Abstract
Breast cancer is the second leading cause of cancer-related death in women. Previously, evidence suggested that ubiquitin-specific protease 14 (USP14) was associated with various signal transduction pathways and tumourigenesis. In this study, we demonstrate that USP14 is a novel therapeutic target in breast cancer. A Western blot analysis of USP14 was performed using seven breast cancer tissues and paired adjacent normal tissues and showed that the expression of USP14 was increased in the breast cancer tissues. Immunohistochemistry was conducted on formalin-fixed paraffin-embedded sections of breast cancer samples from 100 cases. Using Pearson’s χ2 test, it was demonstrated that USP14 expression was associated with the histological grade, lymph node status and Ki-67 expression in the tumour. The Kaplan–Meier analysis revealed that increased USP14 expression in patients with breast cancer was associated with a poorer prognosis. In in vitro experiments, the highly migratory MDA-MB-231 cells that were treated with USP14-shRNA (shUSP14) exhibited decreased motility using Transwell migration assays. Next, we employed a starvation and re-feeding assay, and the CCK-8 assay demonstrated that USP14 regulated breast cancer cell proliferation. Furthermore, we used flow cytometry to analyse cellular apoptosis following USP14 knockdown. Taken together, our results suggested that USP14 was involved in the progression of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the leading type of cancer diagnosed in women worldwide. In 2012, it resulted in 1.68 million cases and 522,000 deaths. Like other cancers, complex biological processes are involved in the occurrence and progression of breast cancer (Liu et al. 2014). It is important to unravel the molecular and cellular mechanisms of breast cancer to develop targeted cancer therapy (Dou et al. 2015).
The ubiquitin–proteasome system (UPS) is the critical intracellular protein degradation system that removes damaged proteins and, when altered, may cause diseases such as spinocerebellar ataxia, oncogenesis and infertility (Ciechanover and Schwartz 2004). The degraded protein is marked by tagging with ubiquitin, a highly conserved 76-amino acid protein, which is involved in processes as varied as signal transduction, endocytosis, and DNA repair (Hallengren et al. 2013). Proteins are ubiquitinated by three enzymes in sequential steps: ubiquitin activating enzyme (E1), ubiquitin conjugase (E2), and ubiquitin ligase (E3) (Jin et al. 2009). Recent studies suggested that ubiquitination is balanced by deubiquitination, which removes and remodels the covalently attached ubiquitin through deubiquitinating enzymes (DUBs). Ubiquitin conjugases activate ATP hydrolysis, as they contain loosely folded domains and their ubiquitin chains bind to the 26S-associated DUBs. The human genome encodes more than 100 putative DUBs, of which the prominent families are the USP (ubiquitin-specific processing protease) and UCH (ubiquitin C-terminal hydrolase) families (Phillips et al. 2013). DUBs play a central role in regulating cellular processes, such as cell growth, proliferation, apoptosis, DNA repair, kinase activation, and transcription, which cause diseases such as cancer and multiple myeloma (MM), as well as the activation of the inflammasome (Adhikari et al. 2007; Lopez-Castejon et al. 2013; Tian et al. 2014; Zhang et al. 2006). Emerging evidence shows that DUBs should be novel targets for cancer therapy (Brnjic et al. 2014; D’Arcy et al. 2011; D’Arcy and Linder 2012; Kapuria et al. 2010).
Ubiquitin-specific protease 14 (USP14) encodes the Ubiquitin carboxyl-terminal hydrolase 14 enzyme, which belongs to the ubiquitin-specific processing (UBP) family of deubiquitinating enzymes (DUBs) with His and Cys domains (Chuensumran et al. 2011). Ubiquitin-specific protease 14 (USP14) mediates protein degradation via its deubiquitination activity and also controls proteasome gate opening (Mialki et al. 2013). Some researchers suggested that USP14 regulated various signal transduction pathways, including the NF-κB pathway and the Wnt signalling pathway (Jung et al. 2013; Mialki et al. 2013). Additionally, the NF-κB signalling pathway has been implicated in the migration and invasion of breast cancer and glioma cells (Shi et al. 2015a; Tao et al. 2012). Growing evidence suggested that USP14 expression was associated with tumourigenesis in epithelial ovarian cancer (Wang et al. 2015), colorectal cancer (Shinji et al. 2006), intrahepatic cholangiocarcinoma (Chuensumran et al. 2011), Waldenström macroglobulinaemia (WM) tumours (Chitta et al. 2015) and lung carcinoma (Wu et al. 2013). However, there is currently no report demonstrating the function and mechanism of USP14 in breast cancer.
In this report, we demonstrated that USP14 was expressed at high levels in certain breast cancer tissues and several cell lines. Furthermore, we investigated the relationship between USP14 expression and the clinicopathological characteristics and survival of breast cancer patients. Moreover, we indicate that USP14 is a novel regulator of breast cancer cell proliferation, migration and apoptosis.
