Abstract
Enamel is the hardest tissue with the highest degree of mineralization protecting the dental pulp from injury in vertebrates. The ameloblasts differentiated from ectoderm-derived epithelial cells are a single cell layer and are important for the enamel formation and mineralization. Wnt/β-catenin signaling has been proven to exert an important role in the mineralization of bone, dentin and cementum. Little was known about the regulatory mechanism of Wnt/β-catenin signaling pathway in ameloblasts during amelogenesis, especially in the mineralization of enamel. To investigate the role of β-catenin in ameloblasts, we established Amelx-Cre; β-catenin∆ex3fl/fl (CA-β-catenin) mice, which could constitutive activate β-catenin in ameloblasts. It showed the delayed mineralization and eventual hypomineralization in the incisor enamel of CA-β-catenin mice. Meanwhile, the amelogenesis-related proteinases Mmp20 and Klk4 were decreased in the incisors of CA-β-catenin mice. These data indicated that β-catenin plays an essential role in differentiation and function of ameloblasts during amelogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The formation of the tooth is under the control of reciprocal signaling between ectoderm-derived epithelium and neural crest-derived mesenchyme (Hasegawa et al. 2016; Lee et al. 2016; Li and Pan 2017; Shi et al. 2016). As the hardest tissue in our body, enamel lines on the surface of teeth and effectively protects the teeth from deterioration (Lee et al. 2016; Moradian-Oldak 2012). Ameloblasts are the major cells that take responsibility for enamel formation. It is well estimated that, differentiated from epithelial cells, ameloblasts form a single cell layer, interact with the inner mesenchyme-derived odontoblasts and initiate the amelogenesis (Simmer et al. 2012). Based on the differences in maturity and functional of the ameloblasts, the process of amelogenesis is divided into two stages: the secretory stage and the maturation stage (Bartlett 2013). During the secretory stage, ameloblasts secrete enamel matrix proteins, which form the thin carbonated apatite mineral ribbons. While during the maturation stage, the further matured ameloblasts remove the matrix protein and water, continuously deposit minerals and control apatite crystal growth at the nanometer scale, and finally convert enamel into the hardest tissue in the human body (Bartlett 2013; Simmer et al. 2012). Until recently, researchers mainly focused on the early amelogenesis, little was done to recover the regulatory mechanism that involved in the later development especially the maturation stage.
It is well estimated that canonical Wnt/β-catenin pathway plays essential roles in enamel initiation and morphogenesis. First of all, multiple Wnt ligands, including Wnt-3, -4, -5a, -6, -7b, -10a, and -10b, are expressed predominantly in the epithelial tissue in the developing tooth germ, and Wnt/β-catenin signaling is active at multiple stages of tooth development (Dassule and McMahon 1998; Liu et al. 2008; Sarkar and Sharpe 1999). Epithelial deletion of Gpr177, which leads to failed secretion of Wnt ligands and absent canonical Wnt signaling activity in the enamel knot, causes defective cell proliferation and tooth morphogenesis (Zhu et al. 2013). Ectopic expression of dickkopf-related protein 1 (DKK1), which is recognized as canonical Wnt inhibitor, results in the arrest of tooth development before the bud stage in the oral epithelium (Andl et al. 2002). As we know, β-catenin is the master signaling transducer for the canonical Wnt pathway. Recent studies demonstrated that the normal tooth patterning and development were greatly disturbed in mice with β-catenin loss- and gain-of-function specifically in the oral epithelium (Jarvinen et al. 2006; Liu et al. 2008). In addition, it was found that the deletion of β-catenin starting from the embryo stage (E14.5) led to the enamel malformation (Yang et al. 2013). Taken together, these studies confirmed that Wnt/β-catenin signaling, lying upstream of multiple key morphogenetic signaling pathways, regulates epithelia cell proliferation and differentiation during early enamel development. However, the exact roles of Wnt/β-catenin signaling in ameloblasts remain to be elucidated.
