Abstract
Chondroitin sulfate proteoglycan (CSPG) is an important component of extracellular matrix (ECM), it is composed of a core protein and one or more chondroitin sulfate glycosaminoglycan side chains (CS-GAGs). To investigate the roles of its CS-GAGs in dentinogenesis, the mouse mandibular first molar tooth germs at early bell stage were cultivated with or without β-xyloside. As expected, the CS-GAGs were inhibited on their incorporation to CSPGs by β-xyloside, accompanied by the change of morphology of the cultured tooth germs. The histological results and the transmission electron microscopy (TEM) investigation indicated that β-xyloside exhibited obvious inhibiting effects on odontoblasts differentiation compared with the control group. Meanwhile the results of immunohistochemistry, in situ hybridization and quantitative RT-PCR for type I collagen, dentin matrix acidic phosphoprotein 1 and dentin sialophosphoprotein, the products of differentiated odontoblasts, further proved that odontoblasts differentiation was inhibited. Collagen fibers detected in TEM decreased and arranged in disorder as well. Thus we conclude that the inhibition of CS-GAGs incorporation to CSPGs can affect odontoblast differentiation in cultured embryonic mouse molars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular matrix (ECM) is a complex network which fills up the extracellular space within all tissues and organs. It is well accepted that the ECM molecules play an important role in the regulation of cell morphology, growth, migration, adhesion, differentiation, and apoptosis (Hynes 2009). It is also believed that the stage-specific ECMs being essential in the epithelial–mesenchymal interactions to control morphogenesis and dentinogenesis during tooth development, although its exact molecular mechanisms are unknown (Thesleff et al. 1991; Rozario and Desimone 2010).
Chondroitin sulfate proteoglycans (CSPGs), as a major member of the ECMs, is reported to be involving in a variety of cell activities such as cellular proliferation, migration and adhesion during embryogenesis (Carulli et al. 2005). CSPGs are composed of a core protein and one or more chondroitin sulfate glycosaminoglycan side chains (CS-GAGs). CS-GAGs consists of glucuronic acid and N-acetylgalactosamine with one or more sulfates attached, 4- or 6-carbons of the galactosamine moiety most commonly (Ponedel’kina et al. 2012). Biochemical studies revealed that during tooth morphogenesis, CS-GAGs were the most abundant glycosaminoglycans in embryonic mouse molars, as cytodifferentiation occurred, the total amount of chondroitin sulfate, however, decreased (Garud et al. 2008). Our previous study and those of others also indicated that CSPGs expressed in the ECM with a specific temporal–spatial pattern and the sulfation pattern of the core protein varies with the process of tooth development (Schwartz 1977; Takagaki et al. 2002; Jiang et al. 2010; Wu et al. 2010), which indicated that the CS-GAGs might act key roles during tooth development.
The main hard tissue in tooth—dentin, is a mineralized connective tissue. A variety of molecular interactions occur in the process of dentin mineralization, including many collagen–noncollagenous protein interactions. Type I collagen, secreted by odontoblasts, is one of the major structural proteins of the dentin ECM, making up 90% of organic matrix proteins. It is known that type I collagen interacts with many non-collagenous proteins, playing a crucial role during dentinogenesis (Veis 1993; Maciejewska et al. 2006). Non-collagenous proteins are also considered to be important components in regulating collagen mineralization and hydroxyapatite crystal growth during dentinogenesis (Zhang et al. 2017). The vast majority of non-collagenous proteins in dentin is the SIBLING (small integrin-binding ligand, n-linked glycoprotein) family, mainly including dentin matrix acidic phosphoprotein 1 (DMP1), dentin sialophosphoprotein (DSPP) which are also secreted by odontoblasts (Fisher et al. 2001).
According to previous studies, we speculate that CS-GAGs might act essential roles during tooth development. To clarify the exact roles of CS-GSGs in dentinogenesis, in the present study we cultured tooth germ organs with or without 4-methylumbelliferyl-β-d-xyloside (Xyl-MU), which could inhibit the CS-GAGs incorporation of CSPGs. Then we investigated and analyzed the effects of Xyl-MU on tooth morphogenesis and odontoblast differentiation, as well as some odontoblast-related genes and proteins.
