Abstract
The nutritional value of broccoli is largely attributed to its abundant secondary metabolites such as phenolic compounds and glucosinolates (GSLs). However, the dynamic relationship between these compounds, including potential synergistic or antagonistic interactions that influence plant physiology and metabolism, remains unclear. In this study, we aimed to elucidate the intricate interplay between phenolic compounds and GSLs in broccoli plants and their consequent effects on primary metabolism and regulatory mechanisms governing water and nutrient uptake. To investigate this, we externally supplied citric phenolic compounds to broccoli plants, and then measured the levels of GSLs and phenolic compounds, along with assessing physiological parameters such as biomass, gas exchange, and nutrient content. Additionally, the expression of genes related to GSLs and phenolics biosynthesis, as well as genes involved in water transport were measured. Our results revealed a complex interrelation between phenolic compounds and GSLs, with phenolic compounds significantly modulating the response of GSLs and influencing the expression of aquaporin genes. This modulation had notable effects on nutrient regulation mechanisms in broccoli plants. Overall, our findings shed light on the regulatory mechanisms underlying the interaction between phenolic compounds, GSLs and growth, providing insights into their roles in plant physiology and metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant metabolites, both primary and secondary, play crucial roles in the growth and survival of plant species, with complex and interconnected metabolic pathways being key. While primary metabolites directly contribute to vital cellular processes, secondary metabolites, such us phenols, terpenes, and nitrogen/sulfur-containing compounds like glucosinolates (GSLs) serve multifunctional roles (Aharoni and Galili 2011). On the one hand, they primarily respond to environmental conditions, but they also play crucial roles in regulating plant growth and development together primary metabolites (Chen et al. 2022; Tariq et al. 2023). Secondary metabolism is often linked to plant responses to abiotic stress conditions, including temperature fluctuations, salinity, water availability, or chemical exposure. Under these conditions, increases in these metabolites (especially phenolic compounds and GSLs) have been linked to primary responses such as water uptake associated with stress tolerance, thus maintaining growth, yield and reproductive capabilities (Mackenzie and Mullineaux 2022; Nicolas-Espinosa et al. 2023). This role of secondary metabolites has led to increased research into their potential as biostimulants (Kisiriko et al. 2021), which could be crucial in dealing with the issues caused by abiotic stresses, exacerbated by climate change. Furthermore, these biostimulants represent a sustainable and environmentally friendly agricultural practice, mainly if they are derived from natural products (Xu and Geelen 2018).
Specifically, phenolic compounds have been reported to be involved in defense mechanism, safeguarding plant constituents (Ray et al. 2024). They have been found in all plant organs as an adaptation to respond to external stressors like ultraviolet radiation, microorganism and insect infestations, and different climatic conditions (Shukla et al. 2023). Several techniques have been developed for the extraction of these metabolites (Gomez-Molina et al. 2024) and the utilization of these compounds for diverse applications including food science, pharmaceuticals, biomaterials, and environmental remediation (Okumura 2021). In plants, it has been reported that the potential biochemical mechanisms effect of the external addition of phenolic compounds in plants primarily involved an increase of the antioxidant system, gas exchange, and nutrient-water uptake (Pollastri and Tattini 2011), but also in maintaining fruit quality and preventing fruit diseases (Liu et al. 2010, 2023). In addition, previously we determined that the foliar application of citrus flavonoids in tomato plants mainly influenced the stimulation of photosynthesis, increasing CO2 fixation, H2O uptake due to increasing stomatal conductance and nutrient uptake leaded to higher growth that was also related to higher levels of the hormone zeatin (Martinez-Alonso et al. 2022, 2023).
However, the diverse molecules within the group of phenolic compounds can lead to variations in their physiological effects. Besides, further research efforts are required to elucidate the interaction between exogenously applied secondary metabolites and the pathways of secondary metabolism in plants. To address these issues, we investigated the application of citrus polyphenols to a plant species with a highly complex secondary metabolism, such as Brassica oleracea L. var. italica (broccoli). Broccoli has been extensively studied due to its numerous beneficial health properties, that have made it a crop of great agronomic interest due to its increasing consumption. It is characterised by a high complexity of secondary metabolites, highlighting GSLs and phenolic compounds, which contributes to its nutritional value and potential health benefits (Moreno et al. 2006). Nevertheless, the levels of these compounds are highly variable, depending on factors such as plant variety, growing conditions and environmental stress (López-Berenguer et al. 2009).