Recently, it is reported that inhibition of odontoblast-drived or ameloblast-drived Wnt ligands resulted in increased enamel production without disrupting the overall size or shape of the incisor (Aurrekoetxea et al. 2012; Jarvinen et al. 2006). Besides, in vitro studies found that β-catenin deletion may inhibit the migration and differentiation of ameloblasts (Guan et al. 2016). These data suggest that ameloblasts are Wnt-responsive and this signaling pathway may be essential for its function and/or maturation. As a result, in order to understand the role of β-catenin in ameloblasts during later enamel formation, mice with ameloblast-specific continuous activation of β-catenin were generated. Systemic evaluation including tooth morphology, histological phenotypes, matrix mineralization and related gene/protein expression of mandibular incisor were carried out for further comparison and analysis. Our results revealed that the specific stabilization of β-catenin in ameloblasts disturbed the expression pattern of several proteinases and gave rise to enamel hypomineralization, suggesting that Wnt/β-catenin signaling is essential for ameloblast differentiation and function during later amelogensis.
Materials and methods
Generation of mouse lines
The animal care and experimental protocols for this study were approved by the Animal Use and Care Committee of Tongji University (No. TJmed-013-31). C57BL/6 were provided by the Shanghai Research Center for Biomodel Organisms (Shanghai, China). β-Catenin-floxed allele mice were donated by Prof Zhaowen Zong and R26R reporter mice (Rosa26mT/mG) were purchased from Shanghai Model Organisms Center, Inc. (Shanghai, China) (Chen et al. 2015). Amelogenin-Cre (Amelx-Cre) transgenic mouse were generated with a 3572 bp promoter fragment (− 2210 to + 1345), which were kind gifts from Prof Yuguang Gao (Jinping et al. 2017). To generate Amelx-Cre; β-catenin∆ex3fl/fl (CA-β-catenin) mice, we crossed Amelx-Cre; β-catenin∆ex3fl/+ mice with β-catenin∆ex3fl/fl mice. The β-catenin∆ex3fl/+ or β-catenin∆ex3fl/fl mice from the same litters created during the crossbreeding regime were used as control. Cre protein distribution was assessed by crossing transgenic mice into a floxed stop tdTomato-EGFP (Rosa26mT/mG) fluorescent reporter mouse line (Muzumdar et al. 2007).
Micro-computed tomography (Micro-CT)
Eight-week-old mice were sacrificed and perfused. The Micro–CT analysis included a high-resolution scan of the whole tooth (10-mm slice increment, n = 4) (Nakayama et al. 2015). Threshold values in the Micro–CT evaluation program (Himed, Bethpage, NY) were set such that the mineralized enamel appeared as a white high-density solid next to dentin as a transparent object. The value of each tooth was detected by micro computed tomography-50 (Scanco Medical, Bassersdorf, Switzerland).
Resin-casted scanning electron microscopy (SEM)
For the SEM analyses, the incisors of 8-week-old mice (n = 4) were dissected and fixed with 4% paraformaldehyde (pH 7.4; Biotech Well, Shanghai, China) in 0.1 M of cacodylate buffer solution (pH 7.4) at 4 °C for 24 h. The tissue specimens were dehydrated and then embedded in methyl methacrylate (MMA, Buehler, Lake Bluff, Illinois, USA). These transverse sections of the hemi-mandibles samples were polished, etched and dried. Then, the samples were coated with gold and palladium and examined using an FEI/Philips XL30 Field emission environmental SEM system (JSM-6010LA, JEOL, Tokyo, Japan) (Liu et al. 2014).
Von Kossa staining
The incisors of 8-week-old mice were embedded in light-cured resin (Exakt 7200 VLC, German) for 13.5 h. Then, the transverse sections were performed at a thickness of 10 µm (Exakt micro section and grinding system, German). Mineralization of the tissue was visualized by 2% silver nitrate staining.
Hematoxylin and eosin (H&E) staining
The mandibles of 2, 8-week-old mice were fixed with 4% paraformaldehyde for 24 h and demineralized in 10% EDTA (pH 7.4; Biotech Well, Shanghai, China) at 4 °C. Sagittal and transverse sections were embedded in paraffin, cut into 5-mm and stained with hematoxylin and eosin (Biotech Well, Shanghai, China) (Nakayama et al. 2015).
