Abstract
Hydroxychloroquine (HCQ) is supposed to have favorable effects in diabetes mellitus (DM). However no previous experimental studies had investigated its effect on the structure of the endocrine pancreas, islets of Langerhans (IOL), in DM. In addition, the mechanism by which HCQ acts in DM is not well understood. In this study, we hypothesized that the possible favorable effects of HCQ in DM at the structural as well as at metabolic levels could be accomplished, in part, by its anti-inflammatory action. A total of 45 rats were divided equally into; control, DM and HCQ + DM groups (received citrate buffer, 27.5 mg/kg single ip STZ and STZ + HCQ 200 mg/kg/w respectively). After 4 weeks, samples from pancreas were histologically studied for the resulting changes. The HCQ + DM group showed preservation of IOL structure, a significant increase (p < 0.05) in the β-cell area, %, mass, IOL proliferation and neogenesis as well as correction of the significantly increased (p < 0.05) α-cell area, %, disturbed glucose homeostasis and lipid profile compared with the DM group. The significantly elevated inflammatory cytokines in the latter were lowered in the HCQ + DM group. Therefore, HCQ showed definite favorable effects on the histological as well as the metabolic profiles in DM which may be partly attributed to its anti-inflammatory action. This notable improvement of DM by HCQ deserves further studies to distinctly approve HCQ as a promising oral hypoglycemic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high prevalence of diabetes mellitus (DM) is considered a global health problem (Guzman-Flores and Lopez-Briones 2012; Richardson et al. 2013). In particular, type 2 diabetes mellitus (T2DM) that frequently accompanied with obesity (Odegaard and Chawla 2012), results from pancreatic β-cell failure to supply sufficient insulin along with decreased sensitivity to insulin (Nugent et al. 2008; Zou et al. 2014).
The relationship between changes in β-cell mass, function, and insulin action leading to T2DM remains unclear (Matveyenko and Butler 2006). β-cell apoptosis and α-cell proliferation are key players in the dysfunctional remodeling of the islets of Langerhans (IOL) and consequent hyperglycemia in T2DM (Guardado-Mendoza et al. 2009).
Additionally, previous studies have proposed that chronic low grade inflammation plays a crucial role in the pathogenesis of T2DM and in insulin resistance (IR), which is considered as the early stage of T2DM (Fresno et al. 2011; Guzman-Flores and Lopez-Briones 2012; Garcia et al. 2015). While proinflammatory cytokines have been studied in T2DM (Herder et al. 2013), further investigation is demanded to establish their exact role (Barbarroja et al. 2012).
Hydroxychloroquine (HCQ), an antimalarial drug with anti-inflammatory properties, is widely used in rheumatic diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (Mercer et al. 2012). HCQ has been reported to have therapeutic effects in a wide range of other non-rheumatic conditions such as DM, dyslipidemias, coagulopathies, infectious diseases and malignancies (Olsen et al. 2013; Costedoat-Chalumeau et al. 2014). It has no serious adverse effects (Wasko et al. 2015) apart from retinal toxicity which is very rare to occur (Costedoat-Chalumeau et al. 2014).
Treatment with HCQ reduces the risk to develop DM in patients with rheumatic diseases (Mercer et al. 2012; Wasko et al. 2015). Although HCQ has a positive effect on insulin sensitivity and β-cell function, the mechanism by which it acts remains unexplained (Wasko et al. 2015). Its considerable metabolic effects, particularly in disorders of glucose homeostasis, deserve further investigation (Pareek et al. 2009; Wasko et al. 2015).
The aim of the current study was to investigate the structural changes occurring in the endocrine pancreas, IOL, in T2DM treated with HCQ and whether these changes are accompanied with alteration in the levels of inflammatory cytokines.
Materials and methods
Experimental animals
Adult Sprague–Dawley rats (245 ± 35 g) were obtained from Mansoura faculty of Pharmacy animal house, where the experiment was performed. Animals (n = 45) were divided equally into 3 groups (n = 15 each) and were maintained on 12/12 h light/dark cycle at 24 ± 2 °C. The experimental procedures were approved by the Institutional Laboratory Animal Care and Use Committee of Mansoura Faculty of Medicine and were performed in accordance with their guidelines.