GSLs and phenolic compounds have been reported to play crucial roles in regulating physiological processes in plants, including growth, development, and responses to biotic and abiotic stresses, as they can modulate gene expression, enzyme activity, and signalling pathways involved in plant defense, stress tolerance, and adaptation to environmental changes (Wang et al. 2022). However, the interplay between these compounds and their synthesis pathways have poorly studied. GSLs and phenolic compounds can interact with each other and with other secondary metabolites in the plant, influencing their biosynthesis, accumulation, and biological activities (Nicolas-Espinosa et al. 2023). Therefore, as brassica plants have sophisticated regulatory mechanisms to balance the production of secondary metabolites as GSLs and phenolic compounds, feedback regulation mechanisms may occur in response to the tightly controlled aimed at optimizing growth.
We hypothesised that external application of phenolic compounds would modify the biosynthesis of secondary metabolites in broccoli and interact with primary metabolism, leading to alterations in the latter. To investigate this, in our study, we externally supplied phenolic compounds extracted from citrus fruits to determine their effects on GSLs and intrinsic phenolics in broccoli. Additionally, we evaluated their impact on physiological parameters such as biomass and gas exchange, as well as on mineral nutrients, primary metabolites, and gene expression related to GSLs, phenolics biosynthesis, nutrient and water transport.
Materials and methods
Experimental design and growth conditions
Broccoli plants were grown following the standard procedures in broccoli production fields. The experiments were conducted from January to March under Mediterranean climate in fields located in the southeast of Spain, specifically in Lorca (Region of Murcia). Two random blocks were selected that included 3 planting rows, and one block received the foliar treatment by spraying once when plant was 1 month old. The foliar treatment consisted of applying a phenolic-enriched extract of Citrus sp. (Citrus Phenolic Extract, CPE containing 12% of flavonoids) mainly naringenin at 20 mg mL − 1, neohesperidin at 18 mg mL− 1 and rutin at 12 mg mL − 1 at a concentration of 3 mL L− 1. The other block was maintained as control plants. The plants were harvested after 2 months from the foliar application.
Fresh and dry weight
Plants were harvested and separated into aerial part and roots. A total of eight plants of each condition (control and CPE) were weighted to obtain fresh weight (FW). Dry weight (DW) was obtained after drying four plants at 75 °C for 48 h.
Gas exchange parameters
Photosynthetic capacity was measured in fully-expanded leaves using a TPS-2 Portable Photosynthesis System (PP Systems, Inc., Amesbury, MA, USA). It was taken 3 different measurements on three different leaves for eight plant of each treatment (control and CPE).
Mineral content analysis
The concentrations of macronutrients (Ca, K, Mg, P and S), micronutrients (B, Fe, Mn, Mo, Si and Zn) and Na were measured in four dried plants, distinguishing between the aerial part and roots. All the samples were grounded into a fine powder. The analyses were conducted at the Ionomics Laboratory (CEBAS-CSIC, Murcia, Spain) as described Nicolas et al., (Nicolas-Espinosa et al. 2024). The nutrient concentrations were expressed as mg 100 g− 1 DW for macronutrients and mg g− 1 DW for micronutient.
The total carbon and nitrogen content were determined using the Elemental Analyzer model TRUSPEC CN628. Lyophilized samples of leaf and fruit from 3 selected plants of each cultivation were ground into a fine powder using a grinder (Taurus Aromatic, Lleida, Spain). For each ground sample, 0.1 g of the solid sample was placed in a tin capsule and then closed and introduced into the autosampler of the equipment. The C/N concentration was obtained directly in g 100 g− 1.
Metabolomic analysis/NMR analysis
50 mg of lyophilized powder of the aerial part of control and treated broccoli plants were mixed with 1,200 µL of a methanol: water mixture (MeOH: H2O; 1:1), and vigorously vortexed. The samples were subjected to three rounds of sonication and were then incubated for 30 min at 4 °C. Following this, the samples were centrifuged at 11,000 x g for 20 min at 4 °C. The supernatant was dried overnight using a Speed-Vacuum. The dried samples were reconstituted in 800 µL of a 100 mM potassium phosphate buffer, pH = 6.0 (diluted in 100% D2O) + 0.58 mM of TSP-d4 (internal standard). Then, the samples were filtered using 0.45 μm pore diameter Nylon filters and 600 µL were loaded into a 5 mm NMR tube for quantification via H-NMR. The analyses were conducted at the Metabolomics Laboratory (CEBAS-CSIC, Murcia, Spain).
