree of the Rodentia. Photograph of a wild vizcacha specimen. Courtesy of Francisco Rebollo Paz (a). Cladogram of the order Rodentia with the suborders Sciuromorpha, Hystricomorpha and Myomorpha. In the Hystricomorpha suborder, within the Hystricognathi infraorder, parvorden Caviomorpha, at Chinchilloidea superfamily, Chinchillidae family, Lagostominae subfamily is located, the species Lagostomus maximus belongs (b)
Up to the present, there have been no studies on the striatum in the vizcacha or other Chinchillidae, which are at the base of rodent phylogeny (Fig. 1b). The aim of the present work was to analyze the structural organization and GABAergic expression of the striatum and globus pallidus of the vizcacha. We also evaluated the possible existence of dopaminergic sexual dimorphism. We discuss the results in the light of habits and behaviour of the species, and the possible evolutionary meaning of the striatum’s anatomy.
Materials and methods
Animals
Adult plains vizcachas were captured from a resident natural population at the Estación de Cría de Animales Silvestres (ECAS), Villa Elisa, Buenos Aires, Argentina using live-traps located at the entrance of their burrows. All experimental protocols concerning animal handling were conducted in accordance with the guidelines published in the National Institutes of Health (NIH) guide for the care and use of laboratory animals (National Research Council USA 2011), and were reviewed and approved by the Institutional Committee on Use and Care of Experimental Animals (CICUAE) from Universidad Maimónides, Argentina (Resolution Nº16/14). The capture and transport of animals were approved by the Ministry of Agriculture Authority of the Buenos Aires Province Government. Appropriate procedures were performed to minimize the number of animals used. Adult non-pregnant non-ovulating females (n = 13; 2.2–3.5 kg body weight) were captured in March according to the natural reproductive cycle described by Llanos and Crespo (1952), and our own previous field expertise (Jensen et al. 2006; Dorfman et al. 2011, 2013; Halperin et al. 2013; Charif et al. 2016, 2017; Inserra et al. 2017). Adult males with testicular recrudescence (n = 7; 4.5–6.5 kg body weight) were captured in June (González et al. 2012a, b, 2018). All animals ranged from 2.5 to 3.5 years old as determined by the dry crystalline lens weight according to Jackson (1986). The data for the single animals were introduced in Table 1.
Tissue collection
Animals were anaesthetized by the intramuscular injection of 13.5 mg/kg body weight ketamine chlorhydrate (Holliday Scott S.A., Buenos Aires, Argentina) and 0.6 mg/kg body weight xylazine chlorhydrate (Richmond Laboratories, Veterinary Division, Buenos Aires, Argentina) and sacrificed by an intracardiac injection of 0.5 ml/kg body weight of Euthanyl™ (Sodic Pentobarbital, SodicDiphenilhidanthoine, Brouwer S.A., Buenos Aires, Argentina); brains were immediately removed and processed (see below).
Histological staining
After removal, brains were serially sectioned in a coronal plate in 5 mm thick blocks, immediately fixed in cold 4% paraformaldehyde (PFA) (Sigma Aldrich Inc., St. Louis, Missouri, USA) in 0.1 M phosphate-buffer saline (PBS) (pH 7.4) for 72 h, dehydrated through a graded series of ethanol and embedded in paraffin. For each specimen, the block containing the brain region comprising the striatum was entirely cut in serial coronal sections (5 µm thick) with a microtome (Leica RM2125RT, Wetzlar, Germany) and mounted individually onto coated slides. Sections were dewaxed in xylene and rehydrated through a decreasing series of ethanol (100, 95 and 70%). One every seven slides was separated to visualize neurons by classical Nissl staining using cresyl violet solution (0.1 g/l crystal violet in 10% glacial acetic acid, pH 7.4), and myelin by Klüver–Barrera staining using 0.1 g/l Luxol fast blue and 0.05 g/l lithium carbonate plus counterstaining with cresyl violet solution (Klüver and Barrera 1953). The location of the striatum was established by comparison with rat (Rattus norvegicus), mouse (Mus musculus), chinchilla (Chinchilla lanigera) and guinea pig (Cavia porcellus) histological brain atlases (Tindal 1965; Luparello et al. 1964; Paxinos and Franklin 2004; Paxinos and Watson 2013); according to the anatomical characteristics, three levels of striatum (rostral, mid, and caudal) were established.
Immunohistochemistry
All the sections from mid-striatum were dewaxed in xylene and rehydrated through a decreasing series of ethanol (100, 95 and 70%). Antigen retrieval was performed boiling sections in 10 mM sodium citrate buffer (pH 6) for 20 min, followed by 20 min cooling at room temperature. Then, endogenous peroxidase activity was blocked with 2% hydrogen peroxide in methanol for 20 min. After that, sections were incubated with blocking solution of PBS containing 10% normal serum (pH 7.4) for 1 h. Immunoreactivity was detected incubating slides overnight at room temperature with the corresponding primary antibody (Table 2). Specificity was corroborated in adjacent sections by omission of the primary antibodies. Immunoreactivity was revealed with biotinylated goat anti-rabbit IgG or with biotinylated horse anti-goat IgG followed by incubation with avidin–biotin complex (ABC Vectastain Elite kit, Vector Laboratories, Burlingame, California, USA). The reaction was visualized with 3,3′diaminobenzidine (DAB) and intensified with nickel ammonium sulphate (DAB kit, Vector Laboratories, Burlingame, California, USA) that yields a black product. Finally, sections were dehydrated through a graded series of ethanol (70%, 95% and 100%), cleared in Neo-Clear (Merck KGaA, Darmstadt, Germany) and cover slipped. Six females and five males were tested.