Immunofluorescence
Immunofluorescence was performed with frozen sagittal sections of mandibular incisors at 2-week-old mice. Hyaluronidase-induced antigen retrieval citrate buffer was performed. The sections were incubated with primary antibodies specific for β-catenin (dilution 1:200; Abcam, USA), and Amelogenin (dilution 1:400; Santa Cruz Biotechnology, CA, USA) overnight at 4 °C. Then sections were incubated with a fluorescently labeled secondary antibody (dilution 1:1000; Alexa 488 goat anti-mouse and Alexa 555 goat anti-rabbit, Abcam, USA).
Immunohistochemistry (IHC)
IHC was performed with an UltraSitive S-P detection kit (MXB, Fuzhou, China). Briefly, paraffin sections were treated with 3% H2O2 to remove endogenous tissue peroxidase activity followed by Hyaluronidase-induced antigen retrieval citrate buffer at 37 °C for 60 min. After blocking with sheep serum for 2 h at room temperature, the slides were incubated with the primary antibodies, including mouse anti-AMELX (dilution 1:400; Santa Cruz Biotechnology, CA, USA), rabbit anti-AMBN (dilution 1:200; Santa Cruz Biotechnology, CA, USA), rabbit anti-KLK4 (dilution 1:200; Santa Cruz Biotechnology, CA, USA), rabbit anti-MMP20 (dilution 1:200; Abcam, USA), and goat anti-ENAM (dilution 1:200; Santa Cruz Biotechnology, CA, USA). After incubating overnight at 4 °C, tissues were incubated with secondary antibody for 10 min. Finally, the sections were treated with an ABC kit (Vector) to detect the expression of these proteins.
Real-time quantitative PCR (RT-qPCR)
For real-time quantitative PCR analyses, total RNA was extracted from mandibular incisors of three 8-week-old mice. The primers used in this study were designed by Primer Premier 5.0. The expression of each gene was normalized to that one of GAPDH. The primer sets used in real-time quantitative PCR are listed in Table 1 (Bae et al. 2018; Jinping et al. 2017). All experiments were repeated three times.
Statistical analysis
All data were expressed as the means ± standard error. Statistical significance was evaluated using SPSS11.0 software. Data were considered significant at P < 0.05.
Results
Validation of Amelx-Cre activity and β-catenin expression
The mouse incisor is a constantly erupting model that allows the developmental stage of the ameloblast to be observed. The cervical loop (CL) region is composed of the outer enamel epithelium, inner enamel epithelium, and the stellate reticulum, which differentiate into secretory and maturation stages ameloblasts that ultimately form enamel (Fig. 1A). First of all, the incisors 2-week-old Rosa26mT/mG mice and Amelx-Cre; Rosa26mT/mG mice were evaluated and compared to confirm the Cre activity. It was found that the Amelx-Cre line exhibited strong Cre recombinase activity mainly in the secretory and the mature ameloblasts, but little in the cells around cervical loop (Fig. 1B). As a result, to investigate the role of Wnt/β-catenin signaling in ameloblasts during later enamel development, the same Amelx-Cre transgenic mice were crossed with β-catenin∆ex3fl/fl for the generation of Amelx-Cre;β-catenin∆ex3fl/fl (CA-β-catenin) offsprings. To identify a successful stabilization of β-catenin in ameloblasts, we examined the expression of β-catenin and amelogenin in CA-β-catenin incisors by immunofluorescence double labeling. As shown in Fig. 1C, both epithelial cells and differentiated ameloblasts expressed β-catenin, while the enhanced β-catenin activity was only found in ameloblasts.