Experimental design
The animals were divided equally into three groups. The control group (received ip citrate buffer alone), diabetic (DM) and HCQ + DM groups. The latter received HCQ at a dose of 200 mg/kg/week orally (hydroquine 200 mg tablet, Minapharm, Egypt) during the whole period of the experiment (4 weeks).
Induction of T2DM in the DM and HCQ + DM groups was done by a single i.p. injection of streptozotocin (STZ) (Sigma, St. Louis, MO; 27.5 mg/kg ip in 0.1 mol/L citrate buffer, pH 4.5) as previously described (Ti et al. 2011). 1 week after STZ administration, rats with FBG ≥ 160 mg/dl in 2 consecutive analyses were considered diabetic animals and were included in the study. Rats of the second and third groups were fed a high-fat diet (34.5 % fat, 17.5 % protein, 48 % carbohydrate) during the whole period of the experiment. On the other hand, the control ones received standard laboratory animal’s chow. In addition, all rats received water ad labitum during the period of the experiment. At the end of the experiment, resection of the pancreas was carried out under strict sterile conditions.
Histological procedure
The resected pancreas was immediately fixated in 10 % formol solution, from which paraffin blocks were prepared. The latter were cut at 4–5 μm thickness for the routine hematoxylin and eosin (H&E) as well as for immunohistochemical staining.
All histological evaluations were performed in a blindfold manner by a light microscope mounted to digital camera connected to a computer.
Immunohistochemical study
Paraffin sections from specimens of the pancreas were immunohistochemically stained by guinea pig anti-insulin (polyclonal, 1/100, A0564, Dako, Glostrup, Denamark), rabbit anti-glucagon antibody (polyclonal, 1/50, ab8055, Abcam, Cambridge, MA) and mouse anti-Ki-67 (monoclonal, 1/20, Clone MIB-5, Dako, Glostrup, Denmark) antibodies according to instructions provided by the manufacturer.
Isolation of the IOL
Isolation of the IOL from pancreata (3 animals in each group) was performed as described by Carter et al. (2009) and Pae et al. (2013). Briefly, the pancreas was perfused directly by a collagenase, as tissue dissociating enzyme, through a cannula inserted in the common bile duct. The removed pancreas was placed into a tube (for digestion at 37 °C for 8 min) and washed by G-solution (1 % BSA containing Hank’s balanced salt solution, HBSS, Cat# 14065056, ThermoFisher Scientific Inc.). Afterwards, the tissue was filtered through a Netwell Polyester Mesh (Cat# 3480, Corning Life Sciences). Then, centrifugation was performed and the pellet was re-suspended with Histopaque 1100 solution (Cat# 10771, 11191, Sigma–Aldrich) for gradient separation. The supernatant was transferred into a new tube and washed with G-solution. The pellet was re-suspended in RPMI 1640 media (Cat# 11875093, ThermoFisher Scientific Inc.), supplemented with 10 % FBS and 1 % Penicillin–Streptomycin mixture and cultured at 37 °C and 5 % CO2 incubator for 4 h. After centrifuged for 5 min at 3000 rpm, supernatant was taken from IOL of each group for cytokines, capspase-3 and Bcl-2 assay.
Islets morphometry
β-cell and α-cell morphometric assessment
IOL from six sections/animal were photographed using a digital camera coupled to a microscope (CX 31, Olympus, Tokyo, Japan). The islet, β-cell and α-cell areas were analyzed using a professional software (Image J. version 1.43, NIH, Bethesda, Maryland). β-cell area % in the islet was measured by dividing the area of all insulin+ cells by the islet area and by multiplying by 100. Similarly, α-cell area % in the islet was measured by dividing the area of all glucagon+ cells by the islet area and by multiplying by 100.