Glucosinolates and phenolic compounds analysis
In order to purified GSLs and phenolic compounds, 100 mg of the lyophilized powder of the aerial part of control and treated broccoli plants were extracted with 1 mL of 70% methanol in water (CH3OH: H2O; 1:10). The extraction process was carried out in a water bath at 72 °C during 30 min, with vortexing every 5 min. Next, the samples were cooled in an ice bath for 15 min and subsequently centrifuged at 10,000 x g for 15 min. The resulting supernatant was collected and filtered through a 0.22 μm diameter Millex Syringe Filter, Durapore (PVDF) by Merck Millipore (Billerica, MA, USA), into vials for HPLC-MS analysis. The analysis was conducted using High-Performance Liquid Chromatography – Mass Spectrometry (HPLC-MS) at the Metabolomic and Proteomic Laboratory (ACTI - Murcia University, Spain) following the procedure described by (Albaladejo-Marico et al. 2024).
RNA extraction and cDNA synthesis
Aerial part and root samples from four plant of each treatment stored at -80 °C were ground into a fine dust using liquid nitrogen. Total RNA was extracted from 50 mg of each sample using the NZY Total RNA Isolation kit (Nzytech, Lisbon, Portugal) following the manufacturer’s instructions. The quantity and purity of RNA was determined using a Nanodrop 1000 Spectrophotometer (ThermoFisher Scientific, Waltham, MA). The RNA was stored at -80 °C until use. The RT Master Mix for qPCR II Kit (HY-K0510A-MedChemExpress, Sollentuna, Sweden) was used to synthesise cDNA from 2 µg of total RNA, according to the protocol provided by the manufacturer.
Quantitative real-time PCR (RT-qPCR) analyses
RT-qPCR was carried out in an Applied Biosystems 7500 Real-Time PCR system utilizing 2 µL of cDNA samples (1:10 diluted) within an 8 µL reaction mixture (600 nM gene-group primers (Table 1) and 5 µL of SYBR Green Master Mix 2X (Applied Biosystems). The amplification protocol comprised a two-step process: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 15 s of denaturation at 95 °C and 1 min of annealing and extension at 60 °C. Subsequently, a dissociation stage was performed. These conditions were used for both target and reference genes. Three technical replicates and three biological samples were tested for each treatment. Transcript levels were calculated using the 2–ΔΔCt method (Livak and Schmittgen 2001) for both target and reference genes.
Statistical analysis
Statistical analyses and data presentation were conducted using Origin(Pro) (Version 2021 software package by OriginLab Corporation, Northampton, MA, USA) and R Studio software (R Core Team 2018). These tests were preceded by a normality test and a Grubbs’ test to identify potential outliers. All the acquired data were subjected to a T-test. Significance levels are denoted as follows: * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and n.s. for no significant differences. All presented values represent means ± SE. R packages FactoMineR (Lê et al. 2008) and factoextra (Kassambara and Mundt 2020) were used to generate Principal Component Analysis (PCA).
Results
Response of biomass and gas exchange parameters to foliar application of CPE in broccoli plants
Broccoli plants that had been foliar treated with CPE significantly reduced their biomass. Both the aerial part and the root showed lower FW and DW (Fig. 1A-B). Untreated plants showed a total FW of 794.7 g and a DW of 85.0 g, while treated plants had a FW of 139.0 g and a DW of 13.2 g. Regarding the gas exchange parameters measured prior to plant harvesting, none of the parameters exhibited significant differences, with evaporation, stomatal conductance (Gs), net photosynthetic rate (An) and substomatal CO2 concentration (Ci) remaining at the same values as those of the untreated broccoli plants (Fig. 1C-F).
- Physiological parameters determined in broccoli plants treated with citric phenolic extract (CPE) and control: (A) Fresh weight (FW) (aerial part and root), (B) Dry weight (DW) (aerial part and root). Photosynthetic parameters: (C) Evaporation, (D) Stomatal conductance (Gs), (E) Net photosynthetic rate (An), and (F) Calculated sub stomatal CO2 concentration (Ci). Each bar represents mean ± SE (n = 8). Significant differences between plants treated with CPE and the control were measured according to T-Test (* means p < 0.05)
Modification of nutrient profile of broccoli plant in response to CPE treatment
The mineral content of control and CPE-treated broccoli plants was analysed to find differences in macronutrients and micronutrients due to externally applied flavonoids. Using multivariate analysis, specifically a PCA, it was determined that foliar treatment with flavonoids caused an alteration in the nutrient profile of the treated plants in both the aerial and root parts (Fig. 2A-B). As for the macronutrients, the groups of both the aerial part and the root part of the plants treated with CPE are in the upper part of the plot, which mainly indicates a positive correlation with the variable K, while the control groups are in the lower part, correlating positively with C (Fig. 2A). In the case of the micronutrients, the groups referring to the treated plants are in the lower part of the plot, being positively correlated with the variables Zn and Cu (Fig. 2B). In addition to the multivariate analysis, individual representations of some elements of interest have been made. The total nitrogen and carbon content were not significantly altered in either the aerial or root part (Fig. 2C-D). Ca concentration decreased significantly in the aerial part of the treated plants (Fig. 2E). As for K, P, and Zn concentrations, these increased significantly in both the aerial and root parts of plants treated with CPE (Fig. 2F-H).