Validation of Cre and β-catenin activity in mouse incisor. A H&E staining of 2-week-old C57BL/6 mice incisor. B Expression of Cre recombinase at the cervical loop cells (yellow dots), secretory stage ameloblasts and maturation stage ameloblasts in Rosa26mT/mG mouse and Amelx-Cre; Rosa26mT/mG mouse. C Immunofluorescence double labeling of amelogenin (green), and β-catenin (red) at the cervical loop cells, secretory stage ameloblasts and maturation stage ameloblasts in 2-week-old control mice and CA-β-catenin mice incisors. Am ameloblasts, CL the cervical loop cells, IE inner enamel epithelium, ma-Am maturation ameloblasts, Od odontoblast, OE outer enamel epithelium, sc-Am secretory ameloblasts, SR stellate reticulum; scale bar = 100 mm. (Color figure online)
Morphological analysis of CA-β-catenin mice
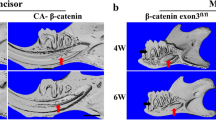
When comparing with the control mice, incisor from CA-β-catenin mice exhibited a typical amelogenesis imperfect (AI)-like phenotype, which displayed chalky enamel with a lack of the typical translucent (Fig. 2A-a, c). Micro-CT images indicated that labial enamel was apparently reduced whereas the incisal dentin was severely exposed and abrade in the CA-β-catenin mice (Fig. 2A-b, d, e, f). In addition, three-dimensional Micro-CT images analysis showed that the shape of enamel was not obviously affected (Fig. 2B). Further statistic data analysis indicated that the enamel thickness at the early erupted portion of CA-β-catenin incisor was not apparently changed. However, its mineral density was significantly decreased when comparing with the control group (Fig. 2C). These observations consistently indicated that although the enamel thickness was not significantly changed, the constitutive stabilization of β-catenin in ameloblasts caused the decreased enamel production and the disturbed mineral density, leading to an AI-like phenotype and the severe abrasion of the incisor. As a result, further experiments were carried out to evaluate whether the enamel structure and/or composition were influenced in CA-β-catenin mice.
Macroscopic evaluation of CA-β-catenin mice. A Gross appearance and micro-CT images of the mandibular incisors of CA-β-catenin mice at 8-weeks-old. B Analysis of enamel mineralization by three-dimensional Micro-CT images in CA-β-catenin mice. C The thickness and enamel mineral density of incisors in CA-β-catenin mice. NS nonsignificant, **P < 0.01, N = 4
Histological phenotypes of CA-β-catenin mice
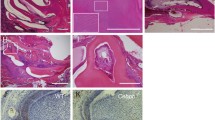
According to the schematic drawing of the location of stages of amelogenesis in mandibular mouse incisors, we picked up the 2, 4 and 8 mm distance from AL to observe secretory, early- and late-maturation stages of enamel (Fig. 3A). At the secretory stage, in both the CA-β-catenin mice and control mice were covered by a continuous enamel layer in enamel space. In the early-maturation stage ameloblasts, the mineralized enamel was demineralized in control mice. However, the delay in enamel mineralization in CA-β-catenin mice was evident by the appearance of an acid-insoluble, eosin-stained organic enamel matrix. Until the late maturation stage, there was residual enamel matrix in the CA-β-catenin mice, suggesting a failure in the removal of matrix proteins and a defect in the mineralization during amelogenesis in the CA-β-catenin mice. In addition, the ameloblasts of CA-β-catenin mice were disorganized and lost their polarity (Fig. 3B). An enamel rod was the mineralized trail left behind as each ameloblast migrates, ameloblasts moved in groups and slid by one another, and this movement culminated in the characteristic decussating enamel prism pattern. The irregular ameloblasts arrangement may lead to disrupted enamel rod pattern.
Histological phenotypes of the mandibular incisor in CA-β-catenin mice. A Schematic drawing of the location of stages of amelogenesis on mandibular mouse incisors. B H&E staining of secretory, early- and late-maturation stages 2, 4 and 8 mm from the apical loop, respectively red line in A in CA-β-catenin mice incisor. Asterisk indicates enamel matrix in enamel space. C Von Kossa staining of different stages in CA-β-catenin mice enamel. White line shows the boundary of dentine and enamel. Red arrows indicate less positivity for von Kossa staining in the incisor enamel of CA-β-catenin mice compared with those of wild type mice. D Scanning electron microscopy images of the interwoven arrangement of the rods (white arrow, rod decussation) with the inter-rod (yellow arrow) enamel at different stages in CA-β-catenin mice incisor. Am ameloblasts, Asterisk enamel matrix, ES enamel space, NS nonsignificant; **P < 0.01, ***P < 0.001, N = 4. (Color figure online)
Mineralization and ultrastructure of undecalcificated incisor enamels
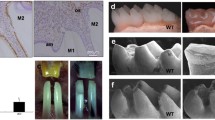
To further assess the enamel matrix characteristics of the CA-β-catenin mice, unmineralized incisor enamel were evaluated by von Kossa staining and backscattered SEM. Compared to enamel stained in black in control mice, the CA-β-catenin enamel turned to be brown or gray in von Kossa staining (Fig. 3C), suggesting that constitutively stabilization of β-catenin in ameloblasts would lead to the enamel hypomineralization. Furthermore, it was found that in the secretory stage of the control enamel, numerous enamel rods were regularly interweaved and composed of certain organic constituent, which was almost totally substituted by hydroxyapatite crystallites during the subsequent early maturation stage. However, in the CA-β-catenin mice, this rod decussation (X-shaped pattern, white arrow dots) was disrupted. Instead, the irregular enamel rods were promiscuously arranged, and both the enamel rod and the interrod matrix failed to deposit enough mineral even until the late maturation stage (Fig. 3D). Taken together, these data revealed that the Wnt/β-catenin pathway in ameloblasts was important for enamel mineralization.