While the islet mass (in mg) was assessed by multiplying the pancreas weight by the % of the total islet area/pancreatic section, the β-cell mass (in mg) was evaluated by multiplying the islet mass by the % of β-cells/islet.
Assessment of β-cell proliferation and neogenesis
The rate of β-cell proliferation was assessed by counting the number of Ki67-positive cell nuclei (Ki67+) in the core IOL (X100) and dividing it by the total number of IOL nuclei. In addition, the frequency of islet cells, immunostained by anti-insulin, that sprout from pancreatic ducts was used as an index of β-cell neogenesis according to the previously reported (Montanya and Tellez 2009).
Biochemical assays
Oral glucose tolerance test (OGTT) and area under the curve (AUC)
Baseline fasting venous blood samples were collected from each rat to perform the OGTT. Glucose was administrated (2.5 g/kg, orally) then blood samples were collected at 30, 60, 90, and 120 min. In addition, area under the curve (AUC) was determined according to the previously described (Psyrogiannis et al. 2003).
Assessment of glucose homeostasis and lipid profile parameters
At the end of the experiment, the fasted animals were anaesthetized using diethyl ether and their sera were separated from the blood collected by cardiac puncture for biochemical evaluations.
While fasting blood glucose (FBG) was measured by Randox kits (Antrim, UK), insulin level was evaluated by ELISA kit purchased from Abnova (Jhongli, Taiwan). Additionally, homeostatic model assessment for β-cell function (HOMA-%β) was calculated according to the previously reported (Akarte et al. 2012).
For lipid profile evaluation, serum levels of triglycerides (TG) and total cholesterol (TC) were determined using Boehringer Mannheim kits (Mannheim, Germany) and that of free fatty acids (FFA) by kit purchased from Abcam (Cambridge, UK). In addition, the level of HDL-C was assessed according to previously reported (Lopes-Virella et al. 1977) using the Boehringer Mannheim kit (Mannheim, Germany) and that of LDL-C was calculated according to the previously described (Friedewald et al. 1972).
Assessment of IOL inflammatory cytokines
Pancreatic IOL supernatant was prepared from the freshly isolated IOL for detection of their levels of interleukin (IL)-1β, IL-6, IL-10, tumor necrosis factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1) by ELISA kits according to the manufacture instructions (eBioscience; Vienna, Austria).
Assessment of serum monocyte chemoattractant protein-1 (MCP-1)
Separated sera of the blood samples of each animal were used for determination of MCP-1 by ELISA kit purchased from RayBiotech Inc. (Norcross, USA).
Assessment of pancreatic IOL caspase-3 and Bcl-2
The pancreatic IOL caspase-3 was assayed by a caspase-3 colorimetric assay kit (BF3100, R&D Systems, Minneapolis, MN, USA) according to guidelines of the manufacturer’s. The pancreatic IOL supernatant (50 μl) was added in a 96-well flat-bottom microplate. Caspase cleaves caspase-3 colorimetric substrate (DEVD-pNA) and releases the chromophore pNA. The latter was evaluated spectrophotometrically by a microplate reader (at a wave length of 405 nm). The results were expressed as fold increase in caspase-3 activity according to the corresponding alterations in the optical density (OD).
Additionally, the level of Bcl-2 in the pancreatic IOL supernatant was evaluated by an ELISA kit according to the instructions provided by the manufacturer (R&D Systems Inc., Minneapolis, MN, USA). The levels were expressed as ng/mg of tissue protein.
Statistical analysis
Values were presented as mean ± SEM. One-way ANOVA test was employed for comparisons between groups followed by Tukey test by using SPSS 16 software (Chicago, IL, USA). A value of p < 0.05 was considered as statistically significant.