- Analysis of mineral nutrients in the aerial part and root of broccoli plants treated with citric phenolic extract (CPE) and control. Principal component analysis (PCA) of (A) macronutrients and (B) micronutrients with arrows indicating loadings of each nutrient; 95% confidence ellipses were plotted for each treatment, each small circle represents the data from an individual sample, while the large circle represents the centroid of mean values. (C) Concentration of nitrogen, N (g/100 g), (D) carbon, C (g/100 g), (E) calcium, Ca (g/100 g), (F) potassium, K (g/100 g), (G) phosphorous, P (g/100 g), (H) zinc, Zn (mg/Kg). In bar plots each value represents mean ± SE (n = 4). Significant differences between plants treated with CPE and the control were measured according to T-Test (* means p < 0.05, ** means p < 0.01)
Response of primary and secondary metabolism to CPE treatment
The primary metabolism of the aerial part of both control and CPE-treated broccoli plants was determined using NMR (Table 2). Concerning organic acids, citrate and malate exhibited a significant increase in the treated plants. Conversely, certain amino acids, such as GABA, arginine, and valine, showed decreased levels. Glutamate levels increased in the treated plants, reaching a concentration of 2.05 mM, while the control plants exhibited a concentration of 1.29 mM. Additionally, the treatment resulted in a significant decrease in maltose levels.
Furthermore, secondary metabolism was analysed in both control and treated broccoli plants, focusing on the main phenolic acids and GSLs of broccoli (Fig. 3). All phenols measured (caffeic acid, sinapic acid and chlorogenic acid) exhibited significant decreases in broccoli plants treated with CPE (Fig. 3A). Regarding GSLs, the total concentration of these compounds measured by NMR decreased in the treated plants compared to the control (Table 2). However, when two specific GSLs (GRA and GBA) were analysed using HPLC, no significant decrease was observed (Fig. 3B).
- Secondary metabolite in the aerial part of broccoli plants treated with citric flavonoid extract (CPE) and control. Concentration (mM) of (A) caffeic, sinapic, and chlorogenic acid; (B) glucoraphanin (GRA) and glucobrassicin(GBA). Each bar represents mean ± SE (n = 6).Significant differences between plants treated with CPE and the control were measured according to T-Test (* means p < 0.05, ** means p < 0.01, *** means p < 0.001)
Global metabolism response
In order to obtain a comprehensive overview of the plant’s response to CPE treatment, two correlation matrices have been made for all the metabolites analysed (Fig. 4). Consequently, a modification in the metabolomic profile of organic acids, amino acids, sugars, and secondary metabolites in the plants treated with CPE was observed. Figure 4 displays that in the control plants, there are negative correlations between amino acids and phenolic compounds, as well as GSLs, whereas in the treated plants, there are positive correlations. Furthermore, if we examine inverse correlations between control and treated plants, we find one between chlorogenate and glucose, which is positive in control plants and negative in treated plants. Overall, it is evident that the two correlation matrices exhibit differences between the control plants and those treated with CPE.
Gene expression modification in response to CPE treatment
In order to further investigate the molecular mechanisms of the effect of CPE on broccoli plants, the expression of certain genes involved in the transport of potassium, water and GSLs, in the synthesis of phenolic compounds and transcription factors related to the synthesis of GSLs was measured both control and CPE-treated plants (Fig. 5). In the aerial part different changes were found, the expression of two transcription factors, one involved in the synthesis of aliphatic GSLs (MYB28) and the other in the synthesis of indole GSLs (MYB34), both of which appear up-regulated in the treated plants. In addition, two genes are down-regulated, a transcription factor (MYB122) involved in the synthesis of indole GSLs and BCAT3, a gene involved in side-chain elongation of aliphatic GSLs (Fig. 5A). The expression of different aquaporins was analysed, finding that PIP2;1 and PIP2;2–3 are up-regulated, while TIP2;1 has a lower expression in treated plants compared to control plants (Fig. 5B). Furthermore, the Phe-Tyr ammonia lyase (PT-AL) gene, involved in the shikimate pathway, which leads to the synthesis of most phenolic compounds, was found to be down-regulated in the aerial part of treated plants (Fig. 5C). Regarding root part several changes were found in the expression of analysed genes. Gene expression of HAK5 and AKT1, two genes related to K transport, was significantly increased in the root of the plants in response to foliar treatment with CPE (Fig. 5D). As for the aquaporins, PIP1;2, PIP2;2–3 and TIP2;1 increased their expression in treated plants compared to control plants (Fig. 5E).