Expression of amelogenesis-related proteins
As we know, enamel formation is exquisitely regulated at the molecular level, with numerous genes being critical for normal development. Several known genes play important roles in regulating the quantity and/or quality of enamel during amelogenesis, including extracellular matrix proteins, such as Amelogenin (Amelx), Ameloblastin (Ambn), and Enamelin (Enam), etc, and critical proteinases are involved in processing the enamel extracellular matrix (ECM), including Matrix metalloproteinase 20 (Mmp20) and Kallikrein4 (Klk4), etc. The expression patterns of these amelogenesis-related proteins were detected using mouse mandibular incisors of CA-β-catenin mice and control mice. Immunohistochemistry staining of ameloblast specific proteins, including AMELX, AMBN and ENAM, showed none obvious differences between CA-β-catenin mice and control mice (Fig. 4A). Whereas the matrix proteases were remarkably down-regulated in the CA-β-catenin mice: the expression level of MMP20 was apparently decreased in both the secretory-stage and the maturation-stage ameloblasts, and KlK4 was decreased in maturation-stage ameloblasts. Klk4 was not present at the secretory ameloblasts in the two types of mice. As shown above the maturation-stage enamel in CA-β-catenin mice was detectable. And within this malformed enamel, there were large amounts of residual protein including both the enamel ECM and the critical proteinase, implying a hypomineralization of this enamel.
Expression of amelogenesis-related genes and proteins. A Quantitative changes expression of AMELX, AMBN, ENAM, MMP20 and KLK4 in secretory and maturation stage of ameloblasts of control and CA-β-catenin mice. Black arrows indicate the down-regulation of positive signals in the incisor ameloblasts of CA-β-catenin mice. B Expression of amelogenesis-related genes in the incisor ameloblasts of CA-β-catenin mice. The data were analysed as the mean ± SD, *P < 0.05. Am ameloblasts; scale bar = 100 mm, N = 5
Expression of amelogenesis-related genes
RNAs were also extracted from the two type mandibular incisors of 8-week-old mice, and the expression levels of these amelogenes-related genes were evaluated by qRT-PCR (Fig. 4B). Consistent with the immunohistochemistry staining results, the gene expression levels of Amelx, Ambn and Enam in CA-β-catenin group didn’t show evident differences when compared with control group. However, Mmp20 and Klk4, which belongs to the matrix protease, were significantly down-regulated in the CA-β-catenin mice. It reflected that the mineralization converting of the immature enamel matrix might be interrupted by an abnormal up-regulation of canonical Wnt signaling activity in ameloblasts.