Materials and methods
Animals
Pregnant ICR mice were purchased from the Shanghai Laboratory Animal Center of the Chinese Academic of Science (Shanghai, China). All animal work was done according to the National Institutes of Health guidebook and approved by the Committee on the Ethics of Animal Experiments of Tongji University. The presence of a vaginal plug was used as an indication of embryonic day 0 (E0). The first mandibular molar tooth germs of E16.5 were dissected under stereomicroscope and used for organ cultures.
Organ culture
Mandibular first molar germs from E16.5 mouse embryos were dissected and cultured on 0.1 μm Omnipore filters (Millipore, America) in a modification of Trowell’s system. Two tooth germs from each mandible were divided into the control and experimental groups. The explants were cultured in DMEM/F12 medium supplemented with 10% FBS, 100 μg/ml ascorbic acid, 50 U/ml penicillin, and 50 μg/ml streptomycin (complete medium, all from Sigma, USA) in a humidified atmosphere of 5% CO2 in air at 37 °C. Medium was changed every other day. Tooth germs were cultured in the presence or absence of 2 mM 4-methylumbelliferyl-β-d-xyloside (Xyl-MU), a selectively inhibitor of the CS-GAGs incorporation (Sigma, USA). The tooth germs were photographed every other day and tooth size (width, cusp height and total height) was measured (n = 12) using the method reported in the previous literature to evaluate their growth (Wu et al. 2010). Each experiment was repeated three times.
Tissue preparations
After 6 days or 8 days’ cultivation, tooth germs were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 °C for 24 h, followed by embedding in paraffin. Serial sections (5 μm) were cut for hematoxylin–eosin (HE) staining, immunohistochemistry and in situ hybridization. HE staining was observed under a microscope (Nikon Eclipse 80i, Japan).
Transmission electron microscopy (TEM)
Tooth germs cultured for 6 days were rinsed in Hanks medium, immersed for 4 h at 4 °C in a fixative solution containing 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), then post-fixed with 1% osmium tetroxide for 30 min at room temperature. The samples were rinsed in the same buffer, dehydrated in graded ethanol, then embedded in Epon 812 (TAAB, Berkshire, UK). Ultrathin sections stained with uranyl acetate and lead citrate were examined with a HITACHI H-800 transmission electron microscope operating at 80 kV.
Antibodies and immunohistochemistry
A SABC kit (Boster, Wuhan, China) was used for immunohistochemistry. 2B6, 3B3 and CS-56 mouse monoclonal antibodies were purchased from Seikagaku (Tokyo, Japan). 2B6 recognizes unsaturated uronic acid linked to N-acetylgalactosamine 4-sulphate (△di-4S) and 3B3 recognizes unsaturated uronic acid coupled to N-acetylgalactosamine 6-sulphate (△di-6S). These epitopes are produced by chondroitinase ABC digestion. CS-56 recognizes the GAG portion of native CSPGs. These antibodies have been widely used in immunohistochemistry for CS-GAGs (Dondi and Muir 1976; Kresse and Schönherr 2001; Septier et al. 2001; Tenório et al. 2003; Zhang et al. 2014; Yang et al. 2016). Rabbit polyclonal antibody against type I collagen, DMP1 and secondary antibody were obtained from Abacam (ab21286, ab103203, ab6720). Briefly, the sections were deparaffinized and rehydrated by gradient elution using xylene and ethanol followed by incubation in 3% H2O2 to suppress endogenous peroxidase activity. Sections were digested or incubated in sodium citrate buffer and heated for antigen retrieval. A concentration of 5% BSA was applied to sections for 20 min at room temperature. Next, primary antibodies were applied to the sections at 4 °C overnight. The sections were then incubated with biotinylated secondary antibody. An SABC kit was used for subsequent staining. Finally, diaminobenzidine (DAB) or 3-amino-9-ethylcarbazole (AEC) was used as color developing agents, after counterstaining with hematoxylin, these sections were mounted with Permount TM Mounting Medium, and observed under microscope.
RNA extraction and quantitative RT-PCR
Total RNA of tooth germs (E16.5 cultured for 8 days, n = 8) were extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to instruction of manufacturer. The amount and the integrity of RNA were assessed by measurement of absorbance at 260 and 280 nm. First-strand cDNA synthesis was performed with a cDNA synthesis kit (Qiagen). The levels of type I collagen, DMP1 and DSPP and were measured by quantitative RT-PCR with SYBR Green Master Mix (TaKaRa Biotech, Dalian, China) and normalized to the level of GAPDH mRNA. These experiments were performed in triplicate. Primer sequences used in quantitative RT-PCR are listed in Table 1.