Materials and methods
Tissue samples
The human breast specimens and clinicopathological data were collected from 100 patients who underwent surgery between 2003 and 2006 at the Department of Pathology, Affiliate Hospital of Nantong University. The specimens were formalin-fixed and paraffin-embedded for the histopathologic diagnosis and immunohistochemical study. The TNM systems of tumour staging and histological grading were performed according to the guidelines of the World Health Organization (Hartmann et al. 1981). The follow-up information was obtained from telephone conversations and interviews. The fresh samples were frozen in liquid nitrogen immediately after surgery and maintained at −80 °C until use in the western blot analysis. All human tissues were collected using protocols approved by the Ethics Committee of Affiliate Hospital of Nantong University. An informed consent agreement was signed by all patients.
Cell cultures
The MDA-MB-231, SKBR-3 and MCF-7 human breast cancer cell lines, which were gifts from the Department of Oncology, Affiliated Cancer Hospital of Fudan University, were used in this study. All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10 % heat-inactivated foetal bovine serum (FBS), 2 mM l-glutamine, and 100 U/ml of a penicillin–streptomycin mixture (Gibco BRL) at 37 °C in a 5 % CO2 incubator.
Antibodies
The antibodies used for immunohistochemistry in this study included: anti-USP14 (Santa Cruz Biotechnology, USA) and anti-Ki-67 (Santa Cruz Biotechnology, USA). The antibodies used for the Western blots included: anti-USP14 (Santa Cruz Biotechnology, USA), anti-cyclin E (Santa Cruz Biotechnology, USA), anti-PCNA (Santa Cruz Biotechnology, USA), anti-cyclin A (Santa Cruz Biotechnology, USA), anti-E-cadherin (Santa Cruz Biotechnology, USA), anti-vimentin (Santa Cruz Biotechnology, USA), anti-Bcl-2 (Santa Cruz Biotechnology, USA), anti-procaspase-3 (Santa Cruz Biotechnology, USA), anti-cleaved caspase-3 (Santa Cruz Biotechnology, USA) and anti-GAPDH (Santa Cruz Biotechnology, USA).
Western blot analysis
Before immunoblotting, the cells were washed three times with ice-cold PBS and resuspended in 2× lysis buffer (50 mM Tris–HCl, 120 mM NaCl, 0.5 % Nonidet P-40, 100 mM NaF, 200 μM Na3VO4, and a protease inhibitor mixture). The frozen tissues were homogenized in lysis buffer (1 % NP-40, 50 mmol/l Tris, pH 7.5, 5 mmol/l EDTA, 1 % SDS, 1 % sodium deoxycholate, 1 % Triton X-100, 1 mmol/l PMSF, 10 mg/ml aprotinin, and 1 mg/ml leupeptin). Then, they were denatured at 100 °C for 15 min. The lysates were clarified by centrifugation (15 min,12,000 rpm, 4 °C). The protein concentrations were determined using a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). The supernatant was diluted in 2× SDS loading buffer containing DDT and boiled at 100 °C for 15 min. All of the protein samples were stored at −20 °C. Prior to SDS-polyacrylamide gel electrophoresis (SDS-PAGE), the samples were denatured at 100 °C for 3 min. Then, the proteins were separated by SDS-PAGE and then transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5 % dried skim milk in TBST (20 mM Tris, 150 mM NaCl, 0.05 % Tween-20) for 2 h at room temperature and then incubated with a primary antibody overnight at 4 °C for 6 to 8 h. After three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:1000; Pierce) for 2 h at room temperature, and the bands were then detected with the ECL (enhanced chemiluminescence) detection systems (Pierce, Rockford, IL, USA).
Immunohistochemistry (IHC)
The surgically excised specimens (4 μm thick) were prepared on glass slides, which were fixed with 10 % formalin and embedded in paraffin. The sections were deparaffinized in xylene and rehydrated in a graded ethanol series and then heated to 121 °C in an autoclave for 3 min to retrieve the antigen using citrate buffer (pH 6.0). After rinsing in PBS (pH 7.2), the sections were then incubated with a polyclonal mouse anti-USP14 antibody and a monoclonal mouse anti-Ki-67 antibody overnight at 4 °C. All slides were processed using the peroxidase-antiperoxidase method (Dako, Hamburg, Germany). After washing the sections with PBS, the peroxidase reaction was visualized by incubating the sections with DAB (0.1 % phosphate buffer solution, 0.02 % diaminobenzidine tetrahydrochloride, and 3 % H2O2). After rinsing in water, the sections were counterstained with haematoxylin, dehydrated with a graded alcohol series and cover slipped. All of the immunostained sections were evaluated by observers who were blinded to the clinical and pathological characteristics of the patients.
Immunohistochemical evaluation
Five fields were selected in each slide, and at least 1000 cells were counted per field at high magnification. To evaluate the USP14 immunoreactivity, the staining intensity was classified as negative staining (0), weak staining (1), moderate staining (2), and strong staining (3). Using the USP14 expression ratio, the intensity of immunostaining in each tumour section was semiquantitatively assessed as negative (1), low (2), moderate (3), and high expression (4) using the following scale: <10 % of cells (1), 10–35 % (2), 35–50 % (3), and >50 % (4) of cells. Therefore, the samples were considered to express high levels of USP14 if the total score was ≥6 and low levels if the score was <6. The Ki-67 immunoreactivity was classified into a high expression group (>50 %) and a low expression group (≤50 %).