NADPH-diaphorasehistochemistry
After removal, brains were coronally sectioned in blocks of 5–6 mm thick and fixed in cold 4% PFA in 0.1 M PBS (pH 7.4) for 72 h, cryoprotected in 30% sucrose in 0.1 M phosphate buffer, frozen with powdered dry ice and stored at – 80 ºC. Brain regions containing the striatum were entirely cut with a cryostat (Leica CM1850, Wetzlar, Germany) to serial coronal sections (20 µm thick), mounted onto coated slides, air dried and stored at – 80 ºC. In order to identify sections containing mid-striatum, classical Nissl staining was performed in one out of ten adjacent sections. Sections were incubated with a solution containing 0.1% β-NADPH and 0.02% nitroblue tetrazolium diluted in 0.1 M phosphate buffer, pH 7.4, with 0.3% Triton X-100, for 60 min at 37 ºC. Negative control was performed omitting NADPH in the incubation mixture. Three females were used.
Image analysis and quantification approach
Anatomically matching areas among animals were selected for the image analysis. Microscope images of histological, histochemical and immunoreactive staining were captured with an optic microscope (BX40, Olympus Optical Corporation, Tokyo, Japan), fitted with a digital camera (390CU 3.2 Megapixel CCD Camera, Micrometrics, Spain), and the image software Micrometrics SE P4 (Standard Edition Premium 4, Micrometrics, Spain). To avoid variations in the determination of the specific immunoreactivity and in the quantification process, all the images for the same marker were obtained the same day and under the same light and contrast intensity. Immunoreactive cells and NADPH-d reactive cells were quantified with Image-Pro Plus software (Image-Pro Plus 6, Media Cybernetics Inc., Bethesda, Maryland, USA). In order to check the distribution of each marker through the mid-striatum three females were used. In these cases, all the tissue sections containing the mid-striatum (50 sections) were analyzed. Each of the six antibodies employed was used in a systematic manner in consecutive sections followed by the two sections used for Nissl staining and for Klüver–Barrera staining. This sequence was systematically six times repeated in order to cover all the mid-striatum. In this way, six sections distributed throughout the whole mid-striatum were stained for each antibody and cells expressing each marker were counted every eight sections. In the remaining three females and in the five males, measures were assayed in just three slices distributed at the rostral, middle and caudal regions of the mid-striatum. In order to be comparable with the former mentioned analyzed slices, sections were chosen in a systematic manner, spaced at an interval of 120 µm from each other within the striatum. At each section, absolutely all the immunostained cells were measured in an exhaustive fashion. The analyses were conducted in both left and right sides of the brains. For each area (caudate, putamen or globus pallidus) the density of immunoreactive cells (NRC, expressed as the average of the number of immunoreactive cells/10 mm2), the somatic reactive area (SRA, expressed in µm2) and the medial diameter of reactive cells (MDRC, expressed in µm) were determined using the staining optical density. Only those cells that had a grey level darker than a defined threshold criteria (defined as the optical density threefold higher than the mean background density) were considered as specific immunoreactive stained cells. The background density was measured in a region devoided of immunoreactivity, immediately adjacent to the analyzed region. The SRA and the MDRC were determined as the surface covered by the pixels that exceed the threshold density criterion. Similar methods were previously employed by our group (Dorfman et al. 2013) and by others (Rey-Funes et al. 2013, 2016). The measurements informed as SRA and MDRC have been corrected by the tissue shrinkage and swelling factor which did not vary between males and females. Regarding to GAD-65, in spite that mild-stained cells with a very low optical density were observed, only those cells showing an optical density that exceed the defined threshold criteria were considered for the quantification (referred to as strong-stained). On the other hand, in order to detect a possible sexual dimorphism, the percentage of the TH-immunoreactive area (IRA = TH-immunoreactive area/caudate plus putamen areas) and the number of TH-immunoreactive neurons/10 mm2 of striatum were determined in male and female vizcachas. TH-immunoreactive area was determined using the optical density. The area that had a grey level darker than the defined threshold criteria was considered for the quantification. Quantifications were done by two independent observers. Adobe Photoshop software (Adobe Photoshop CS5, Adobe Systems Inc., Ottawa, Ontario, Canada) was used for digital manipulation of brightness and contrast when preparing the shown images.
Sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS)-PAGE and Western-blotting
Immediately after brain removal, striatum was homogenized (1:3 w/v) in RIPA buffer (0.1 M PBS with 1% Igepal, 0.5% sodium deoxycolate and 0.1% SDS, pH 7.4), containing 0.1 μM aprotionin, 0.1 μM leupeptin, 0.1 μM pepstatin and 0.1 mM phenyl methyl sulfonyl fluoride (PMSF). All procedures were carried out at 4 °C. Homogenate was centrifuged for 30 min at 14,000×g and supernatant collected. Protein concentration was determined by Bradford method (Bradford 1976) using bovine serum albumin (BSA) as a standard. Fifty micrograms of solubilized proteins were mixed with sample buffer (1 M Tris–HCl with 10% w/v SDS, 30% v/v glycerol, 0.1% w/v bromo phenol blue and 0.15% w/v 2-mercaptoethanol, pH 6.8) and heated for 3 min at 100 °C. Proteins were separated on an SDS–polyacrylamide 10% running gel and 4% stacking gel (29:1 acrylamide: bis acrylamide, Bio-Rad Laboratories, Hercules, California, USA), with 0.25 M Tris–glycine, pH 8.3, as the electrolyte buffer, in an electrophoresis cell (Mini-PROTEAN II Electrophoresis Cell, Bio-Rad Laboratories, Hercules, California, USA). For Western-blot analysis, proteins were electro-transferred to a 0.2 mm polyvinylidene difluoride (PVDF) membrane (Immobilon-P, EMD Millipore Corporation, Billerica, Massachusetts, USA) at 400 mA for 75 min. For protein identification, membranes were blocked 1 h at room temperature with 5% powdered skim milk in PBS containing 0.1% Tween 20. Then, they were incubated overnight at 4ºC with the appropriate primary antibody (1:200 dilution. Table 2). For immunoreactivity development, membranes were incubated with goat anti-rabbit IgG-HRP (1:3000 dilution, 170–6515, Bio-Rad Laboratories, California, USA), with anti-mouse IgG-HRP (1:3000 dilution, sc-516102, m-IgGκ BP-HRP, Santa Cruz Biotechnology, Santa Cruz, California, USA), or with horse anti-goat IgG-HRP (PI-9500, Vector Laboratories, California, USA) as appropriate. For chemiluminiscence development, ECL Plus kit (GE Healthcare Ltd., Amersham Place, Buckinghamshire, United Kingdom) was employed. Membranes were scanned with a ImageQuant 350 Capture Imaging System (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The estimation of the band size was performed using a pre-stained protein ladder (PageRuler, Fermentas UAB, Vilnius, Lithuania) as molecular weight marker. In this technique, two pools of proteins were used: one pool of proteins extracted from two striatum of rats (one male and one female) and another pool of proteins extracted from two striatum of vizcachas (one male and one female).