- Gene expression analysis of different genes expressed as fold change, with respect to the control measured in both control and citric phenolic extract (CPE)-treated plants. A) transcription factors related to the synthesis of GSLs (MYB28, MYB29, MYB34, MYB122, BCAT3 in aerial part, B) aquaporins (PIP1;1, PIP1;2, PIP2;1, PIP2;2–3, and TIP2;1) in aerial part, C) genes involved in in the synthesis of phenolic compounds (shikimate kinase - Shik, citrate sintase – CitrS, and Phe-Tyr ammonia lyase - PT-AL) in aerial part, D) aquaporins (PIP1;1, PIP1;2, PIP2;1, PIP2;2–3, and TIP2;1) in roots, and E) genes involved in the transport of potassium (HAK5 and AKT1) in roots. Each bar represents mean ± SE (n = 3). Significant differences between plants treated with CPE and the control were measured according to T-Test (* means p < 0.05, ** means p < 0.01, *** means p < 0.001)
Discussion
An essential driving force behind research in the field of external plant polyphenolic compounds applications is their ubiquitous presence and distinctive properties, which connect them to one of the primary classes of secondary metabolites found in plants. Polyphenols has been reported to play a crucial role in ensuring plant growth, development, and reproduction, acting as signalling molecules involved in the phytohormones synthesis pathway (Cheynier et al. 2013). Recent studies have explored the impact of phenolic compounds on stimulating seed germination, promoting plant growth and biomass production by enhancing plant metabolism (Tanase et al. 2019). Understanding the influence of these compounds on plant development is crucial for assessing their effect in agriculture as natural extracted growth regulators. Therefore, interactions between externally applied natural compounds and the metabolism of plants need to be studied extensively, as they can lead to direct or indirect stimulatory or inhibitory effects mediated by various chemical compounds produced by the host plant. In our experiments, the drastic reduction in growth indicated that the secondary metabolism of the broccoli plant may have been significantly altered. Although not widely documented, the negative effect of plant extract on growth has been reported. For instance, extracts obtained from various parts of black mustard exhibited reductions of oat plant germination and growth (Turk and Tawaha 2003). However, although the specific effects varied depending on the type of solvent used, aqueous and methanolic extracts of lettuce leaves, as well as fractions isolated from these extracts, demonstrated negative impacts on the germination of various seed types (Chon et al. 2005). Similarly, studies with fractions derived from onion bulbs and leaves, containing phenolic compounds such as ferulic, p-coumaric, vanillic, and syringic acids, resulted in reduced seed germination and plant development in lettuce and wheat plants (Djurdjevic et al. 2004). Additionally, the overall aqueous extracts of wheat exhibited inhibitory effects on wheat and barley seed germination, the extent of which depended on the plant material (roots or leaves) used for the extractions. Therefore, further investigation should be conducted to elucidate the mechanisms underlying these alterations.
Previous studies have reported that citrus phenolic compounds stimulated plant growth by enhancing gas exchange in tomato plants, including photosynthesis and stomatal conductance, while also increasing stomatal density (Martinez-Alonso et al. 2022). This outcome correlated with the expression of aquaporins. However, the fact that gas exchange was not altered in our broccoli plants indicates that these parameters were not involved in the inhibitory effect observed in CPE treated plants.
According to the mineral nutrient analysis, a decrease in Ca was observed in CPE treated plants. Calcium plays an important role in regulation plant growth and signal transduction as well as the accumulation of secondary metabolites in plants (Kudla et al. 2010). Although the effect of external phenolic application on Ca has not been extensively studied, the effect of Ca treatment has been reported to increase the total phenolic acids and anthocyanins in carrot (Singh et al. 2012), and glucosinolate biosynthesis in broccoli by upregulating the expression of related genes (Liu et al. 2010). Although K, P, and Zn have been linked to phenolics and GSLs in broccoli, the observed increase in K, P, and Zn in CPE-treated plants suggests a possible association between lower growth and higher nutrient concentrations. This negative correlation is commonly observed under salinity conditions (Pascale et al. 2005). Also, the PCA analysis of the mineral nutrients revealed that, unlike the gas exchange parameters, there are indeed differences in the nutritional profile between the treated and control plants, which allows us to relate the decrease in plant size to a nutritional imbalance triggered by the CPE treatment.