Discussion
Numerous studies have confirmed that Wnt/β-catenin signaling pathway is widely expressed in ameloblasts, odontoblasts, cementum and osteoblasts, and exerts an important role in the in vivo mineralization of hard tissues, including enamel, dentin, cementun and bone (Chen et al. 2015; Guan et al. 2016; Han et al. 2015; Lerner and Ohlsson 2015). Briefly, through binding to the extracellular receptors, Wnt ligands elicit diverse intracellular responses by allowing β-catenin to enter the nucleus and interact with Lef/Tcf transcription factors to activate downstream target genes (van Amerongen and Nusse 2009). In the absence of Wnts, β-catenin is maintained at low levels and targeted for proteasome degradation through a destruction complex (Yu et al. 2005; Zeng et al. 1997). So as the key signal transducer of the Wnt signaling pathway, it is predictable that β-catenin is essential for the development and mineralization of hard tissue, such as enamel. However, direct evidence is still inadequate in recovering the biological functions of β-catenin during amelogenesis. Recently, ameloblast-lineage cells were used by Dr. Guan to investigate the potential roles of β-catenin in cell migration. They found that the ablation of β-catenin caused the up-regulation of E-cadherin expression and inhibited cell migration, leading to enamel malformation (Guan et al. 2016). Nevertheless, a more systematic analysis in vivo is still required for further discovering the roles of β-catenin especially in regulating enamel mineralization and ameloblast function. In this study, we created the Amelx-Cre; β-catenin∆ex3fl/fl mice, in which β-catenin was continuously activated in the cells expressing amelogenin. The morphology, histology, mineralization and gene/protein expression were detected and analyzed. Our results showed that this mouse model developed a severe disturbance in the mineralization of enamel, suggesting that Wnt/β-catenin signaling pathway in amelobalsts is also very important in regulating mineralization in vivo.
It is widely accepted that the highly controlled expression of ECM proteins and enzymes is the key for achieving an organized amelogenesis (Moradian-Oldak 2012; Smith et al. 2011). Data from our experiments indicated that the continuous activation of β-catenin specifically in ameloblasts did not affect the expression of the ECM proteins, such as AMELX, AMBN and ENAM. In addition, since the full layer of the enamel is established during the secretory stage (Bartlett and Simmer 2014; Hu et al. 2014, 2016; Moradian-Oldak 2012), the thickness of the mutant enamel also didn’t show a significant change in our study. Taken together, it is highly suggested that the enamel initiation and the subsequent formation of secretory stage-enamel are not controlled by Wnt/β-catenin signaling pathway. In another word, Wnt/β-catenin signaling in ameloblasts is not crucial for regulating their differentiation and secretary function. However, our further evaluations found that the mineralization of CA-β-catenin enamel was strikingly interfered. Moreover the expression of proteinases, including MMP20 and KLK4, were also significantly decreased in the mutant mice, implying the degradation of enamel ECM might be affected. MMP20, mainly expressed in secretory-stage and maturation-stage enamel, is one of key proteinases for hydrolyzing newly secreted enamel ECM proteins into stable intermediate products (Bartlett et al. 2011; Caterina 2002; Shin et al. 2014). KLK4, expressed in the maturation-stage ameloblasts, removes the mass of previously secreted and partially hydrolyzed (by Mmp20) ECM proteins of the enamel, so as to facilitate the growth of the hydroxyapatite crystallites and promote their fusions with each other (Simmer et al. 2009). As a result, it was proved by our work that Wnt/β-catenin is important in regulating the maturation and mineralization function of ameloblasts during the maturation stage of enamel. Specifically, by adjusting the activity of Wnt/β-catenin signaling at a certain level, ameloblasts will further mature and secret proteinase containing MMP20 and KLK4, which digest the proteolytic products of AMELX, AMBN and ENAM, etc., provide enough space for crystal growth.
Interestingly, the most striking mineralization abnormality was restricted within the incisor enamel, and there was not an obvious malformation of enamel in CA-β-catenin molars. This difference may be due to the special growth characteristics of mandibular incisor. It is reported that mouse incisor is a rapidly and continuously erupting tooth (Simmer et al. 2009), which involved several mechanisms what differ from molar. Some researchers reported that the activity of Wnt/β-catenin was undetectable in ameloblasts of the mouse molar, while others confirmed that β-catenin was expressed in the ameloblasts of the mouse incisor, indicating Wnt/β-catenin signaling might plays a different role in molar and incisor amelogenesis, or the activation levels of this signaling is distinctly required for molar and incisor enamel mineralization (Lohi et al. 2010; Suomalainen and Thesleff 2010).