In situ hybridization
In situ hybridization used digoxigenin-labeled RNA probes for type I collagen (Kindly supplied by Prof. Shunichi Shibata, Tokyo Medical and Dental University, Tokyo, Japan) and the Nucleic Acid Detection Kit (Roche Diagnostics, Mannheim, Germany) was performed. Briefly, deparaffinized sections were washed in PBS and fixed with freshly prepared 4% paraformaldehyde (PFA) in PBS for 15 min. These sections were treated in proteinase K at 37 °C and fixed again with PFA in PBS for another 15 min. After washing in PBS, they were treated with 0.2 m HCl for 10 min and were acetylated in 0.25% acetic anhydride in 0.1 m triethanolamine (pH 8.0) for 10 min. Hybridization was performed at 50 °C for more than 16 h in a humid chamber. After hybridization, sections were washed with 2× SSC and 50% formamide solution. Hybridization signal was detected by adding alkaline phosphatase substrate (NTB/BCIP; Roche Diagnostic, Dallas, TX) in detection buffer (10% polyvinyl alcohol 70–100 kD, 100 mmol/l Tris, pH 9, 100 mmol/l NaCl, 2 mmol/l Levamisole; Sigma-Aldrich, St. Louis, MO). The duration of hybridization signal development was for 1 h at room temperature. Each section was examined after counterstaining with methyl green and sense probes were used as the negative control. We examined three to four different explants for each group (cultured for 8 days), in order to confirm the consistency of the findings.
Statistical analysis
Data were shown as the arithmetic mean ± the standard error of the mean. Statistical analysis was carried out using IBM SPSS Statistics 22 for Windows (IBM Corporation, Armonk, NY, USA). The significance of differences between groups was tested using Student’s t test. Differences were considered significant when p < 0.05.
Results
CS-GAGs synthesis were inhibited by Xyl-MU
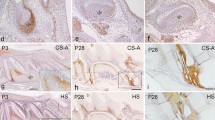
The epitopes of the CS-GAG chains, 2B6, 3B3 and CS-56 were detected by immunohistochemical method on tooth germs after 6 days’ cultivation. The distribution patterns of them in the control group were identical to those observed at comparable developmental stages in vivo as previous report (Schwartz 1977; Takagaki et al. 2002; Wu et al. 2010). The immunostaining of 2B6 was detected in the stellate reticulum and the cuspal region of the dental papilla while CS-56 was detected in the dental papilla but not in the stellate reticulum in the control group, the 3B3 immunostaining pattern was similar to CS-56 but decreased in the cuspal region (Fig. 1a–c). However, in the Xyl-MU treated tooth germs, 3B3, 2B6 and CS-56 immunostaining were considerably reduced. 3B3 (Fig. 1d) and 2B6 (Fig. 1e) were completely devoid, and CS-56 was nearly abolished (Fig. 1f).
Immunohistochemistry. A Immunohistochemical staining of tooth germs after 6 days’ cultivation for 2B6, 3B3 and CS-56. The immunostaining of 2B6 was detected in the stellate reticulum and the cuspal region of the dental papilla while 3B3 and CS-56 were detected in the dental papilla in the control group (triangle in a, b, c). In the Xyl-MU treated group, they were considerably reduced that 3B3 and 2B6 (d, e) were completely devoid and CS-56 was nearly abolished (arrow in f). B Immunohistochemical staining of tooth germs after 8 days’ cultivation for type I collagen. Type I collagen was detected in the dental papilla of both group. But in the predentin of the control group, type I collagen staining was thicker and stronger than that of Xyl-MU treated group (arrow in g and h). C Immunohistochemical staining of tooth germs after 6 days’ cultivation for DMP1. DMP1 was detected in odontablast located regions in the control group, while immunostaining was weak in the Xyl-MU treated group (arrow in i and j). Scale bar 100 μm
Xyl-MU caused morphological variation of cultured tooth germs
Tooth molar germs were treated with or without 2 mM Xyl-MU in organ culture system. Under a normal condition, after 6 days’ culture, tooth germs of E16.5 mice grew and showed well developed cusps (Fig. 2a). In contrast, in the presence of Xyl-MU, the growth of the cultured tooth germs and cusp formation were inhibited (Fig. 2b). Meanwhile, after measurement, the tooth germ width, cusp height and total height were inhibited by 17, 70 and 11% respectively (n = 12) (Fig. 2B).