Transient transfection
The USP14-specific shRNAs were chemically synthesized by Genechem (Shanghai, China). The USP14-specific shRNA target sequences included: USP14-shRNA#0 (sh0)—5′-CAGAGTTGAAATAATGGAA-3′, USP14-shRNA#1 (sh1)—5′-AGCCAAATACAAGTGACAA-3′, USP14-shRNA#2 (sh2)—5′-TTGGTAACACTTGTTACAT3′, and USP14-shRNA#3 (sh3)—5′-CAAGATTCAGCAGTCAGAT-3′. The MDA-MB-231 cells were transfected with the USP14-shRNA or control-shRNA according to the manufacturer’s instructions. The transfected cells were used for the subsequent experiments 48 h after transfection.
Cell proliferation assay
Cell proliferation was measured using the cell counting kit-8 (CCK-8) assay according to the manufacturer’s instructions. The cells were serum starved for 24 h, transfected with the shRNA for 48 h, and then 5 × 104 cells were seeded into a 96-well cell culture cluster (Corning Inc., Corning, NY) in volumes of 100 μl and incubated for 24 h. The CCK-8 reagents (Dojindo, Kumamoto, Japan) were added to a subset of wells at different time points and then incubated for 1.5 h at 37 °C in the dark. The absorbance was quantified on a microplate reader (Bio-Rad). This experiment was repeated at least three times.
Transwell migration assays
The cells that had been transfected with the USP14 shRNAs were starved overnight in DMEM media with 0.1 % FBS and then trypsinized and resuspended in DMEM containing 0.1 % bovine serum albumin. The cells (1 × 105) were added to the top chambers of the Transwells (Corning, 8 mm pore size) in 24-well plates, and DMEM with 10 % FBS was added to the bottom chambers. After an overnight incubation, the cells that remained in the top chamber (nonmigrated) were removed, and the cells in the bottom chamber (migrated) were fixed and stained with crystal violet to visualize the nuclei. The number of migrating cells was counted in five fields at 200× magnification, and the means for each chamber were determined. All experiments were conducted in triplicate and repeated twice.
Flow cytometry analysis of cellular apoptosis
The MDA-MB-231 cells transfected with USP14-shRNA#2 and the control-shRNA were cultured for 48 h and harvested. Then, 60 μl of Muse™ Annexin V & Dead Cell Reagent (Part No. 4700-1485, 100 tests/bottle) were added to each tube and then 60 μl of suspended cells were added to each tube. After a 20 min incubation at room temperature in the dark, the apoptosis assay was performed using the Muse™ cell analyser (EMD Millipore Corporation) according to the manufacturer’s instructions.
Statistical analysis
USP14 expression and the clinicopathological features were analysed by the Chi square (χ2) test. Kaplan–Meier survival plots were generated to analyse the survival data, and a log-rank test was performed. Multivariate analysis was performed using Cox’s proportional hazards model. For all statistical analyses, the level of significance was set at P < 0.05. The SPSS17.0 and sigmaplot12 statistical programmes were used for all statistical analyses. All values were expressed as the mean ± SD. Each experiment consisted of at least three replicates per condition (Ji et al. 2013).
Results
The expression of USP14 in human breast cancer
To analyse the expression of USP14 in breast cancer, we first examined USP14 by Western blotting. The results showed that USP14 is overexpressed in human breast cancer, but expressed at low or undetectable levels in the paired adjacent normal tissues (Fig. 1a, b). Next, we selected three appropriate breast cancer cell lines, MCF-7, MDA-MB-231, and SKBR-3, for the Western blot analysis. Among these cell lines, the expression of USP14 was highest in the MDA-MB-231 cells compared to the other cells (Fig. 1c, d). Therefore, in the subsequent experiments, we chose to use the MDA-MB-231 cell line. Furthermore, MDA-MB-231 cells exhibit certain malignant cancer features such as high invasion, metastasis and a mesenchymal phenotype (Wang et al. 2012).
Ubiquitin-specific protease 14 (USP14) is expressed in breast cancer tissues. a The expression of USP14 in seven paired adjacent normal tissues (N1–N7) and breast carcinoma tissues (T1–T7) was assessed by Western blotting. b The expression levels of USP14 in the adjacent and cancer tissues were displayed with the aid of density photometry. c The expression of USP14 in three breast cancer cell lines was analysed by Western blotting. d The expression levels of the USP14 protein in the three breast cancer cell lines were displayed with the aid of density photometry. The same experiment was repeated at least three times
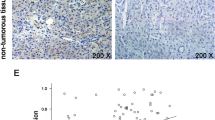
To reveal the role of USP14 in breast cancer, we examined the expression and distribution of USP14 and Ki-67 in 100 paraffin-embedded breast cancer specimens by immunohistochemistry. We found that USP14 was mainly located in the cytoplasm of the breast cancer cells, while Ki-67 was localized in the nucleus (Fig. 2). Interestingly, USP14 was highly expressed in the breast cancer tissue.