The metabolomics analysis revealed an increase of several primary and secondary metabolites (Fig. 6). Furthermore, in the correlation plot we observe a marked shift in the overall metabolic profile of the treated plants, whose alterations, when specifically analysed, may lead to elucidate the cause of the reduced growth and development of the CPE-treated plants. According to the organic acids, an increase of malate and citrate was observed in plants treated with CPE. Malate is a crucial metabolite with specific role in redox exchange between cell organelles (Selinski and Scheibe 2019), and citrate is produced mainly in leaf mitochondria when the TCA cycle operates at night to obtain energy. In this way, it has been reported that DICARBOXYLATE CARRIER 2 (DIC2) enable mitochondrial malate-citrate exchange in Arabidopsis plants and depletion of this transporter reduced the growth of vegetative tissues (Lee et al. 2021). Therefore, the reduced interchange of malate and citrate between the mitochondrial and the other organelles or cellular compartments could be the responsible for the malate and citrate accumulation in our plants.
Gamma-aminobutyric acid (GABA) is a non-protein amino acid that have a role as signalling molecule in plants (Roberts 2007), acting in several physiological and molecular responses as mediation of plant growth and development that usually related mineral nutrients (Daş et al. 2016). The cytosolic enzyme glutamate decarboxylase (GAD; EC 4.1.l.15), catalyses the synthesis of GABA via glutamate. Therefore, in our results, in plants treated with CPE, the decrease of GABA was related to the decrease of growth, but also, it is in accordance with the increase of glutamate.
The decreases of arginine, leucine and valine could be related to alterations in metabolism related to growth and development (Sohail et al. 2021b). The impact of five amino acids -valine, glycine, cysteine, methionine, and phenylalanine- on the prevention of oxidation in potatoes revealed that valine showed greater inhibition (Ali et al. 2016). On the other hand, the effects of arginine, cysteine, and methionine on the delay of senescence in broccoli were studied (Sohail et al. 2021a). Senescence, characterized by the loss of green colour, ethylene production, and respiration rate, was found to be equally inhibited by the three amino acids as assessed. Also, reductions in the level of free amino acids were reported to be related to the decreased response to stress conditions in broccoli, being cultivar dependent (Gomes and Rosa 2000). In our experiments we were able to give the main role to arginine, leucine, and valine that, connected with the increases of citrate and malate, provide the indications that their synthesis was reduced through the enzymes pathway. Although the central role of this amino acid in broccoli development along with the connections to key metabolic pathways, need to be studied in depth, since the connection to secondary metabolism is clear in our results and in previous report (Trovato et al. 2021).
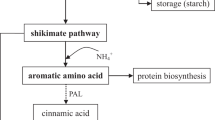
A decrease of phenolic compounds and GSLs has been observed in our broccoli plants. The fact that all the phenolic compounds such as caffeic, sinapic and chlorogenic acid were significantly decreased in these plants could bring the idea that the synthesis pathway was directly inhibited by the externally added phenolics as naringenin, neohesperidin and rutin. However, previous investigation with tomato plants after addition of the same formulation of phenolics reported an increase in the total phenolic content by 46% (Martinez-Alonso et al. 2022). The results were explained by the putative stimulation of the salicylic acid synthetic pathway inside the plant. It is well known that salicylic acid plays a key role in the regulation of plant growth and development (Sharma et al. 2023). But in broccoli plants, the fact that expression of phenylalanine ammonia lyase (PT-AL) was reduced could be the important differential key factor. Since, it is the first enzyme of the phenylpropanoid pathway that catalyses the biotransformation of L-phenylalanine into different phenolic compounds (Reyes Jara et al. 2022). Downstream, the enzyme chalcone isomerase catalyses the reaction that turns naringenin chalcone into naringenin has been reported in Brassica oleracea (Zhang et al. 2015). As naringenin was supplied in our experiments, the results indicate the importance of understanding the biochemical and molecular regulation pathway of the phenolic synthesis.
The fact that precursors of aliphatic (methionine) or indolic GSLs (tryptophan) did not change revealed that the connection should be in the intermediate synthesis pathway or in the degradation. Also, MYB122 transcription factors that are known to regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana and gene BCAT3, involved in the biosynthesis of glucoraphanin (Tian et al. 2021). As both were found to be repressed could be consistent with the results obtained with glucosinolate analysis.