In general, by generating an appropriate mouse model for the in vivo study of Wnt/β-catenin during the later stages of enamel formation, we confirmed that the constitutive activation of β-catenin in ameloblasts did not affect the development of secretory stage-enamel, but severely inhibited its further mineralization. It was confirmed that β-catenin played as a key factor for promoting ameloblast maturation and function by regulating the secretion of MMP20 and KLK4 during maturation stage of amelogenesis, so as to take part in the enamel ECM protein digestion and crystallites growth. Nevertheless, future studies are encouraged to discover the related underlying mechanisms.
References
Andl T, Reddy ST, Gaddapara T, Millar SE (2002) WNT signals are required for the initiation of hair follicle development. Dev Cell 2:643–653
Aurrekoetxea M, Lopez J, Garcia P, Ibarretxe G, Unda F (2012) Enhanced Wnt/beta-catenin signalling during tooth morphogenesis impedes cell differentiation and leads to alterations in the structure and mineralisation of the adult tooth. Biol Cell 104:603–617. https://doi.org/10.1111/boc.201100075
Bae J-M et al (2018) Specificity protein 7 is required for proliferation and differentiation of ameloblasts and odontoblasts. J Bone Miner Res. https://doi.org/10.1002/jbmr.3401
Bartlett JD (2013) Dental enamel development: proteinases and their enamel matrix substrates. ISRN Dent 2013:684607. https://doi.org/10.1155/2013/684607
Bartlett JD, Simmer JP (2014) Kallikrein-related peptidase-4 (KLK4): role in enamel formation and revelations from ablated mice. Front Physiol 5:240. https://doi.org/10.3389/fphys.2014.00240
Bartlett JD, Skobe Z, Nanci A, Smith CE (2011) Matrix metalloproteinase 20 promotes a smooth enamel surface, a strong dentino-enamel junction, and a decussating enamel rod pattern. Eur J Oral Sci 119(Suppl 1):199–205. https://doi.org/10.1111/j.1600-0722.2011.00864.x
Caterina JJ (2002) Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598–49604. https://doi.org/10.1074/jbc.M209100200
Chen S et al (2015) Adverse effects of osteocytic constitutive activation of ss-catenin on bone strength and bone growth. J Bone Miner Res 30:1184–1194. https://doi.org/10.1002/jbmr.2453
Dassule HR, McMahon AP (1998) Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol 202:215–227. https://doi.org/10.1006/dbio.1998.8992
Guan X, Xu M, Millar SE, Bartlett JD (2016) Beta-catenin is essential for ameloblast movement during enamel development. Eur J Oral Sci 124:221–227. https://doi.org/10.1111/eos.12261
Han P, Ivanovski S, Crawford R, Xiao Y (2015) Activation of the canonical Wnt signaling pathway induces cementum regeneration. J Bone Miner Res 30:1160–1174. https://doi.org/10.1002/jbmr.2445
Hasegawa K et al (2016) Facioscapulohumeral muscular dystrophy (FSHD) region gene 1 (FRG1) expression and possible function in mouse tooth germ development. J Mol Histol 47:375–387. https://doi.org/10.1007/s10735-016-9680-5
Hu JC et al (2014) Enamelin is critical for ameloblast integrity and enamel ultrastructure formation. PLoS ONE 9:e89303. https://doi.org/10.1371/journal.pone.0089303
Hu Y, Smith CE, Richardson AS, Bartlett JD, Hu JC, Simmer JP (2016) MMP20, KLK4, and MMP20/KLK4 double null mice define roles for matrix proteases during dental enamel formation. Mol Genet Genomic Med 4:178–196. https://doi.org/10.1002/mgg3.194
Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I (2006) Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 103:18627–18632. https://doi.org/10.1073/pnas.0607289103
Jinping Z et al (2017) Overexpression of constitutively active MAP3K7 in ameloblasts causes enamel defects of mouse teeth. Arch Oral Biol 84:169–175. https://doi.org/10.1016/j.archoralbio.2017.09.020
Lee HK, Park JW, Seo YM, Kim HH, Lee G, Bae HS, Park JC (2016) Odontoblastic inductive potential of epithelial cells derived from human deciduous dental pulp. J Mol Histol 47:345–351. https://doi.org/10.1007/s10735-016-9676-1
Lerner UH, Ohlsson C (2015) The WNT system: background and its role in bone. J Intern Med 277:630–649. https://doi.org/10.1111/joim.12368