Statistical analysis
Values are expressed as the average among animals ± mean standard deviation. Results of dimorphism were evaluated using t-test for comparisons between two groups. Differences were considered significant when p < 0.05. Statistical analysis was performed using Prism 4.0 software (GraphPad Software Inc., San Diego, California, USA).
Results
Anatomical and histochemical description of the striatum of the vizcacha
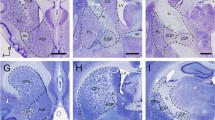
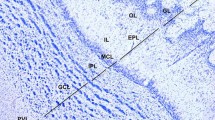
The striatum was dorsally delimited by the extension of the white matter that separates the striatum from the brain cortex, laterally by the extension of the white matter forming the external capsule and internally by the lateral ventricle and a band of white matter forming the internal capsule, and ventrally by the amygdaloid area (Fig. 2). The morphology of the striatum was studied in a rostro–caudal orientation, and the rostral-, mid-, and caudal-striatum levels were analyzed. Both rostral- and caudal-striatum showed homogenous morphology forming a unique caudate–putamen structure (Fig. 2a–c, g–i). However, at the mid-striatum level (spanning about 300 µm), an internal capsule dividing the caudate–putamen structure in caudate and putamen was observed (Fig. 2d–f). At the three levels, caudate and putamen showed a similar organization to a striosome-matrix compartmentalization. A schematic representation of coronal sections at rostral-, mid- and caudal-striatum, and the regionalization of the mid-striatum into caudate and putamen areas is shown in Fig. 2c, f, i.
Structural organization of the striatum of the vizcacha, Lagostomus maximus. Representative images of coronal hemi-sections showing the localization of the rostral-striatum (a–c), mid-striatum (d–f) and caudal-striatum (g–i). a, d and g Nissl staining; b, e and h Klüver–Barrera technique; c, f, i Schematic representation showing the striatum regionalization in caudate nucleus and putamen. White matter (lined), gray matter (white) and ventricles (grey) are shown. ac: anterior commissure; C: caudate nucleus, cc: corpus callosum, ccg: corpus callosum genu, cing: cingulate, CP: caudate-putamen, CTX: cortex, ec: external capsule, fx: fornix column, GP: globus pallidus, ic: internal capsule, IIIV: 3rd ventricle, lot: lateral optical tract, LV: lateral ventricle, MS: medial septal nucleus, och: optic chiasm, PT: putamen. Scale bars: 5 mm
GABAergic system expression in the mid-striatum and globus pallidus of the vizcacha
Considering the regionalization of the mid-striatum of the vizcacha, the distribution and the relative abundance of cells expressing different components of the GABAergic system were analyzed. GABAergic expression was determined in the three areas caudate, putamen and globus pallidus of non-pregnant females. Homogenous distribution of each marker was determined along the rostro–caudal extension of complete mid-striatum. Three females were used (Fig. 3). Considering this homogenous distribution, GABAergic system inspection for the remaining animals was developed in three representative tissue sections corresponding to rostral, middle, and caudal regions of the mid-striatum respectively. Specific expression was determined at caudate, putamen and globus pallidus for each studied marker.
Distribution of GABAergic cells throughout the entire section of mid-striatum and globus pallidus of female vizcacha, Lagostomus maximus. Parvalbumin (a), calretinin (b), calbindin (c), GAD-65 (d), and GABA-A (e)—immunoreactivity was evaluated in the entire section of mid-striatum and globus pallildus of female vizcacha. The complete mid-striatum was studied in its rostro-caudal orientation and all the sections (a total of 50) were stained for immunohistochemistry. Each antibody was used in consecutive sections, and this sequence was seven times repeated in order to cover all the mid-striatum. In this way, cells expressing each marker were systematically counted every seven sections. For each marker, bars represent the number of immunoreactive cells corresponding to the whole striatum, whereas the number of immunoreactive cells in caudate nucleus, putamen and globus pallidus was white, grey and black represented. CB calbindin, CR calretinin, GABA-A γ-aminobutiric acidionotropicreceptor, GAD-65 glutamic acid decarboxilase 65, IR immunoreactive, S section, PV parvalbumin
Parvalbumin
Parvalbumin-immunoreactivity, which indicates the presence of GABAergic aspiny interneurons, was observed in cells localized in the matrix of the caudate and putamen as well in the globus pallidus (Fig. 4a–c). The putamen showed the largest number of immunoreactive cells whereas the caudate displayed the lowest number (Table 3). In addition, cells from globus pallidus were smaller than the cells from other two areas (Table 3).
Distribution of parvalbumin, NADPH-d and calretinin expression in the mid-striatum and globus pallidus of female vizcacha, Lagostomus maximus. Parvalbumin-immunoreactivity is observed in cells of the caudate nucleus (a), the putamen (b) and the globus pallidus (c). NADPH-d staining is observed in cells and matrix of the caudate nucleus (d) and the putamen (e); in the globus pallidus, NADPH-d staining was only detected in microvessels (f). Calretinin-immunoreactivity is differentially distributed in the striatum areas. In the caudate nucleus, calretinin-immunoreactivity was detected in cells and neurofibers (g) and in the putamen, calretinin-immunoreactivity was detected in neurofibers of patches (h). In the globus pallidus, calretinin-immunoreactivity was detected in neuropil (i). Details of each immunoreactivity are shown in insets. Somatic immunoreactivity (arrowhead), neurofibrillar immunoreactivity (arrow) and NADPH-reactive microvessel (asterisk) are indicated. LV: lateral ventricle. Scale bars: 50 µm; insets scale bars: 10 µm
NADPH-d
NADPH-d stained cells, which evidences the presence of GABAergic aspiny interneurons, were distributed in the matrix of both caudate and putamen (Fig. 4d–e). Big and scarse NADPH-d stained bipolar or multipolar neurons, with primary and secondary ramifications, were detected. Caudate and putamen exhibited a similar number of NADPH-d stained neurons (8 ± 2 and 9 ± 3, respectively), whereas NADPH-d cells at both areas showed similar size (caudate: 104.09 ± 25.02 μm2 somatic reactive area and 11.45 ± 1.37 μm medial diameter; putamen: 144.06 ± 52.04 μm2 somatic reactive area and 13.75 ± 3.01 μm medial diameter). The globus pallidus was devoid of NADPH-d stained cells. Only randomly distributed microvessels were detected in this area (Fig. 4f).