Measurement of cultured tooth germs. A Tooth germs of E16.5 were cultured with or without 2 mM Xyl-MU for 6 days. The tooth germ and cusps were well developed in the control group while the tooth germ was smaller and cusps formation was inhibited in Xyl-MU group (arrow in a, b). B Tooth germ width, cusp height and total height were measured and found to be inhibited in Xyl-MU group. (n = 12) *p < 0.05, **p < 0.01 (Student’s t test). Scale bar 100 μm (a, b)
Xyl-MU affected the histogenesis of cultured tooth germs
In addition to the morphological defects, Xyl-MU also affected the histogenesis of cultured tooth germs (Dondi and Muir 1976). In the control explants, after 6 days’ culture, the base membrane disappeared and be replaced by an evident predention deposition, odontoblasts were fully differentiated in the main cusp (Fig. 3a, b). Whereas in the Xyl-MU treated tooth germs, the base membrane could still be seen clearly and the underlying dental mesenchyme cells was flat, the odontoblast differentiation was inhibited and, as a consequence, the deposition of predentin was severely affected and nearly absent (Fig. 3c, d).
Histogenesis and ultrastructure of cultured tooth germs. A HE staining of tooth germs after 6 days’ cultivation. The base membrane disappeared, the predention deposition was evident and odontoblasts were fully differentiated in the control group, while the base membrane could still be seen clearly, the odontoblast differentiation was inhibited and the predentin deposition was nearly absent in the Xyl-MU treated group (arrow in b, d). B TEM images of tooth germs after 6 days’ cultivation. The thickness of the predentin (pd) in the control group was almost five times thicker than that in the Xyl-MU treated groups. Odontoblasts (ob) showed a cube or flat shape and odontoblast processes (△ in f) could not be observed in the Xyl-MU treated group. Collagen fibers (* in f, h) were regularly arranged in the control group but were in disorder in the Xyl-MU treated group. pd predentin, ob odontoblast. Scale bar 100 μm (a–d). Scale bar 5 μm (e, g). Scale bar 1 μm (f, h)
Xyl-MU altered the ultrastructure of cultured tooth germs
TEM results indicated that the odontoblasts at the cusp tip area were arranged in parallel in proximal region and odontoblast process could be seen in the predentin. While, odontoblasts in the Xyl-MU treated groups showed a cube or flat shape, and there were no odontoblast processes observed. The predentin in the control group could be obviously observed and the thickness of the predentin could reach 10 μm at some certain place after cultured for 6 days, almost five times thicker than that in the Xyl-MU treated group. (Fig. 3e, g). Meanwhile, collagen fibers were regularly arranged in the predentin in the control group (Fig. 3f), but they were disorder-arranged in the Xyl-MU treated group (Fig. 3h).
The effects of Xyl-MU on the related gene and proteins of odontoblast
Immunohistochemistry
Type I collagen was detected in dental papilla cells of both group. In the predentin of the control group, type I collagen staining was strong. But in the predentin of the Xyl-MU treated explants, the staining was weaker (Fig. 1g, h). DMP1 was detected in odontoblasts-located regions in the control group, while immunostaining was weak in the Xyl-MU treated explants (Fig. 1i, j).
Quantitative RT-PCR
Total mRNA of type I collagen, DMP1 and DSPP from tooth germs cultured for 8 days from two groups were isolated and subjected to quantitative RT-PCR. Comparing with the control explants, the expression level of type I collagen, DMP1 and DSPP were decreased in the samples of the Xyl-MU treated tooth germs (Fig. 4A).