Results
Histological results
The control group showed the typical view of the islets of Langerhans (IOL) which were formed of anastomosing cords of epithelial cells surrounded by the exocrine pancreatic acini (Fig. 1a). Disrupted appearance of the IOL was obviously demarcated in the DM group in the form of cellular as well as nuclear degeneration. The cytoplasm of many cells was heterogeneous and vacuolated along with appearance of hyperchromatic enlarged nuclei which occasionally disappeared in some cells. In addition, the matrix in between cells was widened by hyaline deposition and was infiltrated by mononuclear cell infiltrate mostly at the periphery of the islets (Fig. 1b). However in the group treated with HCQ (Fig. 1c), preservation of the IOL structure was observed to a wide degree. While almost all the cellular component of the IOL was intact, the matrix in between showed a minimal hyaline deposition and nearly absent inflammatory cells compared with the DM group.
Representative photomicrographs of the pancreas in various groups. Obvious degenerative changes in the islets of Langerhans (IOL) are distinct in the DM group (b) compared with the normal appearing ones in the control group (a). The cellular component of the IOL in DM shows abnormal cytoplasmic vacuoles and nuclear abnormalities in the form of karyomegaly, dense nuclei or complete disappearance of the nuclei. The intercellular matrix is condensed by hyaline deposits (arrow heads) and is infiltrated by mononuclear inflammatory cells (arrows) particularly at the periphery of the islets. On the other hand in the group administrated HCQ (c), preservation of the IOL structure is seen together with normal appearance of both the cellular and matrix components of the islets which are nearly similar to those of the control group. (H&E a–c ×400, Scale bar = 20 μm)
Immunohistochemical results
Most of the cells of IOL of the control group (Fig. 2a) showed an intense widely distributed insulin immunoexpression all over the islets. On the other hand, the IOL of the DM group (Fig. 2b) exhibited fewer less intense insulin expressing cells. Minimal affection of the IOL was denoted in the HCQ + DM group (Fig. 2c) with preservation of most of their insulin expressing cells which appeared numerous and had intense reaction compared with the DM one.
Representative photomicrographs of the pancreas in various groups. The insulin expressing cells are the predominant ones seen in the islets of the control group (a) showing an intense positive stain of wide distribution all over the islets. However in the DM group (b), they appear obviously fewer in number and less intensely stained than the control ones. On administrating HCQ + DM group (Fig. c), more numerous insulin expressing cells are observed compared with the DM group. (Anti-insulin immunostaining, a–c ×400, Scale bar = 20 μm)
On the other hand, the glucagon secreting cells were located mainly in the periphery of the IOL of the control group (Fig. 3a) and were obviously fewer than those expressing insulin. However in the IOL of the DM group (Fig. 3b) the distribution and the intensity of the reaction of the glucagon expressing cells were obviously increased. Nonetheless, the typical appearance of the few peripheral glucagon expressing cells were restored in the HCQ + DM group (Fig. 3c).
Representative photomicrographs of the pancreas in various groups. The IOL show that glucagon expressing cells are mostly in the periphery of the islets and are less predominant in the control (a) and HCQ + DM (c) groups unlike those in islets of the DM group (b), which are noticeably more numerous and more intensely stained. (Anti-glucagon immunostaining, a–c ×400, Scale bar = 20 μm)
In addition, the DM group showed a relatively decreased Ki67 expression compared with the control and HCQ + DM groups (Fig. 4c). Interestingly the IOL of the latter, containing increased Ki+ cells, were seen sprouting from the nearby pancreatic ducts.
Representative photomicrographs of the pancreas in all groups. Relatively decreased Ki67+ cells are observed in the IOL of the DM group (b) compared with the control (a) and HCQ + DM (c). In the latter, a pancreatic duct (arrow) is seen close to the nearby sprouting IOL. (Anti-Ki67 immunostaining, a–c ×400, Scale bar = 20 μm)
IOL morphometric results
The favorable effect of HCQ was noticeably demarcated on almost all the morphometric parameters of the islets. The total β-cell area, % and mass as well as the total α-cell area and % were reversed in the HCQ + DM group to approximate the control levels without detectable change in the total islet area or mass (Fig. 5a, b; Table 1). In addition, the significantly decreased % Ki67+ cells/total islet cells and islets neogenesis frequency were corrected in the HCQ + DM group (Fig. 5c, d).