Calretinin
Calretinin, a marker of GABAergic aspiny interneurons, was differentially detected in the matrix or in the patches depending of the nucleus. In the caudate, clusters of round-shaped calretinin-immunostained neurons with labelling in the proximal segment of some dendrites, were distributed in the matrix (Fig. 4g, and Table 3), whereas the putamen showed calretinin-immunoreactive neurofibers with patch localization (Fig. 4h). The globus pallidus revealed calretinin-immunoreactivity in neuropil (Fig. 4i).
Calbindin
Calbindin, a marker of GABAergic spiny neurons, presented immunoreactivity at neuropil and cells of matrix. Abundant round-shaped neurons with the proximal segment of their dendrites positively labeled for calbindin were localized in the matrix of both caudate and putamen with similar abundance and size (Fig. 5a–c and Table 3).
Distribution of calbindin, GAD-65 and GABA-A expression in the mid-striatum and globus pallidus of female vizcacha, Lagostomus maximus. Calbindin-immunoreactivityis observed in the somas of the matrix of the caudate nucleus (a) and the putamen (b); the globus pallidus showed only neuropil labeling (c). GAD-65-immunoreactivity is observed in cells located in the matrix of the striatum (d–e); and in cells of the globus pallidus (f). Two subpopulations of GAD65-immunoreactive cells were detected: mild immunoreactive cells (white arrowheads) and strong immunoreactive cells (black arrowheads). GABA-A immunoreactivity is observed in neuropil and neurofibers of the matrix in the caudate nucleus (g) and the putamen (h). In addition, GABA-A immunoreactive neurofibers and bipolar cells were detected in the globus pallidus (i). Detail of each immunoreactivity is shown in insets. Somatic (arrowhead) and neurofibrillar immunoreactivity (arrow) is indicated. Scale bars: 50 µm; insets scale bars: 10 µm
Glutamic acid decarboxilase 65 (GAD65)
The expression of GAD65, the enzyme involved in γ-aminobutiric acid (GABA) synthesis, showed two sub-populations of GAD-65 neurons, with mild- or strong-staining, in striatum and globus pallidus (Fig. 5d–f). Only the big GAD-65 immunoreactive cells with six times higher optical density than the background were quantified (referred to as strong-stained). The caudate nucleus and the putamen depicted abundant mild stained round multipolar neurons together with scarce strong stained cells, whereas the globus pallidus showed scarce mild stained cells together with abundant strong stained fusiform-bipolar neurons (Fig. 5d–f and Table 3).
GABA ionotropic receptor A (GABA-A)
Immunoexpression of GABA-A was detected in the neuropil of the caudate nucleus and putamen, but GABA-A immunolabeled cells were not detected in those areas (Fig. 5g–i). The globus pallidus displayed large scarse bipolar GABA-A immunoreactive neurons inmersed among immunopositive neurofilaments (Fig. 5i and Table 3).
Specific immunoreactivity was not detected when adjacent slides were incubated in the same conditions but omitting the primary antibodies (Fig. 6g–i). In addition, single bands corresponding to the expected molecular weights of parvalbumin, calretinin, calbindin, GAD 65, and GABA-A were detected by Western-blot in a protein extract from the striatum of a female vizcacha and a female rat (Fig. 6a–e). This reproducible pattern between vizcacha and rat, and the single band obtained for each marker, reinforced the specificity of the employed antibodies.
Specific expression of parvalbumin, calretinin, calbindin, GAD-65, GABA-A, and tyrosine hydroxylase in the striatum of vizcacha, Lagostomus maximus. Representative images of protein expression of parvalbumin (a), calretinin (b), calbindin (c), GAD-65 (d), GABA-A (e), and tyrosine hydroxylase (f) determined by Western-blot in the striatum of non-pregnant vizcacha and non-pregnant rat (Sprague–Dawley). A pool of striatum proteins of one male rat and one female rat (Rat), and a pool of striatum proteins of one male vizcacha and one female vizcacha (Viz) were used. Representative images of negative controls (primary antibodies omitted) of immunohistochemistry are shown in g (incubated with biotinylated anti-rabbit IgG), h (incubated with biotinylated anti-mouse IgG), and i (incubated with biotinylated anti-goat IgG)
Sexual dimorphism for tyrosine hydroxylaseexpression in the mid-striatum of the vizcacha
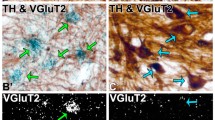
Tyrosine hydroxylase (TH) expression, a marker of dopamine sinthesis intermediary, was studied throughout striatum. The darkest TH optical density was detected at the matrix of caudate, whereas the matrix of putamen showed a lower intensity of TH-immunoreactivity (Fig. 7a). A fibrillar TH-immunoreactive pattern was observed in the matrix of the striatum and in the globus pallidus (Fig. 7b–d). In the caudate nucleus, TH-immunoreactive nerve endings were deteted around TH immunonegative neurons (Inset Fig. 7b), whereas a few and small TH-Immunoreactive cells were detected in putamen (Fig. 7c). In the globus pallidus, sparse TH-immunoreactive axonic varicosities were observed (Fig. 7d). TH-immunoreactivity was not detected when adjacent slides were incubated in the same conditions but omitting the primary antibody (Fig. 6g). Since TH-immunoreactivity was previously shown to be sexually dimorphic in other rodents (Leranth et al. 2000; Daubner et al. 2011), here we compared TH expression between male and female vizcachas. Females showed a significant larger mid-striatum TH-immunoreactive area than males (10% of increment) (Fig. 7e) and a significantly higher abundance of TH-immunoreactive cells that were only identified in the putamen (Fig. 7f). Significant differences between sexes were not found in the size of TH-immunoreactive cells (females: 11.26 ± 3.87 μm2 somatic reactive area and 3.79 ± 0.56 μm medial diameter; males: 12.13 ± 2.18 μm2 somatic reactive area and 4.01 ± 0.42 μm medial diameter).