Immunohistochemical staining reveals USP14 and Ki-67 expression in paraffin-embedded breast cancer tissues. a–b The expression of USP14 and Ki67 was low in grade I tissues. c–d The expression of USP14 and Ki67 was moderate in grade II tissues. e–f The expression of USP14 and Ki67 was high in grade III tissues. The images in a–f were captured at ×400 magnification
Correlation between USP14 expression and the clinicopathological characteristics and survival of breast cancer patients
To gain a better understanding of the pathophysiological significance of USP14, we analysed the relationship between USP14 expression and the clinicopathological characteristics of the tumours, which are described in Tables 1 and 2. According to Table 1, which shows the statistical analysis of USP14 and Ki-67 expression, USP14 expression was significantly associated with the histological grade (P = 0.001), lymph node status (P = 0.015) and Ki-67 expression (P = 0.008), whereas there was no correlation observed with the patients’ age (P = 0.827), tumour size (P = 0.507), or histology (P = 0.161). Next, we implemented the survival curve analysis to correlate the survival status and clinicopathological parameters of 100 breast carcinoma specimens and demonstrated that the histological grade (P = 0.030), lymph node status (P = 0.040), Ki-67 expression (P = 0.040) and USP14 expression (P = 0.002) were substantially associated with the patients’ overall survival (Table 2). Furthermore, Cox’s proportional survival analysis suggested that the histological grade (P = 0.024), lymph node status (P = 0.020), USP14 expression (P = 0.016) and Ki-67 expression (P = 0.001) were independent prognostic indicators for the overall patient survival (Table 3). In addition, we a performed Kaplan–Meier analysis to assess the relationship between USP14 expression and the 100 patients’ survival status. The survival curves indicated that the median survival for patients with high USP14 expression levels was significantly shorter (Fig. 3).
USP14 expression in the different phases of the MDA-MB-231 cell cycle
We found that high levels of USP14 expression were associated with a poor prognosis in patients with breast cancer. Thus, we assumed that USP14 participated in the cell cycle progression of breast cancers. To verify our hypothesis, we employed a serum starvation and re-feeding process in the MDA-MB-231 cells. The flow cytometry analysis showed that and the cells were arrested in G1 phase after serum deprivation for 48 h (Fig. 4a). After serum re-feeding, the cells reentered S phase (Fig. 4a). We collected the proteins from the MDA-MB-231 cells at different time points for western blot analysis. We found that USP14 expression was substantially increased in the MDA-MB-231 cells as early as 4 h after serum re-feeding. The expression of the cell proliferation marker PCNA showed a similar trend (Fig. 4b, c). These results indicated that USP14 played a critical role in the regulation of cell proliferation and allowed G1/S progression.
The expression of USP14 and cell cycle-related molecules in proliferating breast cancer cells. a Flow cytometry quantitation of the cell cycle progression of the MDA-MB-231 cells. The cells were synchronized at G1 phase and progressed into S phase when serum was added after 0, 4, 8, 12 or 24 h. The results are the mean ± SD of three independent experiments. *, # P < 0.05 compared to the control cells that were serum-starved for 48 h (S48 h). b, c The cell lysates were prepared and analysed by Western blotting using antibodies against USP14, PCNA and GAPDH (loading control). The results are presented as the mean ± SD (n = 3, *, # P < 0.05 compared to the control cells that were serum-starved for 48 h: S48 h). S standard serum starvation, R standard serum release
USP14 knockdown reduced the proliferation of the MDA-MB-231 cells
To further determine the effect of USP14 on cell proliferation, we transfected the MDA-MB-231 cells with a control-shRNA, USP14-shRNA#0, USP14-shRNA#1, USP14-shRNA#2, or USP14-shRNA#3 to knock down the endogenous USP14. USP14 expression was examined by Western blotting (Fig. 5a). The results show that the USP14 shRNAs (USP14-shRNA#1, USP14-shRNA#2, and USP14-shRNA#3) could knock down the expression of USP14 compared to the negative control shRNA (control-shRNA and USP14-shRNA#0), and USP14-shRNA#2 could obviously decrease the expression of USP14. Then, we transfected the MDA-MB-231 cells with USP14-shRNA#2 or the control-shRNA and then detected the levels of PCNA, cyclin A and cyclin E by Western blotting (Fig. 5b). Moreover, we tested the cell proliferation using the CCK-8 assay (Fig. 5c). In addition, we collected the cells transfected with USP14-shRNA#2 or the control-shRNA and examined the proportions of cells in G0/G1 and S phases by flow cytometry (Fig. 5d). The results revealed that the number of cells in G0/G1 phase increased, while the number of cells in S phase decreased in the USP14-shRNA#2 group compared to the control group. Therefore, we assumed that USP14 played a positive role in regulating breast cancer cell proliferation.