In other side, the increase in the expression of K transporter and channel in the root part was supported by the increase in potassium in both the aerial and root parts. CPE treatment may have resulted in increased gene expression of AKT1 and HAK5 genes probably due to altered secondary metabolism which could leaded to nutritional impairment.
Plant aquaporins (membrane channels that allow water transport across cell membranes) are crucial for regulating water to maintain the water balance, which is essential for growth, development, and adaptation to changing environmental conditions (Li et al. 2014; Afzal et al. 2016). They have an essential role in hydraulic regulation and nutrient transport, in both roots and leaves (Li et al. 2014). In addition, they have been reported to be involved in the long-distance GSLs and phenolic compounds transport (Nicolas-Espinosa et al. 2023). However, there are no reports relating all those transporters in cells. In our experiments, the plasma membrane aquaporins both PIP and TIP changed their expression. In aerial part, PIP1 (PIP1;1 and PIP1;2) did not changed with the CPE treatments and both PIP2 (PIP2;1 and PIP2;2–3) showed an increase probably as an attempt to compensate the decreased metabolic results. However, a reduction of the expression of TIP2;1 was observed. As vacuole serves for storing nutrients as GSLs and phenolics, probably the lower facilitation of movement of water into and out of the vacuole, could be a response to lower concentrations found in CPE plants. As for the expression of aquaporins in the roots, it was observed that plants treated with the extract increased their expression, which could be a response of the plant to maintain water homeostasis due to the metabolic imbalance caused in the aerial part by the foliar treatment with flavonoids. In fact, stomatal conductance and transpiration did not change in the treated plants despite the drastic reduction in growth.
Conclusions
The large abundance of polyphenolic compounds in all organs of plants and the availability of numerous raw materials produced in agriculture, confer advantages in testing and assessing the novel bioactive effect of these compounds on plant physiology. The benefits of polyphenols and the aspiration to substitute synthetic antioxidants with natural alternatives have led investigations to intensify their efforts to uncover and utilise fruits and vegetables as sources of this bioactive compounds. However, the complexity of the secondary metabolism of plants needs to be considered. Therefore, our research revealed that flavonoids, mainly naringenin, could be mainly responsible for growth reduction by altering mineral nutrients (K, P and Zn levels) and metabolomic composition, specifically the synthesis of citrate, malate and GABA together with the synthesis of some amino acids such as arginine, glutamate, leucine and valine which are the precursors of phenolic compounds and GSLs. In addition, the regulation of genes responsible for the synthesis of GSLs (MYB122 and BCAT3), phenolics (PT-AL) and tonoplast aquaporins could reveal that feedback regulation could be taking place. Therefore, Brassicaceae family has a secondary metabolism that underscores the importance of interdisciplinary research involving genetics, biochemistry, physiology, and agronomy to determine the underlying mechanisms that will be altered when certain compounds are applied.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- An:
-
net photosynthetic rate
- Ci:
-
substomatal CO2 concentration
- CitrS:
-
citrate synthase
- CPE:
-
Citrus Phenolic Extract
- DW:
-
dry weight
- FW:
-
fresh weight
- GSLs:
-
glucosinolates
- HPLC-MS:
-
High-Performance Liquid Chromatography – Mass Spectrometry
- GBA:
-
glucobrassicin
- GRA:
-
glucoraphanin
- Gs:
-
stomatal conductance
- PCA:
-
Principal Component Analysis
- PT-AL:
-
Phe-Tyr ammonia lyase
- Shik:
-
shikimate kinase
References
Afzal Z, Howton T, Sun Y, Mukhtar M (2016) The roles of aquaporins in plant stress responses. J Dev Biol 4:9. https://doi.org/10.3390/jdb4010009
Aharoni A, Galili G (2011) Metabolic engineering of the plant primary–secondary metabolism interface. Curr Opin Biotechnol 22:239–244. https://doi.org/10.1016/j.copbio.2010.11.004