Distribution of tyrosine hydroxylase (TH) expression and TH sexual dimorphism in the mid-striatum of vizcacha, Lagostomus maximus. TH-immunoreactivityis observed in the matrix of the caudate nucleus and the putamen (a–c). Caudate and putamen TH-immunoreactivity is enclosed by a black line (a). TH-immunoreactive terminals (arrow) surrounding a non-immunoreactive cell (white arrowhead) are observed in the inset (b). Small TH-immunoreactive cells are observed in the putamen (c). TH-immunoreactive neurofibers (arrow) are observed in the globus pallidus (d). Significantly larger mid-striatum (caudate nucleus plus putamen) TH-immunoreactive area and significantly higher abundance of TH-immunoreactive cells was determined in females compared to males (e, f). Bars indicate average value ± mean standard deviation. T-test was used to determine statistically significant differences between groups. Asterisks (*) indicate significant differences with p < 0.05. C: caudate nucleus. PT: putamen. Scale bars = (A): 5 mm (B): 100 µm (C-D): 50 µm, inset (B): 5 µm, inset (C): 20 µm
In a Western-blot assay, single bands corresponding to the expected molecular weight were detected for TH (60 kDa) in the striatum of vizcacha and rat (Fig. 6f). This reproducible pattern between vizcacha and rat, and the single band obtained, reinforced the specificity of the anti-TH employed antibody.
Discussion
The present work describes for the first time the striatum of the South American plains vizcacha showing its regionalization, compartmentalization and GABAergic expression in the striatum. The striatum structural organization greatly differs from that described for miomorph rodents and it relates the basal histricognathe vizcacha to other taxa sharing a common ancestor. Besides, TH immunoreactivity revealed the existence of dopaminergic sexual dimorphism.
GABAergic characterization of mid-striatum
In order to describe the GABAergic expression in the mid-striatum of the vizcacha, several markers previously used for striatal characterization in other species like mouse, rat, cat (Felis silvestris), gerbil (Meriones unguiculatus), tree shrew (Anathana ellioti), monkey (Saimiri sciureus) and human were studied (Sandell et al. 1986; De Las Heras et al. 1994; Holt et al. 1997; Wu and Parent 2000; Rice et al. 2011; Bae et al. 2015). Spiny and aspiny GABAergic neurons have been described in the mid-striatum of the vizcacha: spiny neurons expressing calbindin and sending projections from both caudate and putamen regions towards the globus pallidus and the substantia nigra, and aspiny interneurons expressing parvalbumin, NADPH-d, or calretinin, and controlling the action of projection neurons (Kawaguchi et al. 1995). This complex network of GABAergic cell bodies and processes detected in the striatum of the vizcacha had been previously found in other mammals (Kawaguchi et al. 1995; Rice et al. 2011). In addition, the immunoreactivity of the three calcium binding proteins parvalbumin, calretinin and calbindin, revealed a similar organization to the classical striosome–matrix cytoarchitecture in the striatum of vizcacha. The three neurochemical markers revealed ovoid cells, most of them devoid of visible immunoreactive dendrites, and a homogeneous matrix distribution without differences of staining intensity among caudate and putamen as previously described for tree shrews (Rice et al. 2011). The differences in the distribution among calretinin-, calbindin-, and parvalbumin-immunoreactive cells may indicate different GABAergic pathways. Strikingly, GAD65-immunoreactive cells were much less abundant and bigger than calretinin-, calbindin-, and parvalbumin-immunoreactive cells, and the size is in range with the previously reported for other rodents, but is smaller than that described for the tree shrew and primates (Mensah and Deadwyler 1974; Yelniket al. 1991; Rice et al. 2011). However, the different size between GAD65-immunoreactive cells and calretinin-, calbindin-, and parvalbumin-immunoreactive cells may be related to GAD67 activity which is, as well as GAD65, implicated in GABA synthesis. It should be considered that we did not study the expression of the enzyme GAD67. On the other hand, the presence of GABA-A immunoreactive cells exclusively located in the globus pallidus, confirms the inhibitory GABAergic modulation over this area (Parent and Hazrati 1995). In addition, neurons expressing the calcium binding proteins here analyzed (parvalbumin, calretinin and calbindin) were much more abundant and with a smaller size than the NADPH-d reactive neurons which showed similar size than GAD65-immunoreactive neurons. A similar distribution of NADPH-d staining at cells and neuropil of the caudate nucleus and the putamen was previously described in rat, cat and primates (Vincent and Johansson 1983; Sandell et al. 1986; Wu and Parent 2000).