A graph of the measurements of pancreatic morphometric parameters including: % of β-cell/islet, % of α-cell/islet (a), islet mass, β-cell mass mg/islet (b), % Ki67+ cells/total islet cells (c) and islets neogenesis frequency (d) in various groups. p < 0.05 is significant. aSignificant versus the control, bsignificant versus the HCQ + DM, csignificant versus the DM. Values are represented as mean ± SEM in each group (n = 12 rats)
Biochemical assay results
Although they did not reach the control levels, a noticeable improvement in the parameters of glucose homeostasis and lipid profile was detected in the HCQ + DM group compared with DM one. The OGTT curve became more flattened in the HCQ + DM compared with the diabetic OGTT curve (Fig. 6a). Similarly, the levels of FBG and AUC decreased significantly in the HCQ + DM group compared with the DM ones. On the other hand, the insulin levels and % of β-cell function, which decreased in the DM group, were raised in the HCQ + DM group to approximate those of the control (Fig. 6b, c).
A graph of the measurements of various serum biochemical markers including: OGTT (a), AUC (b), FBG (mg/dl), insulin (µU/ml), % β-cell function (c) as well as lipid profile parameters (d): TG (mg/dl), TC (mg/dl), FFA (µM), LDL-C (mg/dl) and HDL-C (mg/dl) in various groups. p < 0.05 is significant. aSignificant versus the control, bsignificant versus the HCQ + DM, csignificant versus the DM. Values are represented as mean ± SEM in each group (n = 15 rats)
In addition, the favorable effect of HCQ on the lipid profile was observed by lowering of the significantly elevated serum levels of TG, TC, FFA, LDL-C in the DM group, unlike the level of HDL-C which increased by HCQ but did not reach control one (Fig. 6d).
Inflammatory cytokines results
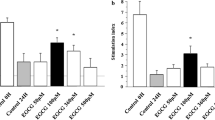
Significant increase (p < 0.05) in the level of IOL cytokines; IL-1β, IL-6, TNF-α and TGF-β1, as well as the serum level of MCP-1 were detected in the DM group compared with the control and HCQ + DM groups. On the other hand, the nearly detectable IL-10 remained unchanged in all groups (Fig. 7).
A graph of the measurements of pancreatic IOL IL-1β, IL-6, IL-10, TNF-α and TGF-β1 (pg/g tissue) (a) as well as the serum level of MCP-1 (pg/ml) (b) assayed in each group. p < 0.05 is significant. aSignificant versus the control, bsignificant versus the HCQ + DM, csignificant versus the DM. Values are represented as mean ± SEM in each group (n = 15 rats in MCP-1 assessment, n = 3 in the other cytokines assessment)
Caspase-3 and Bcl-2 assay results
The DM group showed a significant increase (p < 0.05) in the IOL cleaved capsase-3 accompanied with a significant decrease (p < 0.05) in the Bcl-2 level compared with the other groups (Fig. 8).
A representative graph of the ELISA assay of the cleaved caspase-3 (a) and Bcl-2 (b) (ng/mg protein) in the pancreatic IOL of various groups. p < 0.05 is significant. aSignificant versus the control, bsignificant versus the HCQ + DM, csignificant versus the DM. Values are represented as mean ± SEM in each group (n = 3 rats)
Discussion
In this study, there were degenerative changes in the IOL accompanied with a significant decrease in the total β-cell area, % and a significant increase in the total α-cell area and % in the DM group compared with the other groups. While β-cell mass deficit can reach up to 70 % in T2DM (Matveyenko and Butler 2006; Tomita 2010), α-cells become the predominant cells in diabetic IOL (Tomita 2012).
Early changes in T2DM include IOL hypertrophy/hyperplasia followed by degenerative changes, their atrophy and infiltration by inflammatory cells particularly macrophages (Jones et al. 2010; Katsuda et al. 2014). This supports our finding of the matrix infiltration by mononuclear cells in the DM group.