Quantitative RT-PCR and in situ hybridization analysis. A Quantitative RT-PCR for type I collagen, DMP1 and DSPP of tooth germs after 8 days’ cultivation. The expression level of type I collagen, DMP1 and DSPP were significantly decreased in the samples of Xyl-MU treated tooth germs. Data were shown as the mean ± SD; *p < 0.05, **p < 0.01 (Student’s t test). B In situ hybridization for type I collagen mRNA of tooth germs after 8 days’ cultivation. Type I collagen mRNA was expressed obviously in predentin of the control group, while it was very weak in Xyl-MU treated group (arrow in a, b). Scale bar 100 μm (a, b)
In situ hybridization analysis
The results of the in situ hybridization for type I collagen demonstrated that its expression in the two groups were quite different. In the control group, type I collagen mRNA expressed obviously in predentin, while it was detected very weak in the predetin of Xyl-MU treated tooth germs (Fig. 4a, b). This outcome was coinciding with the finding of TEM, immunohistochemistry and quantitative RT-PCR.
Discussion
The process of tooth development depends on well-regulated reciprocal interaction between dental epithelium and dental mesenchyme which acts as an indispensable initiator (Thesleff and Nieminen 1996). The stage-specific ECMs plays a critical role during the epithelial–mesenchymal interactions. CSPGs, as a major family of noncollagenenous macromolecules in the ECMs, also make significant contributions during tooth development. It is reported that the CS-GAGs are important components of CSPGs, and many of its functions are accomplished by the CS-GAGs alone or in conjunction with the core protein (Wight 2002). Our previous study also showed that the expression of CS-GAGs changed in different stages of tooth development (Jiang et al. 2010).
The prolongation of CS-GAGs synthesis depends on glycosyltransferase, but β-xyloside can compete with the xylosided–proteoglycan core protein for the glycosyltransferase thus selectively inhibits the glycosaminoglycan side chains incorporation of chondroitin sulfate proteoglycans (Garud et al. 2008). Therefore, in order to further clarify the role of CS-GAGs during tooth development, we cultured tooth germs with or without Xyl-MU. Our immunohistochemistry result showed a reduced immunostaining of 3B3, 2B6 and CS-56, which recognized CS-GAG chains in the Xyl-MU treated group. This result indicated that Xyl-MU could inhibit the incorporation of CS-GAGs to CSPGs in the cultured tooth germs.
The morphological changes discovered in the present study showed that the tooth germs growth and cusp formation were significantly inhibited compared with the control group when treated with Xyl-MU. It is known that tooth germs develop from the dental lamina stage through the bud, cap, bell stage to the formation of hard tissue, and finally form the crown and roots (Thesleff and Nieminen 1996). Tooth germs of E16.5 are in the early bell stage, which is a critical period for tooth development to change the shape of the tooth, since the inner enamel epithelium is growing and folding towards underlying mesenchymal growth and deep mesenchymal cells are gathering in the future cusp region, then mesenchymal cells start to polarize to form odontoblasts (Linde and Goldberg 1993; Morita et al. 2014). According to our experimental findings, the control group basically consistent with the histological changes shown in those previous studies, however, the Xyl-MU treated group appeared inhibition effect on cusp formation and odontoblast differentiation. Similar changes were observed in Mark’s research as well (Mark et al. 1990). To provide more microscopic evidence for Mark’s and our finding, we performed transmission electron microscopy method, the results showed a more clear and direct image of differentiation-inhibited and depolarized odontoblasts in the Xyl-MU treated group, as well as the collagens synthesized in disorder and the thinner predentin. These results indicated that the inhibition of synthesis of CS-GAGs would lead to the inhibition of odontoblast differentiation, thus affecting the formation of the predentin and dentin matrix.
When odontoblasts are fully differentiated, there are usually some specific molecular markers, such as type I collagen, DMP1, and DSPP (D’souza et al. 1997; Embery et al. 2001; Lee et al. 2016). The result of quantitative RT-PCR of those markers, as well as in situ hybridization, showed an expression decrease in the Xyl-MU treated tooth germ, which is in consistent with the findings of the immunohistochemical and TEM results. The decrease was caused by the inhibition of odontoblast differentiation. Functional differentiated mature odontoblasts would become less when they were inhibited, then type I collagen, DMP1 and DSPP which were secreted by odontoblasts would decrease as well. Since the mineralization of dentin occurs within the type I collagen network (Yamauchi et al. 1996), and it also needs those noncollagenous protein like DMP1 and DSPP, so it resulted in the reduction of predentin synthesis consequently.