Sexual dimorphism of TH expression
The expression of tyrosine hydroxylase (TH), the enzyme involved in the conversion of tyrosine in l-3,4 dihidroxifenil alanina (l-DOPA), an intermediate of dopamine synthesis, has been reported in the striatum of mice and primates including humans (Dubach et al. 1987; Meredith et al. 1999; Cossette et al. 2005; Pickel et al. 1975; Xenias et al. 2015). Here we detected some scarce TH-immunoreactive cells in the putamen of male and female vizcachas. It has been described that gonadal hormones modulate TH and dopaminergic activity in the striatum. Accordingly, female rats and mice exhibit higher dopamine release than males (Walker et al. 1999; Arvidsson et al. 2014). Estradiol enhances striatal dopamine release in female rats without effects in males (McDermott et al. 1994; Becker 1999; Leranth et al. 2000; Orendain-Jaime et al. 2016), whereas castrated male rats show higher striatum dopamine concentration than ovariectomized female rats (Xiao and Becker 1994). Here we showed sexual dimorphic expression of TH with a major expression area in the mid-striatum of females. Considering the modulatory effect of estrogens on dopamine induced sensorimotor function and behavior (Becker et al. 1987), and that we have recently shown the presence of aromatase in the brain of vizcacha (Charif et al. 2017), the sexual differences observed in this work may be related to the levels of estradiol and/or neuroestradiol available in the brain of vizcachas. Finally, the sex differences determined in the mid-striatum of vizcacha may have benefited the female survival. Female vizcachas take risks staying for long times outside the vizcachera searching for food and supervision of juvenile; unlike males spend most of the time inside the vizcachera or in its entrance fulfilling the role of observing and alerting through vocalization at the presence of predators (Branch 1993b; Jackson et al. 1996). Considering that the striatum is involved in the initiation and regulation of voluntary movements and motor learning, where dopamine is associated with motor control, responses as reaction time for movements, decision making processes, motor learning, etc., could be related to the differences in tyrosine hydroxylase that would play a key role in influencing and favoring the specific female behavior in response to predators.
Evolutionary considerations
The striatum of vizcachas exhibits a clear regionalization resulting from the presence of an internal capsule that separates the striatum into the two well-defined structures: caudate nucleus and putamen. This strikingly differs from the undivided caudate–putamen structure seen in murids like mouse or rat and even in closer evolutionary related caviomorpha such as the guinea pig (Cavia porcellus) (Tindal 1965; Paxinos and Watson 2013). This regionalization of the striatum in L. maximus, reminds the anatomical organization found in other orders like Primates and Scandentia (Graybiel and Ragsdale 1978; Carpenter 1994; Snell 2007; Rice et al. 2011). It is worth to note that Primates, Scandentia, Rodentia and Lagomorpha originate from the same evolutionary clade. The presence of an internal capsule in the basal histricognathe L. maximus, makes a striking difference with laboratory conventional rodents such as mice and rats belonging to the myomorpha terminal clade in rodent phylogeny (Churakov et al. 2010; Voloch et al. 2013). In this way, the greater level of regionalization provided by the presence of the internal capsule may be correlated to a segregation of the motor and cognitive skills allowing the division of the striatal functions that would provide an adaptive advantage to the vizcacha (Daw et al. 2005; Dayan et al. 2006; Kreitzer and Malenka 2008; Liljeholm and O’Doherty 2012).
As it was suggested for the presence of brain convolutions that are lost in more recently evolved rodents, i.e. Myomorpha (Kelava et al. 2013), it seems reasonable to hypothesize a similar situation for the compartmentalization of the striatum. This feature would be an example of an evolutionary/phylogenetic constraint that emerged early and was retained in almost all groups except the murine rodents which likely underwent further modification to develop their unique striatal morphology. The greater degree of regionalization could be interpreted as an evolutive retention that enables the vizcacha´s lifestyle, since it allows an increase of motor skills for the construction of their caves and the manipulation of their food. Rodents with subterranean habits show morphological, physiological and ethological modifications as an adaptative response to the hypogeous environment that might still have impacted in their striatal biology as an adaptative advantage favouring escape from predators (Nevo 1979; Contreras 1981; Bee de Speroni and Pellegrini de Gastaldo 1988).
References
Arvidsson E, Viereckel T, Mikulovic S, Wallen-Mackenzie A (2014) Age- and sex-dependence of dopamine release and capacity for recovery identified in the dorsal striatum of C57/BI6J mice. PLoS ONE 9(6):e99592
Bae EJ, Chen BH, Shin BN, Cho JH, Kim IH, Park JH, Lee JC, Tae HJ, Choi SY, Kim JD, Lee YL, Won MH, Ahn JH (2015) Comparison of immunoreactivities of calbindin-D28k, calretinin and parvalbumin in the striatumbetween young, adult and aged mice, rats and gerbils. Neurochem Res 40(4):864–872
Becker JB (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64:803–812
Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ (1987) The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav 27:53–59
Bee de Speroni N, Pellegrini de Gastaldo A (1988) Encefalización y composición cerebral en tres roedores sudamericanos (Dolichotis patagonum, Lagostomus maximus y Calomys musculinus). Physis 46(111):31–39
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branch LC (1993a) Social organization and mating system of the plains viscacha (Lagostomus maximus). J Zool (London) 229:473–491
Branch LC (1993b) Seasonal patterns of activity and body mass in plains vizcacha, Lagostomus maximus (family Chinchillidae). Can J Zool 71:1041–1045
Branch LC (1995) Observations of predation by pumas and Geoffroy's cats on the plains vizcacha in semiarid scrub of central Argentina. Mammalia 59:152–156
Carpenter MB (1994) Neuroanatomía: fundamentos 4th edition. Medica Panamericana. 448 pag. ISBN: 9788479031732.
Charif SE, Inserra PIF, Schmidt AR, Di Giorgio NP, Cortasa SA, Gonzalez CR, Lux-Lantos V, Halperin J, Vitullo AD, Dorfman VB (2017) Local production of neurostradiol affects gonadotropin-releasing hormone (GnRH) secretion at mid-gestation in Lagostomus maximus (Rodentia, Caviomorpha). Physiol Rep 5(19):e13439
Charif SE, Inserra PIF, Di Giorgio NP, Schmidt AR, Lux-Lantos V, Vitullo AD, Dorfman VB (2016) Sequence analysis, tissue distribution and molecular physiology of the GnRH preprogonadotrophin in the South American plains vizcacha (Lagostomus maximus). Gen Comp Endocrinol 232:174–184
Churakov G, Sadasivuni MK, Rosenbloom KR, Huchon D, Brosius J, Schmitz J (2010) Rodent evolution: back to the root. Mol Biol Evol 27:1315–1326
Contreras JR (1981) El tunduque: Un modelo de ajuste adaptativo. Serie Científica 22–25.