In addition, Tomita (2010) has showed that IOL in T2DM contain amyloid deposits in the stroma displacing the residual islet cells. This may explain the increased hyaline deposits in the matrix of the IOL observed in the DM group in our study. Amyloid deposit is a characteristic feature seen in more than 70 % of T2DM (Hayden 2002) and its main component is the islet amyloid polypeptide (Tomita 2012).
Similar to our findings, Tomita 2010 has demonstrated that IOL in T2DM have a higher caspase-3 expression accompanied by increased amyloid deposits which initiate β-cell apoptosis or even necrosis to form interstitial deposits that signify end-stage IOL in T2DM (Matveyenko and Butler 2006; Tomita 2012). The latter is characterized not only by IOL degeneration but also by impaired insulin secretion and action resulting in hyperglycemia (Matveyenko and Butler 2006; Nugent et al. 2008). This was manifested in our study by the significantly impaired glucose homeostasis that accompanied the histological findings.
Butler et al. (2003) have demonstrated that β-cell mass responds to IR by increasing IOL neogenesis and replication as well as by decreasing their apoptosis. Failed compensatory expansion of β-cell mass in DM might be attributed to the increase in β-cell apoptosis (Butler et al. 2003). This supports our finding of the upregulated capsase-3 expression accompanied with a significant decrease in the Bcl-2 levels in DM group which can overwhelm the futile compensatory mechanisms. The latter were clearly denoted in the significantly decreased β-cell mass, area, %, Ki67+ cells %, and islets neogenesis in the DM group compared with the other ones.
In the current study, there was an obvious improvement in the histological structure of IOL and OGTT curve in the HCQ + DM group accompanied with a significant decrease in the FBG and AUC compared with the DM group. In addition, the decreased insulin levels and % of β-cell function in the DM group were adjusted in the HCQ + DM group to approximate those of the control state.
Wasko et al. (2015) have proposed that HCQ has a favorable effect on insulin sensitivity and β-cell function. This effect is demonstrated on FBG, glucose tolerance and glycated hemoglobin (HbA1c) (Gerstein et al. 2002; Wasko et al. 2015). The positive glycemic outcome of HCQ has been also displayed in SLE and RA cases (Penn et al. 2010; Rekedal et al. 2010).
Interestingly, HCQ improves glycemic control in DM without rheumatic disease (Rekedal et al. 2010) and in sulfonylurea-refractory T2DM cases (Gerstein et al. 2002). Unexpectedly, combining insulin therapy with HCQ in decompensated, refractory T2DM reduces the daily insulin dose and provides a better management of the disease (Quatraro et al. 1990).
Pareek et al. (2009) have shown that HCQ synergism by its combination with atorvastatin affords a better glucose homeostasis in a dose–response fashion. In addition, Wasko et al. (2007) have demonstrated that the incidence of DM is reduced by increasing the duration of HCQ use.
In our study, the positive effect of HCQ on the lipid profile was observed by lowering of the significantly elevated levels of TG, TC, FFA and LDL-C in the DM group. Although some studies have revealed small improvement in the lipid profile by HCQ use (Solomon et al. 2014), other ones have established the favorable effect of HCQ on lipid profile which accompanies that on glucose homeostasis (Araiza-Casillas et al. 2013) and can add a benefit of cardiovascular risk reduction (Penn et al. 2010).
Our data indicated that HCQ lowered the pancreatic levels of IL-1β, IL-6, TNF-α and TGF-β1, as well as the serum level of MCP-1 which were significantly elevated in the DM group. The main cytokines involved in the pathogenesis of T2DM are IL-1β, TNF- α, IL-6, and others such as MCP-1(Spranger et al. 2003; Alexandraki et al. 2006). TNF-α and IL-6 are demonstrated to alter insulin sensitivity by affecting crucial steps in its signaling pathway (Bastard et al. 2006).