Taken together, our present study hinted that CS-GAGs would affect odontoblast differentiation during tooth development. But the specific mechanism is not clear yet, so here we put forward some conjectures. It is reported that there were three main ways to regulate epithelial–mesenchymal interaction in morphogenesis cell differentiation of tooth germ: (1) the direct contact between the cells; (2) the interaction of extracellular matrix molecules and their receptors; (3) the interaction between signal molecules like growth factors (Thesleff et al. 1996; Kazuto et al. 1999; Du et al. 2016). Moreover, studies have shown that CS-GAGs of CSPGs could not only offer support for the cell growth, but more importantly provided space for cytokines storage and transportation (Wight 2002; Xie et al. 2015; Shi et al. 2016). Thus, we speculate that it left insufficient intercellular space for odontoblasts to proliferate and polarize when the CS-GAG chains were inhibited, then the odontoblasts differentiation were affected. The unique structure of CS-GAGs extending from the core protein could create a loose and hydrated matrix environment according to Landolt’s research (Landolt et al. 1995; Sotoodehnejadnematalahi and Burke 2013), so it may offer help to storage growth factors and cytokines in such microenvironment. The enamel knot, a signaling or organizing center of the tooth, has been reported to produce a range of molecular signals such as fibroblast growth factors (FGF), bone morphogenetic proteins (BMP) and Wnt signals. These molecular signals can direct the growth of the surrounding epithelium and mesenchyme thus provide positional information for tooth morphogenesis and regulates the growth of tooth cusps (Jernvall et al. 1994; Vaahtokari et al. 1996). Therefore, when the CS-GAG chains were inhibited, the growth factors and cytokines within the enamel knot might run off and brought impact to the epithelium and mesenchymal cells growth, resulting in defective odontoblast differentiation and cusp formation. However, the possible effect of Xyl-Mu on enamel knot formation needs our further investigation.
In sum, we conclude that the inhibition of CS-GAGs incorporation of CSPGs by β-xyloside can affect the differentiation of odontoblasts, lead to the decreased secretion of type I collagen, DMP1 and DSPP, finally altered tooth morphogenesis and dentinogenesis in cultured embryonic mouse molars. It highlights the significance of CS-GAGs of CSPGs during the development of the tooth germs, which needs our sustained attention and further study about their secrets.
References
Carulli D, Laabs T, Geller HM et al (2005) Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol 15(1):116–120
D’souza RN, Cavender A, Sunavala G et al (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res 12(12):2040–2049
Dondi P, Muir H (1976) Collagen synthesis and deposition in cartilage during disrupted proteoglycan production. Biochem J 160(1):117–120
Du J, Wang Q, Yang P et al (2016) FHL2 mediates tooth development and human dental pulp cell differentiation into odontoblasts, partially by interacting with Runx2. J Mol Histol 47(2):195–202
Embery G, Hall R, Waddington R et al (2001) Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med 12(4):331–349
Fisher LW, Torchia DA, Fohr B et al (2001) Flexible structures of sibling proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun 280(2):460–465
Garud D, Tran V, Victor X et al (2008) Inhibition of heparan sulfate and chondroitin sulfate proteoglycan biosynthesis. J Biol Chem 283(43):28881–28887
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326(5957):1216–1219
Jernvall J, Kettunen P, Karavanova I et al (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 38(3):463
Jiang BZ, Yokohama-tamaki T, Wang ZL et al (2010) Expression, localisation and synthesis of versican by the enamel organ of developing mouse molar tooth germ: an in vivo and in vitro study. Arch Oral Biol 55(12):995–1006
Kazuto HD, Kemmotsu S, Takeuchi Y et al (1999) The primary calcification in bones follows removal of decorin and fusion of collagen fibrils. J Bone Miner Res 14(2):273–280
Kresse H, Schönherr E (2001) Proteoglycans of the extracellular matrix and growth control. J Cell Physiol 189(3):266–274
Landolt RM, Vaughan L, Winterhalter KH et al (1995) versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development 121(8):2303–2312