Cossette M, Lecomte F, Parent A (2005) Morphology and distribution of dopaminergic neurons intrinsic to the human striatum. J Chem Neuroanat 29:1–11
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508(1):1–12
Daw ND, Niv Y, Dayan P (2005) Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 8:1704–1711
Dayan P, Niv Y, Seymour B, Daw ND (2006) The misbehavior of value and the discipline of the will. Neural Netw 19:1153–1160
De las Heras S, Hontanilla B, Mengual E, Giménez-Amaya JM (1994) Immunohistochemical distribution of calbindin D-28k and parvalbumin in the head of the caudate nucleus and substantia nigra of the cat. J Morphol 221(3):291–307
DeLong M, Alexander GE, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT (1984) Role of basal ganglia in limb movements. Hum Neurobiol 2:235–244
DeLong MR, Georgopoulos AP (1981) Motor functions of the basal ganglia. Handb Physiol 3:1017–1061
Dorfman VB, Fraunhoffer N, Inserra PIF, Loidl CF, Vitullo AD (2011) Histological characterization of gonadotropin-releasing hormone (GnRH) in the hypothalamus of the South American plains vizcacha (Lagostomus maximus). J Mol Histol 42:311–321
Dorfman VB, Saucedo L, Di Giorgio NP, Inserra PIF, Fraunhoffer N, Leopardo NP, Halperin J, Lux-Lantos V, Vitullo AD (2013) Variation in progesterone receptors and GnRH expression in the hypothalamus of the pregnant South American plains vizcacha, Lagostomus maximus (Mammalia, Rodentia). Biol Reprod 89(5):115–125
Dubach M, Schmidt R, Kunkel D, Bowden DM, Martin R, German DC (1987) Primate neostriatal neurons containing tyrosine hydroxylase: immunohisto-chemical evidence. Neurosci Lett 75:205–210
Fujiyama F, Unzai T, Nakamura K, Nomura S, Kaneko T (2006) Difference in organization of corticostriatal and thalamostriatal synapsesbetween patch and matrix compartments of rat neostriatum. Eur J Neurosci 24:2813–2824
Gerfen CR (1984) The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311:461–464. https://doi.org/10.1038/311461a0
Gerfen CR, Wilson CJ (1996) The Basal Ganglia. In: Hokfelt T, Swanson LW (eds) Handbook of Chemical Neuroanatomy. Elsevier, Amsterdam, pp 365–462
Gillies A, Willshaw D, Li Z (2002) Subthalamic-pallidal interactions are critical in determining normal and abnormal functioning of the basal ganglia. Proc Biol Sci 269(1491):545–551
Gonzalez CR, Muscarsel Isla ML, Fraunhoffer NA, Leopardo NP, Vitullo AD (2012a) Germ cell differentiation and proliferation in the developing testis of the South American plains viscacha, Lagostomus maximus (Mammalia, Rodentia). Zygote 20(3):219–227
Gonzalez CR, Muscarsel Isla ML, Leopardo NP, Willis MA, Dorfman VB, Vitullo AD (2012b) Expression of androgen receptor, estrogen receptors alpha and beta and aromatase in the fetal, perinatal, prepubertal and adult testes of the South American plains vizcacha, Lagostomus maximus (Mammalia, Rodentia). J Reprod Dev 58(6):629–635
Gonzalez CR, Muscarsel Isla ML, Vitullo AD (2018) The balance between apoptosis and autophagy regulates testis regression and recrudescence in the seasonal-breeding South American plains vizcacha, Lagostomus maximus. PLoS ONE 13(1):e0191126
Graybiel AM (1983) Compartmental organization of the mammalian striatum. Prog Brain Res 58:247–256
Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13:244–254. https://doi.org/10.1016/0166-2236(90)90104-I
Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31:359–387. https://doi.org/10.1146/annurev.neuro.29.051605.112851
Graybiel AM, Ragsdale CW (1978) Histochemically distinct compartments in the striatum of human, monkey and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci 75(11):5723–5726
Halperin J, Dorfman VB, Fraunhoffer N, Vitullo AD (2013) Estradiol, progesterone and prolactin modulate mammary gland morphogenesis in adult female plains vizcacha (Lagostomus maximus). J Mol Histol 44(3):299–310
Herkenham M, Pert CB (1981) Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature 291:415–418. https://doi.org/10.1038/291415a0
Holt DJ, Graybiel AM, Saper CB (1997) Neurochemical architecture of the human striatum. J Comp Neurol 384:1–25
Inserra PIF, Charif SE, Di Giorgio NP, Saucedo L, Schmidt AR, Fraunhoffer N, Halperin J, Gariboldi MC, Leopardo NP, Lux-Lantos V, Gonzalez CR, Vitullo AD, Dorfman VB (2017) ERα and GnRH co-localize in the hypothalamic neurons of the South American plains vizcacha, Lagostomus maximus (Rodentia, Caviomorpha). J Mol Histol 48(3):259–273
Jackson JE (1986) Determinación de edad en la vizcacha (Lagostomus maximus) en base al peso del cristalino. Vida Silvestre Neotropical 1:41–44
Jackson JE, Lyn C, Villarreal D (1996) Lagostomus maximus mammalian. Species 543:1–6
Jensen F, Willis MA, Albamonte MS, Espinosa MB, Vitullo AD (2006) Naturally suppressed apoptosis prevents follicular atresia and oocyte reserve decline in the adult ovary of Lagostomus maximus (Rodentia, Caviomorpha). Reproduction 132:301–308
Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18(12):527–535
Kelava I, Lewitus E, Huttner WB (2013) The secondary loss of gyrencephaly as an example of evolutionary phenotypical reversal. Front Neuroanat 7:16
Kita H, Jaeger D (2016) Organization of the Globus Pallidus. Handb Behav Neurosci 24:259–276. https://doi.org/10.1016/B978-0-12-802206-1.00013-1
Kubota Y, Inagaki S, Kito S (1986) Innervation of substance P neurons by catecholaminergic terminals in the neostriatum. Brain Res 375:163–167. https://doi.org/10.1016/0006-8993(86)90969-8
Kubota Y, Kawaguchi Y (1993) Spatial distributions of chemically identified intrinsic neurons in relations to patch and matrix compartments of rat neostriatum. J Comp Neurol 332:499–513. https://doi.org/10.1002/cne.903320409
Klüver H, Barrera E (1953) A method for the combined staining of cells and fibers in the nervous system. J Nueropathol Exp Neurol 12:400–403
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60(4):543–554
Lavoie B, Parent A (1990) Immunohistochemical study of the serotonergic innervation of the basal ganglia in the squirrel monkey. J Comp Neurol 1:1. https://doi.org/10.1111/eip.12846
Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE Jr (2000) Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s Disease and memory. J Neurosci 20(23):8604–8609
Liljeholm M, O’Doherty JP (2012) Contributions of the striatum to learning, motivation and performance: an associative account. Trends Cogn Sci 16(9):467–475
Llanos AC, Crespo JA (1952) Ecología de la vizcacha (Lagostomus maximus maximus Blainv.) en el nordeste de la Provincia de Entre Ríos. Revista de Investigaciones Agrícolas 6:289–378
Luparello TJ, Stein M, Park CD (1964) A stereotaxic atlas of the hypothalamus of the guinea pig. J Comp Neurol 122:201–217
McDermott JL, Liu B, Dluzen DE (1994) Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol 125:306–311
Mensah P, Deadwyler S (1974) The caudate nucleus of the rat: cell types and the demonstration of a commissural system. J Anat 117:281–293
Meredith GE, Farrell T, Kellaghan P, Tan Y, Zahm DS, Totterdell S (1999) Immunocytochemical characterization of catecholaminergic neurons in the rat striatum followingdopamine-depleting lesions. Eur J Neurosci 10:3585–3596
Micheli FE and Luquin-Piudo ME (2012) Functional organization of basal ganglia. Abnormal movements. Ed: Medica Panamericana, Chapter 2.