Although some studies have proposed that the circulating levels of IL-1β, IL-6, and TNF-α may not be affected in DM (Wasko et al. 2015), others have displayed the important role of IL-1β and IL-6 in IR (Wieser et al. 2013) and in T2DM (Spranger et al. 2003; Wang et al. 2013). The expression of these inflammatory cytokines increases in obese diabetics and is accompanied by systemic inflammation, IR (Wieser et al. 2013; Garcia et al. 2015) and progression of the diabetic nephropathy (Navarro-Gonzalez and Mora-Fernandez 2008).
Of activated innate immune cells, macrophages release these cytokines and create a pro-inflammatory environment that blocks insulin action, contributing to the development of IR, β-cell damage and eventually T2DM (Galic et al. 2010; Harford et al. 2011; Odegaard and Chawla 2012). Besides, other immune cells such as T and B cells can be involved in the resulting low grade inflammation (Guzman-Flores and Lopez-Briones 2012).
Additionally, the exact role of IL-10, an anti-inflammatory cytokine produced from macrophages (Harford et al. 2011), is not clearly distinct in T2DM (Herder et al. 2013). In our study, we did not detect significant changes in IL-10 level in various groups. On the other hand, the significant increase in TGF-β1 in the DM group may indicate a compensatory counter-regulation against the proinflammatory environment (Herder et al. 2013).
Conclusion
It could be concluded that hydroxychloroquine has a favorable effect on the structure of endocrine pancreas as well as on the metabolic profile in a type 2 diabetic model. This outcome may be accomplished by its anti-inflammatory action along with suppression of β-cells apoptosis. However, further studies are required to establish hydroxychloroquine as an efficient hypoglycemic agent.
References
Akarte AS, Srinivasan BP, Gandhi S (2012) A novel long acting DPP-IV inhibitor PKF-275-055 stimulates beta-cell proliferation resulting in improved glucose homeostasis in diabetic rats. Biochem Pharmacol 83:241–252
Alexandraki K, Piperi C, Kalofoutis C et al (2006) Inflammatory process in type 2 diabetes: the role of cytokines. Ann N Y Acad Sci 1084:89–117
Araiza-Casillas R, Diaz-Molina R, Gonzalez-Ortiz M et al (2013) Effects of hydroxychloroquine on insulin sensitivity and lipid profile in patients with rheumatoid arthritis. Rev Med Chile 141:1019–1025
Barbarroja N, Lopez-Pedrera C, Garrido-Sanchez L et al (2012) Progression from high insulin resistance to type 2 diabetes does not entail additional visceral adipose tissue inflammation. PLoS one 7:e48155
Bastard JP, Maachi M, Lagathu C et al (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12
Butler AE, Janson J, Soeller WC et al (2003) Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52:2304–2314
Carter JD, Dula SB, Corbin KL et al (2009) A practical guide to rodent islet isolation and assessment. Biol Proced Online 11:3–31
Costedoat-Chalumeau N, Dunogue B, Morel N et al (2014) Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med (Paris, France:1983) 43:e167–e180
Fresno M, Alvarez R, Cuesta N (2011) Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem 117:151–164
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–139
Garcia FA, Reboucas JF, Balbino TQ et al (2015) Pentoxifylline reduces the inflammatory process in diabetic rats: relationship with decreases of pro-inflammatory cytokines and inducible nitric oxide synthase. J Inflamm (London, England) 12:33
Gerstein HC, Thorpe KE, Taylor DW et al (2002) The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas—a randomized trial. Diabetes Res Clin Pract 55:209–219
Guardado-Mendoza R, Davalli AM, Chavez AO et al (2009) Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci USA 106:13992–13997
Guzman-Flores JM, Lopez-Briones S (2012) Cells of innate and adaptive immunity in type 2 diabetes and obesity. Gac Med Mex 148:381–389
Harford KA, Reynolds CM, McGillicuddy FC et al (2011) Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc 70:408–417
Hayden MR (2002) Pancreatic islet amyloid in type 2 diabetes mellitus: a clinical and historical review. Mo Med 99:495–498