Lee HK, Park JW, Seo YM et al (2016) Odontoblastic inductive potential of epithelial cells derived from human deciduous dental pulp. J Mol Histol 47(3):345–351
Linde A, Goldberg M (1993) Dentinogenesis. Crit Rev Oral Biol Med 4(5):679–728
Maciejewska I, Spodnik JH, Domaradzkapytel B et al (2006) Fluoride alters type I collagen expression in the early stages of odontogenesis. Folia Morphol 65(4):359–366
Mark MP, Karcher-Djuricic V, Baker JR et al (1990) Effects of beta-d-xyloside on morphogenesis and cytodifferentiation in cultured embryonic mouse molars. Cell Differ Dev 32(1):1–16
Morita W, Yano W, Nagaoka T et al (2014) Patterns of morphological variation in enamel-dentin junction and outer enamel surface of human molars. J Anat 224(6):669–680
Ponedel’kina IY, Khaibrakhmanova EA, Odinokov VN (2012) Isolation of chondroitin-6-sulfate from a mixture with dermatan sulfate selectively oxidized by NaOCl–NaBr–2,2,6,6-tetramethylpiperidine-1-oxyl. Chem Nat Compd 48(1):112–113
Rozario T, Desimone DW (2010) The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 341(1):126–140
Schwartz NB (1977) Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem 252(18):6316–6321
Septier D, Hall RC, Embery G et al (2001) Immunoelectron microscopic visualization of pro- and secreted forms of decorin and biglycan in the predentin and during dentin formation in the rat incisor. Calcif Tissue Int 69(1):38–45
Shi L, Li L, Wang D et al (2016) Spatiotemporal expression of caveolin-1 and EMMPRIN during mouse tooth development. J Mol Histol 47(3):337–344
Sotoodehnejadnematalahi F, Burke B (2013) Structure, function and regulation of versican: the most abundant type of proteoglycan in the extracellular matrix. Acta Med Iran 51(11):740–750
Takagaki K, Iwafune M, Kakizaki I et al (2002) Cleavage of the xylosyl serine linkage between a core peptide and a glycosaminoglycan chain by cellulases. J Biol Chem 277(21):18397–18403
Tenório DM, Santos MF, Zorn TM (2003) Distribution of biglycan and decorin in rat dental tissue. Braz J Med Biol Res 36(8):1061–1065
Thesleff I, Nieminen P (1996) Tooth morphogenesis and cell differentiation. Curr Opin Cell Biol 8(6):844–850
Thesleff I, Partanen AM, Vainio S (1991) Epithelial-mesenchymal interactions in tooth morphogenesis: the roles of extracellular matrix, growth factors, and cell surface receptors. J Craniofac Genet Dev Biol 11(4):229–237
Thesleff I, Vaahtokari A, Vainio S et al (1996) Molecular mechanisms of cell and tissue interactions during early tooth development. Anat Rec 245(2):151–161
Vaahtokari A, Aberg T, Jernvall J et al (1996) The enamel knot as a signaling center in the developing mouse tooth. Mech Dev 54(1):39–43
Veis A (1993) Mineral-matrix Interactions in bone and dentin. J Bone Miner Res 8(S2):S493–S497
Wight TN (2002) Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 14(5):617–623
Wu N, Iwamoto T, Yu S et al (2010) Pdgfs regulate tooth germ proliferation and ameloblast differentiation. Arch Oral Biol 55(6):426–434
Xie M, Xing G, Hou L et al (2015) Functional role of EMMPRIN in the formation and mineralisation of dental matrix in mouse molars. J Mol Histol 46(1):21–32
Yamauchi M, Chandler GS, Tanzawa H et al (1996) Cross-linking and the molecular packing of corneal collagen. Biochem Biophys Res Commun 219(2):311–315
Yang G, Jiang B, Cai W et al (2016) Hyaluronan and hyaluronan synthases expression and localization in embryonic mouse molars. J Mol Histol 47(4):413–420
Zhang Z, Guo Q, Tian H et al (2014) Effects of WNT10A on proliferation and differentiation of human dental pulp cells. J Endod 40(10):1593–1599
Zhang H, Jani P, Liang T et al (2017) Inactivation of bone morphogenetic protein 1 (Bmp1) and tolloid-like 1 (Tll1) in cells expressing type I collagen leads to dental and periodontal defects in mice. J Mol Histol 48(2):83–98
Acknowledgements
Financial support for this study was provided by Shanghai Science and Technology Commission Program (Nos. 124119A7400 and 15411965800).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors approve the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Liu, L., Chen, W., Li, L. et al. Inhibition of chondroitin sulfate glycosaminoglycans incorporation affected odontoblast differentiation in cultured embryonic mouse molars. J Mol Hist 48, 337–345 (2017). https://doi.org/10.1007/s10735-017-9732-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-017-9732-5