USP14 knockdown inhibited MDA-MB-231 cell proliferation in vitro. a The expression of the USP14 protein in the MDA-MB-231 cells was detected by Western blotting 48 h after transfection. The transfected cells were subjected to a Western blot analysis with antibodies against USP14 and GAPDH (loading control). The bar chart below the blots indicates the ratio of USP14 to GAPDH, as measured by densitometry. The data are presented as the mean ± SD (*P < 0.05 compared to the control). b Western blot analysis of a series of cell cycle-related molecules, including PCNA, cyclin A and cyclin E, in MDA-MB-231 cells transfected with USP14-shRNA#2 and control-USP14. GAPDH was used as a loading control. c The CCK-8 assay was used to measure cell proliferation and showed that USP14 knockdown inhibited cell proliferation. The cell counting kit-8 reagents were added to the medium and incubated for an additional 2 h. The absorbance was measured at each of the indicated time points (0, 12, 24, 48 and 72 h). The data from each time point were derived from three independent experiments. The data are presented as the mean ± SD (*P < 0.05). d USP14 expression was knocked down in the MDA-MB-231 cells transfected with USP14-shRNA#2; the adherent cells were collected and examined by flow cytometry, which showed a delay in the G1-S transition and significant arrest at G1 phase. The data are presented as the mean ± SD. *, # P < 0.05 compared to the control. The results are a combination of the data from three independent experiments
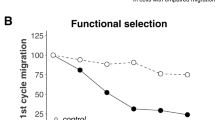
USP14 was associated with breast cancer cell migration
It has been noted that some deubiquitinating enzymes (DUBs), such as USP33 or UCH-L1, regulate cell migration (Frisan et al. 2012; Huang et al. 2015). We postulated that USP14 may be associated with breast cancer metastasis. USP14 knockdown inhibited cell migration to the bottom chambers of the Trans-well compared to the control groups (Fig. 6a, b). To confirm this result, we performed a Western blot analysis to measure the levels of the USP14 protein in the cells that were subjected to the same treatments. It has been reported that E-cadherin and vimentin are markers of epithelial cells and mesenchymal cells, respectively; therefore, we also analysed their levels in the control-shRNA and USP14-shRNA#2 groups. We observed that the expression of E-cadherin was increased, while the expression of vimentin was decreased (Fig. 6c). Based on these results, we speculated that knocking down the expression of USP14 would inhibit breast cancer cell migration by initiating the EMT process. Taken together, we can conclude that USP14 is involved in breast cancer cell migration.
Effect of USP14 knockdown on migration and apoptosis. a USP14 knockdown inhibited cell migration in the Transwell assays. The control-shRNA-transfected cells exhibited an increased ability to migrate through the membrane compared to the USP14-shRNA#2-transfected cells. b The number of cells that migrated through the member was counted in 10 fields using a 40× objective lens. The data are presented as the mean ± SD. *P < 0.05 compared to the control. C Western blot analysis of E-cadherin, vimentin and GAPDH (loading control) in the MDA-MB-231 cells transfected with control-USP14 or USP14-shRNA#2. The levels of the tumour migration markers were significantly decreased in the cells in which USP14 expression was knocked down. The results are presented as the mean ± SD of three independent experiments (*P < 0.05 compared to the control). d The MDA-MB-231 cells were transfected with control-shRNA or USP14-shRNA#2, respectively, and then assayed by the Muse™ Cell Analyser according to the manufacturer’s instructions. e Western blotting was used to analyse the levels of the anti-apoptosis marker Bcl-2, pro-apoptotic marker procaspase-3 and cleaved caspase-3 in control-shRNA- and USP14-shRNA#2-transfected MDA-MB-231 cells
USP14 knockdown induced breast cancer cell apoptosis
Previous evidence had proven that the loss of USP14 expression could induce epithelial ovarian cancer cells apoptosis (Wang et al. 2015). Therefore, we speculated that USP14 knockdown might induce breast cancer cell apoptosis. We performed flow cytometry to analyse apoptosis in the MDA-MB-231 cells that were transfected with the control-shRNA or USP14-shRNA#2. The results showed that the percentage of apoptotic cells was higher in the USP14-shRNA#2 group compared to the control-shRNA group (Fig. 6d). Next, we analysed some markers of cellular apoptosis by Western blotting, including procaspase-3, cleaved caspase-3 and Bcl-2, and noticed that the levels of the anti-apoptosis marker Bcl-2 and pro-apoptotic marker procaspase-3 were significantly decreased and the levels of the pro-apoptotic marker cleaved caspase-3 were increased in the cells transfected with USP14-shRNA#2 (Fig. 6e). All of these results indicated that USP14 knockdown could induce breast cancer cell apoptosis.
Discussion
Breast cancer is the second leading cause of cancer-related death in women. With the comprehensive utilization of B ultrasound examinations, breast molybdenum photography and puncture biopsies, it is possible for us to diagnose and treat breast cancer much earlier. The treatments for breast cancer include surgery, chemotherapy, radiation therapy, hormone therapy and targeted therapy, but the prognosis of breast cancer remains unsatisfactory (Adamczyk et al. 2014; Runowicz et al. 2015). It is essential to unravel the molecular and cellular mechanisms of breast cancer progression and metastasis to develop targeted therapy for breast cancer.