Middleton FA, Strick PL (1994) Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 226:452–461
Nevo E (1979) Adaptive convergence and divergence of subterranean mammals. Annu Rev Ecol Evol Syst 10:269–308
National Research Council USA (2011) Guide for the care and use of laboratory enimals, 8th edn. The National Academies Press, Washington
Orendain-Jaime EN, Ortega-Ibarra JM, López-Pérez SJ (2016) Evidence of sexual dimorphism in D1 and D2 dopaminergic receptors expression in frontal cortex and striatum of young rats. Neurochem Int 100:62–66
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127
Paxinos G, Watson C (2013) The rat brain in stereotaxic coordinates. AP press, Amsterdam
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam
Rey-Funes M, Dorfman VB, Ibarra ME, Peña E, Contartese DE, Goldstein J, Acosta JM, Larráyoz I, Martínez-Murillo R, Martínez A, Loidl CF (2013) Hypothermia prevents gliosis and angiogenesis development in an experimental model of ischemic proliferative retinopathy. Invest Ophthalmol Vis Sci 54(4):2836–2846
Rey-Funes M, Larráyoz I, Fernández J, Contartese D, Rolón F, Inserra PIF, Martínez-Murillo R, López-Costa J, Dorfman VB, Martínez A, Loidl CF (2016) Methylene blue prevents retinal damage in an experimental model of ischemic proliferative retinopathy. Am J Physiol Regul Integr Comp Physiol 310(11):R1011–1019
Rice MW, Roberts RC, Melendez-Ferro M, Perez-Costas E (2011) Neurochemical characterization of the tree shrew dorsal striatum. Front Neuroanat 5:1–16
Sandell JH, Graybiel AM, Chesselet MF (1986) A new enzyme marker for striatal compartmentalization: NADPH diaphorase activity in the caudate nucleus and putamen of the cat. J Comp Nneurol 243:326–334
Smith Y, Bevan MD, Shink E, Bolam JP (1998) Microcircuitry of the direct and indirect pathwaysof the basal ganglia. Neuroscience 86(2):353–387
Snell RS (2007) Neuroanatomía clínica 6 ed. Medica Panamericana, p 594
Tindal JS (1965) The forebrain of the guinea pig in stereotaxic coordinates. J Comp Neurol 124:259–256
Vincent SR, Johansson O (1983) Striatal neurons containing both somatostatin and avian pancreatic polypeptide APPI-like immunoreactivities and NADPH diaphorase activity: a light and electron microscopic study. J Comp Neurol 217:264–270
Voloch CM, Vilela JF, Loss-Oliveira L, Schrago CG (2013) Phylogeny and chronology of the major lineages of New World hystricognath rodents: insights on the biogeography of the Eocene/Oligocene arrival of mammals in South America. BMC Res Notes 6:160
Walker QD, Rooney MB, Wightman RM, Kuhn CM (1999) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95(4):1061–1070
Wu Y, Parent A (2000) Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain Res 863(1–2):182–191
Xenias HS, Ibáñez-Sandoval O, Koós T, Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci 35(16):6584–6599
Xiao L, Becker JB (1994) Quantitative microdialysis determination of extracellular striatal dopamine concentrations in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 180:155–158
Yelnik J, Francois C, Percheron G, Tande D (1991) Morphological taxonomy of the neurons of the primate striatum. J Comp Neurol 313:273–294
Acknowledgements
We are especially grateful to the Ministerio de AsuntosAgrarios, Dirección de Flora y Fauna, Buenos Aires Province Government for enabling animal capture, to the personnel of ECAS for their invaluable help in trapping and handling the animals, to MV. Sergio Ferraris and MV. Fernando Lange and their veterinarian staff for their essential help on vizcachas handling and anesthetizing, to Ms. Sol ClausiSchettini for her excellent technical assistance in tissue processing, and Mr. Santiago Cicculli for his microscopy technical assistance.This work was funded by theNational Scientific and Technical Research Council(CONICET): PIP No. 110/14, National Scientific and Technical Ministry (MINCyT): PICT-1281/2014, and by FundaciónCientífica Felipe Fiorellino, Universidad Maimónides, Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schmidt, A.R., Inserra, P.I.F., Cortasa, S.A. et al. Structural organization, GABAergic and tyrosine hydroxylase expression in the striatum and globus pallidus of the South American plains vizcacha, Lagostomus maximus (Rodentia, Caviomorpha). J Mol Hist 50, 515–531 (2019). https://doi.org/10.1007/s10735-019-09845-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-019-09845-9