Li S, Pan Y (2017) Differential expression of transforming growth factor-beta1, connective tissue growth factor, phosphorylated-SMAD2/3 and phosphorylated-ERK1/2 during mouse tooth development. J Mol Histol 48:347–355. https://doi.org/10.1007/s10735-017-9733-4
Liu F et al (2008) Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 313:210–224. https://doi.org/10.1016/j.ydbio.2007.10.016
Liu P, Zhang H, Liu C, Wang X, Chen L, Qin C (2014) Inactivation of Fam20C in cells expressing type I collagen causes periodontal disease in mice. PLoS ONE 9:e114396. https://doi.org/10.1371/journal.pone.0114396
Lohi M, Tucker AS, Sharpe PT (2010) Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn 239:160–167. https://doi.org/10.1002/dvdy.22047
Moradian-Oldak J (2012) Protein-mediated enamel mineralization. Front Biosci 17:1996–2023
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L (2007) A global double-fluorescent Cre reporter mouse. Genesis 45:593–605. https://doi.org/10.1002/dvg.20335
Nakayama Y, Holcroft J, Ganss B (2015) Enamel hypomineralization and structural defects in amelotin-deficient mice. J Dent Res 94:697–705. https://doi.org/10.1177/0022034514566214
Sarkar L, Sharpe PT (1999) Expression of Wnt signalling pathway genes during tooth development. Mech Dev 85:197–200
Shi L, Li L, Wang D, Li S, Chen Z, An Z (2016) Spatiotemporal expression of caveolin-1 and EMMPRIN during mouse tooth development. J Mol Histol 47:337–344. https://doi.org/10.1007/s10735-016-9675-2
Shin M et al (2014) Matrix metalloproteinase-20 over-expression is detrimental to enamel development: a Mus musculus model. PLoS ONE 9:e86774. https://doi.org/10.1371/journal.pone.0086774
Simmer JP, Hu Y, Lertlam R, Yamakoshi Y, Hu JC (2009) Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J Biol Chem 284:19110–19121. https://doi.org/10.1074/jbc.M109.013623
Simmer JP, Richardson AS, Hu YY, Smith CE, Ching-Chun Hu J (2012) A post-classical theory of enamel biomineralization… and why we need one. Int J Oral Sci 4:129–134. https://doi.org/10.1038/ijos.2012.59
Smith CE, Hu Y, Richardson AS, Bartlett JD, Hu JC, Simmer JP (2011) Relationships between protein and mineral during enamel development in normal and genetically altered mice. Eur J Oral Sci 119(Suppl 1):125–135. https://doi.org/10.1111/j.1600-0722.2011.00871.x
Suomalainen M, Thesleff I (2010) Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn 239:364–372. https://doi.org/10.1002/dvdy.22106
van Amerongen R, Nusse R (2009) Towards an integrated view of Wnt signaling in development. Development 136:3205–3214. https://doi.org/10.1242/dev.033910
Yang Z et al (2013) Cessation of epithelial Bmp signaling switches the differentiation of crown epithelia to the root lineage in a beta-catenin-dependent manner. Mol Cell Biol 33:4732–4744. https://doi.org/10.1128/MCB.00456-13
Yu HM et al (2005) The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132:1995–2005. https://doi.org/10.1242/dev.01786
Zeng L et al (1997) The mouse fused locus encodes axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181–192
Zhu X et al (2013) Intra-epithelial requirement of canonical Wnt signaling for tooth morphogenesis. J Biol Chem 288:12080–12089. https://doi.org/10.1074/jbc.M113.462473
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos: 81570966, 81371141), the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No: 20130072110020) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Linlin Fan, Yuguang Gao, Chun-Hung Chu and Qi Zhang contributed to conception, design, data acquisition analysis and interpretation, drafted and critically revised the manuscript; Shijian Deng, Xin Sui, Mengmeng Liu, Shuhua Cheng, Yunfei Wang contributed to conception, design and data acquisition.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest are disclosed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, L., Deng, S., Sui, X. et al. Constitutive activation of β-catenin in ameloblasts leads to incisor enamel hypomineralization. J Mol Hist 49, 499–507 (2018). https://doi.org/10.1007/s10735-018-9788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-018-9788-x