The ubiquitin–proteasome system (UPS) provides the cell with a pathway to clear damaged and misfolded cellular proteins (Anderson et al. 2005). Protein ubiquitination is critical in numerous cellular processes, including cell cycle control, DNA repair, signal transduction, and the regulation of protein transport. The ubiquitin-specific processing (UBP) family can prolong the life of the substrate protein through deubiquitination. Ubiquitin-specific protease 14 (USP14) is a member of the ubiquitin-specific processing (UBP) family of deubiquitinating enzymes (DUBs) with His and Cys domains (Chuensumran et al. 2011). Previous research suggested that several DUBs were related to cancer progression and could be novel targets for anti-cancer therapies (Daviet and Colland 2008). A clinical study demonstrated that low USP-11 expression was independently correlated with better breast cancer survival (Bayraktar et al. 2013). SiRNA interference studies on three DUBs in cancer cells suggested that RNAi of USP14 can inhibit cellular protein degradation (Koulich et al. 2008). Studies on the expression of USP14 also assumed that USP14 was associated with cancer progression. In colorectal carcinoma and epithelial ovarian cancer, the high levels of USP14 expression were correlated with patient survival or the tumour stage. Furthermore, high levels of USP14 expression were observed in patients with colorectal carcinoma with lymph node metastasis and liver metastasis, which indicated that USP14 may promote tumour metastasis (Shinji et al. 2006; Wang et al. 2015). A previous study revealed that USP14 modulates cancer cell motility by deubiquitinating the chemokine receptor CXCR4 (Mines et al. 2009). In our study, we collected breast cancer specimens from 100 cases and performed immunohistochemistry to analyse the expression of USP14 and Ki67 in the breast cancer tissue; we demonstrated that high levels of USP14 expression were related to the histological grade, lymph node status and shorter survival. The pathogenesis of cancer metastasis is a highly complicated multi-step process and involves various factors. Therefore, a better understanding of the molecular mechanism of cancer metastasis would provide novel therapeutic opportunities for cancers (Cui et al. 2014). Experimental and clinical evidence indicates that the aberrant activation of the epithelial-mesenchymal transition (EMT) contributes to various pathological conditions, including cancer progression and metastasis (Acloque et al. 2009; Thiery et al. 2009). During the EMT, epithelial cells lose their polarity and cell–cell adhesion and are morphologically and functionally converted into mesenchymal stem cells, which are characterized by migratory and invasive phenotypes (Hay 1995; Lyons et al. 2008; Thiery 2002). The EMT promotes tumour progression by enhancing cancer cell migration and metastasis in distant sites, which is underlined by the loss of epithelial phenotype markers such as tight junctions, desmosomes, and cytoskeletal elements (Foroni et al. 2012). At the molecular level, the EMT is associated with a decrease in the expression of cell junction proteins, such as E-cadherin and integrins, and an increase in the expression of the mesenchymal markers N-cadherin and vimentin; these changes lead to actin rearrangements, ultimately resulting in increased cell motility (Shi et al. 2015b; Voulgari and Pintzas 2009).Using the Trans-well assay, we confirmed that USP14 is also involved in breast cancer metastasis. Moreover, we performed shRNA to knock down the expression of USP14 in an in vitro experiment and demonstrated that the loss of USP14 decreased the proliferation of MDA-MB-231 cells.
Some previous studies have shown that oxidative stress was associated with tumour cell apoptosis mediated by the proteasome deubiquitinase b-AP15 (Brnjic et al. 2014; Wang et al. 2014). Moreover, b-AP15 is an inhibitor of USP14 and can promote the accumulation of polyubiquitin, induce tumour cell apoptosis, and inhibit tumour procession. Studies on b-AP15-treated HCT-116 colorectal carcinoma cells showed that the number of hypodiploid cells was increased, which was related to the expression of many apoptotic markers, including activated caspase-3, caspase-cleaved poly-ADP ribose polymerase (PARP) and cytokeratin-18 (CK18). Additional studies reported that treatment with b-AP15 increased the levels of cell cycle regulatory proteins, including inhibitors of the cyclin-dependent kinases CDKN1A and CDKNIB, the tumour suppressor TP53, and arrested the cell cycle in G2/M phase (D’Arcy et al. 2011). Another small-molecule DUB inhibitor, WP1130, could directly inhibit USP14, which downregulated anti-apoptotic proteins and upregulated pro-apoptotic proteins, such as MCL-1 and p53 (Kapuria et al. 2010). P53 is ceaselessly degraded by the ubiquitin–proteasome system, which keeps this protein in a microscale state in normal cells (Liu et al. 2012). In the present study, we utilized the starvation-release assay and then detected the cell cycle markers cyclin A and cyclin E (Zhu et al. 2014). We concluded that USP14 might induce breast cancer cell proliferation by promoting cell cycle progression. Furthermore, the flow cytometry analysis of breast cancer cell apoptosis suggested that USP14 knockdown induced tumour cell apoptosis. In a future supplementary study, we will explore whether USP14 inhibitors can induce breast cancer cell apoptosis and inhibit tumour progression.
In conclusion, we demonstrated that USP14 was highly expressed in breast cancer tissues and that knocking down the expression of USP14 could inhibit proliferation and migration and induce apoptosis of breast cancer cells in vitro. Our studies show that USP14 may be a potentially effective therapy against breast cancer.