Herder C, Carstensen M, Ouwens DM (2013) Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes Metab 15(Suppl 3):39–50
Jones HB, Nugent D, Jenkins R (2010) Variation in characteristics of islets of Langerhans in insulin-resistant, diabetic and non-diabetic-rat strains. Int J Exp Pathol 91:288–301
Katsuda Y, Ohta T, Miyajima K et al (2014) Diabetic complications in obese type 2 diabetic rat models. Exp Anim Jpn Assoc Lab Anim Sci 63:121–132
Lopes-Virella MF, Stone PG, Colwell JA (1977) Serum high density lipoprotein in diabetic patients. Diabetologia 13:285–291
Matveyenko AV, Butler PC (2006) Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55:2106–2114
Mercer E, Rekedal L, Garg R et al (2012) Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthritis Res Ther 14:R135
Montanya E, Tellez N (2009) Pancreatic remodeling: beta-cell apoptosis, proliferation and neogenesis, and the measurement of beta-cell mass and of individual beta-cell size. Methods Mol Biol (Clifton, N.J.) 560:137–158
Navarro-Gonzalez JF, Mora-Fernandez C (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol JASN 19:433–442
Nugent DA, Smith DM, Jones HB (2008) A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol Pathol 36:529–551
Odegaard JI, Chawla A (2012) Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med 2:a007724
Olsen NJ, Schleich MA, Karp DR (2013) Multifaceted effects of hydroxychloroquine in human disease. Semin Arthritis Rheum 43:264–272
Pae EK, Ahuja B, Kim M et al (2013) Impaired glucose homeostasis after a transient intermittent hypoxic exposure in neonatal rats. Biochem Biophys Res Commun 441:637–642
Pareek A, Yeole PG, Tenpe CR et al (2009) Effect of atorvastatin and hydroxychloroquine combination on blood glucose in alloxan-induced diabetic rats. Indian J Pharmacol 41:125–128
Penn SK, Kao AH, Schott LL et al (2010) Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol 37:1136–1142
Psyrogiannis A, Kyriazopoulou V, Symeonidis A et al (2003) Relative iron “overload” in offspring of patients with type 2 diabetes mellitus: a new component in the conundrum of insulin resistance syndrome? Hormones (Athens, Greece) 2:161–168
Quatraro A, Consoli G, Magno M et al (1990) Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. A new job for an old drug? Ann Intern Med 112:678–681
Rekedal LR, Massarotti E, Garg R et al (2010) Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum 62:3569–3573
Richardson VR, Smith KA, Carter AM (2013) Adipose tissue inflammation: feeding the development of type 2 diabetes mellitus. Immunobiology 218:1497–1504
Solomon DH, Garg R, Lu B et al (2014) Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res 66:1246–1251
Spranger J, Kroke A, Mohlig M et al (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52:812–817
Ti Y, Xie GL, Wang ZH et al (2011) TRB3 gene silencing alleviates diabetic cardiomyopathy in a type 2 diabetic rat model. Diabetes 60:2963–2974
Tomita T (2010) Immunocytochemical localisation of caspase-3 in pancreatic islets from type 2 diabetic subjects. Pathology 42:432–437
Tomita T (2012) Islet amyloid polypeptide in pancreatic islets from type 2 diabetic subjects. Islets 4:223–232
Wang X, Bao W, Liu J et al (2013) Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36:166–175
Wasko MC, Hubert HB, Lingala VB et al (2007) Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 298:187–193
Wasko MC, McClure CK, Kelsey SF et al (2015) Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: a randomised trial. Diabetologia 58:2336–2343
Wieser V, Moschen AR, Tilg H (2013) Inflammation, cytokines and insulin resistance: a clinical perspective. Archivum Immunologiae et therapiae Experimentalis 61:119–125
Zou CY, Gong Y, Liang J (2014) Metabolic signaling of insulin secretion by pancreatic beta-cell and its derangement in type 2 diabetes. Eur Rev Med Pharmacol Sci 18:2215–2227
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdel-Hamid, A.A.M., El-Firgany, A.ED.L. Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokines. J Mol Hist 47, 183–193 (2016). https://doi.org/10.1007/s10735-016-9664-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-016-9664-5