References
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119(6):1438–1449. doi:10.1172/JCI38019
Adamczyk A, Niemiec JA, Ambicka A, Mucha-Malecka A, Mitus J, Rys J (2014) CD44/CD24 as potential prognostic markers in node-positive invasive ductal breast cancer patients treated with adjuvant chemotherapy. J Mol Histol 45(1):35–45. doi:10.1007/s10735-013-9523-6
Adhikari A, Xu M, Chen ZJ (2007) Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26(22):3214–3226. doi:10.1038/sj.onc.1210413
Anderson C, Crimmins S, Wilson JA, Korbel GA, Ploegh HL, Wilson SM (2005) Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. J Neurochem 95(3):724–731. doi:10.1111/j.1471-4159.2005.03409.x
Bayraktar S, Gutierrez Barrera AM, Liu D et al (2013) USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J 19(1):10–17. doi:10.1097/PPO.0b013e3182801b3a
Brnjic S, Mazurkiewicz M, Fryknas M et al (2014) Induction of tumor cell apoptosis by a proteasome deubiquitinase inhibitor is associated with oxidative stress. Antioxid Redox Signal 21(17):2271–2285. doi:10.1089/ars.2013.5322
Chitta K, Paulus A, Akhtar S et al (2015) Targeted inhibition of the deubiquitinating enzymes, USP14 and UCHL5, induces proteotoxic stress and apoptosis in Waldenstrom macroglobulinaemia tumour cells. Br J Haematol. doi:10.1111/bjh.13304
Chuensumran U, Saelee P, Punyarit P et al (2011) Ubiquitin-specific protease 14 expression associated with intrahepatic cholangiocarcinoma cell differentiation. Asian Pac J Cancer Prev: APJCP 12(3):775–779
Ciechanover A, Schwartz AL (2004) The ubiquitin system: pathogenesis of human diseases and drug targeting. Biochim Biophys Acta 1695(1–3):3–17. doi:10.1016/j.bbamcr.2004.09.018
Cui X, Liu Y, Wan C et al (2014) Decreased expression of SERPINB1 correlates with tumor invasion and poor prognosis in hepatocellular carcinoma. J Mol Histol 45(1):59–68. doi:10.1007/s10735-013-9529-0
D’Arcy P, Linder S (2012) Proteasome deubiquitinases as novel targets for cancer therapy. Int J Biochem Cell Biol 44(11):1729–1738. doi:10.1016/j.biocel.2012.07.011
D’Arcy P, Brnjic S, Olofsson MH et al (2011) Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 17(12):1636–1640. doi:10.1038/nm.2536
Daviet L, Colland F (2008) Targeting ubiquitin specific proteases for drug discovery. Biochimie 90(2):270–283. doi:10.1016/j.biochi.2007.09.013
Dou X, Wei J, Sun A et al (2015) PBK/TOPK mediates geranylgeranylation signaling for breast cancer cell proliferation. Cancer Cell Int 15(1):27. doi:10.1186/s12935-015-0178-0
Foroni C, Broggini M, Generali D, Damia G (2012) Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev 38(6):689–697. doi:10.1016/j.ctrv.2011.11.001
Frisan T, Coppotelli G, Dryselius R, Masucci MG (2012) Ubiquitin C-terminal hydrolase-L1 interacts with adhesion complexes and promotes cell migration, survival, and anchorage independent growth. FASEB J Off Publ Fed Am Soc Exp Biol 26(12):5060–5070. doi:10.1096/fj.12-211946
Hallengren J, Chen PC, Wilson SM (2013) Neuronal ubiquitin homeostasis. Cell Biochem Biophys 67(1):67–73. doi:10.1007/s12013-013-9634-4
Hartmann WH, Ozzello L, Sobin LH, Stalsberg H (1981) Histological typing of breast tumours. In: International classification of tumours. World Health Organization, Geneva, pp 15–25
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat 154(1):8–20
Huang Z, Wen P, Kong R et al (2015) USP33 mediates Slit–Robo signaling in inhibiting colorectal cancer cell migration. Int J Cancer J Int Du Cancer 136(8):1792–1802. doi:10.1002/ijc.29226
Ji L, Li H, Gao P et al (2013) Nrf2 pathway regulates multidrug-resistance-associated protein 1 in small cell lung cancer. PLoS One 8(5):e63404. doi:10.1371/journal.pone.0063404
Jin C, Yang YA, Anver MR, Morris N, Wang X, Zhang YE (2009) Smad ubiquitination regulatory factor 2 promotes metastasis of breast cancer cells by enhancing migration and invasiveness. Cancer Res 69(3):735–740. doi:10.1158/0008-5472.CAN-08-1463
Jung H, Kim BG, Han WH et al (2013) Deubiquitination of Dishevelled by Usp14 is required for Wnt signaling. Oncogenesis 2:e64. doi:10.1038/oncsis.2013.28
Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ (2010) Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res 70(22):9265–9276. doi:10.1158/0008-5472.CAN-10-1530
Koulich E, Li X, DeMartino GN (2008) Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell 19(3):1072–1082. doi:10.1091/mbc.E07-10-1040
Liu Y, Chen Y, Lu X et al (2012) SCYL1BP1 modulates neurite outgrowth and regeneration by regulating the Mdm2/p53 pathway. Mol Biol Cell 23(23):4506–4514. doi:10.1091/mbc.E12-05-0362
Liu X, Ni Q, Xu J et al (2014) Expression and prognostic role of SKIP in human breast carcinoma. J Mol Histol 45(2):169–180. doi:10.1007/s10735-013-9546-z
Lopez-Castejon G, Luheshi NM, Compan V et al (2013) Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J Biol Chem 288(4):2721–2733. doi:10.1074/jbc.M112.422238
Lyons JG, Lobo E, Martorana AM, Myerscough MR (2008) Clonal diversity in carcinomas: its implications for tumour progression and the contribution made to it by epithelial-mesenchymal transitions. Clin Exp metastasis 25(6):665–677. doi:10.1007/s10585-007-9134-2
Mialki RK, Zhao J, Wei J, Mallampalli DF, Zhao Y (2013) Overexpression of USP14 protease reduces I-kappaB protein levels and increases cytokine release in lung epithelial cells. J Biol Chem 288(22):15437–15441. doi:10.1074/jbc.C112.446682
Mines MA, Goodwin JS, Limbird LE, Cui FF, Fan GH (2009) Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation. J Biol Chem 284(9):5742–5752. doi:10.1074/jbc.M808507200
Phillips AH, Zhang Y, Cunningham CN et al (2013) Conformational dynamics control ubiquitin–deubiquitinase interactions and influence in vivo signaling. Proc Natl Academy Sci USA 110(28):11379–11384. doi:10.1073/pnas.1302407110
Runowicz CD, Leach CR, Henry NL et al (2015) American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol off J Am Soc Clin Oncol. doi:10.1200/JCO.2015.64.3809
Shi M, Cao M, Song J et al (2015a) PinX1 inhibits the invasion and metastasis of human breast cancer via suppressing NF-kappaB/MMP-9 signaling pathway. Mol Cancer 14(1):66. doi:10.1186/s12943-015-0332-2
Shi Y, Liu X, Sun Y et al (2015b) Decreased expression and prognostic role of EHD2 in human breast carcinoma: correlation with E-cadherin. J Mol Histol 46(2):221–231. doi:10.1007/s10735-015-9614-7
Shinji S, Naito Z, Ishiwata S et al (2006) Ubiquitin-specific protease 14 expression in colorectal cancer is associated with liver and lymph node metastases. Oncol Rep 15(3):539–543
Tao T, Cheng C, Ji Y et al (2012) Numbl inhibits glioma cell migration and invasion by suppressing TRAF5-mediated NF-kappaB activation. Mol Biol Cell 23(14):2635–2644. doi:10.1091/mbc.E11-09-0805
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454. doi:10.1038/nrc822
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. doi:10.1016/j.cell.2009.11.007
Tian Z, D’Arcy P, Wang X et al (2014) A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 123(5):706–716. doi:10.1182/blood-2013-05-500033
Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796(2):75–90. doi:10.1016/j.bbcan.2009.03.002
Wang Y, Yang S, Ni Q et al (2012) Overexpression of forkhead box J2 can decrease the migration of breast cancer cells. J Cell Biochem 113(8):2729–2737. doi:10.1002/jcb.24146
Wang X, Stafford W, Mazurkiewicz M et al (2014) The 19S Deubiquitinase inhibitor b-AP15 is enriched in cells and elicits rapid commitment to cell death. Mol Pharmacol 85(6):932–945. doi:10.1124/mol.113.091322
Wang Y, Wang J, Zhong J et al (2015) Ubiquitin-specific protease 14 (USP14) regulates cellular proliferation and apoptosis in epithelial ovarian cancer. Med Oncol 32(1):379. doi:10.1007/s12032-014-0379-8
Wu N, Liu C, Bai C, Han YP, Cho WC, Li Q (2013) Over-Expression of deubiquitinating enzyme USP14 in lung adenocarcinoma promotes proliferation through the accumulation of beta-catenin. Int J Mol Sci 14(6):10749–10760. doi:10.3390/ijms140610749
Zhang D, Zaugg K, Mak TW, Elledge SJ (2006) A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 126(3):529–542. doi:10.1016/j.cell.2006.06.039
Zhu T, Ji Z, Xu C et al (2014) Expression and prognostic role of SGTA in human breast carcinoma correlates with tumor cell proliferation. J Mol Histol 45(6):665–677. doi:10.1007/s10735-014-9586-z
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81302285) and the Natural Scientific Foundation of Nantong University Grant (No. 12Z009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Lianxin Zhu and Shuyun Yang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, L., Yang, S., He, S. et al. Downregulation of ubiquitin-specific protease 14 (USP14) inhibits breast cancer cell proliferation and metastasis, but promotes apoptosis. J Mol Hist 47, 69–80 (2016). https://doi.org/10.1007/s10735-015-9650-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9650-3