Albaladejo-Marico L, Carvajal M, Yepes-Molina L (2024) Involvement of glucosinolates and phenolics in the promotion of broccoli seedling growth through the modulation of primary and secondary metabolism. Plant Sci 112205. https://doi.org/10.1016/j.plantsci.2024.112205
Ali HM, El-Gizawy AM, El-Bassiouny REI, Saleh MA (2016) The role of various amino acids in enzymatic browning process in potato tubers, and identifying the browning products. Food Chem 192:879–885. https://doi.org/10.1016/j.foodchem.2015.07.100
Chen D, Mubeen B, Hasnain A et al (2022) Role of promising secondary metabolites to Confer Resistance Against Environmental Stresses in crop plants: current scenario and future perspectives. Front Plant Sci 13:881032. https://doi.org/10.3389/fpls.2022.881032
Cheynier V, Comte G, Davies KM et al (2013) Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem 72:1–20. https://doi.org/10.1016/j.plaphy.2013.05.009
Chon S-U, Jang H-G, Kim D-K et al (2005) Allelopathic potential in lettuce (Lactuca sativa L) plants. Sci Hortic 106:309–317. https://doi.org/10.1016/j.scienta.2005.04.005
Daş ZA, Dimlioğlu G, Bor M, Özdemir F (2016) Zinc induced activation of GABA-shunt in tobacco (Nicotiana tabaccum L). Environ Exp Bot 122:78–84. https://doi.org/10.1016/j.envexpbot.2015.09.006
Djurdjevic L, Dinic A, Pavlovic P et al (2004) Allelopathic potential of Allium ursinum L. Biochem Syst Ecol 32:533–544. https://doi.org/10.1016/j.bse.2003.10.001
Gomes MH, Rosa E (2000) Free amino acid composition in primary and secondary inflorescences of 11 broccoli (Brassica oleracea var italica) cultivars and its variation between growing seasons. J Sci Food Agric 81:295–299. https://doi.org/10.1002/1097-0010(200102)81:3%3C295::AID-JSFA811%3E3.0.CO;2-%23
Gomez-Molina M, Albaladejo-Marico L, Yepes-Molina L et al (2024) Exploring Phenolic compounds in Crop By-Products for Cosmetic Efficacy. Int J Mol Sci 25:5884. https://doi.org/10.3390/ijms25115884
Kassambara A, Mundt F (2020) Extract and visualize the results of multivariate data analyses [R package factoextra version 1.0. 7]. Comprehensive R Archive Network (CRAN)
Kisiriko M, Anastasiadi M, Terry LA et al (2021) Phenolics from Medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 26:6343. https://doi.org/10.3390/molecules26216343
Kudla J, Batistič O, Hashimoto K (2010) Calcium signals: the Lead Currency of Plant Information Processing. Plant Cell 22:541–563. https://doi.org/10.1105/tpc.109.072686
Lê S, Josse J, Husson F (2008) FactoMineR: an R Package for Multivariate Analysis. J Stat Softw 25:1–18. https://doi.org/10.18637/jss.v025.i01
Lee CP, Elsässer M, Fuchs P et al (2021) The versatility of plant organic acid metabolism in leaves is underpinned by mitochondrial malate–citrate exchange. Plant Cell 33:3700–3720. https://doi.org/10.1093/plcell/koab223
Li G, Santoni V, Maurel C (2014) Plant aquaporins: roles in plant physiology. Biochim Biophys Acta 1840:1574–1582. https://doi.org/10.1016/j.bbagen.2013.11.004
Liu HM, Guo JH, Liu P et al (2010) Inhibitory activity of tea polyphenol and Candida Ernobii against Diplodia natalensis infections. J Appl Microbiol 108:1066–1072. https://doi.org/10.1111/j.1365-2672.2009.04511.x
Liu X, Wei L, Miao C et al (2023) Application of exogenous phenolic compounds in improving Postharvest Fruits Quality: classification, potential biochemical mechanisms and synergistic treatment. Food Rev Int 1–20. https://doi.org/10.1080/87559129.2023.2233599
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 4:402–408. https://doi.org/10.1006/meth.2001.1262
López-Berenguer C, Martínez-Ballesta MDC, Moreno DA et al (2009) Growing hardier crops for better health: salinity tolerance and the nutritional value of broccoli. J Agric Food Chem 57:572–578. https://doi.org/10.1021/jf802994p
Mackenzie SA, Mullineaux PM (2022) Plant environmental sensing relies on specialized plastids. J Exp Bot 73:7155–7164. https://doi.org/10.1093/jxb/erac334
Martinez-Alonso A, Garcia-Ibañez P, Bárzana G, Carvajal M (2022) Leaf Gas Exchange and Growth responses of tomato plants to External flavonoids Application as Biostimulators under Normal and Salt-stressed conditions. Agronomy 12:3230. https://doi.org/10.3390/agronomy12123230
Martinez-Alonso A, Yepes-Molina L, Guarnizo AL, Carvajal M (2023) Modification of Gene Expression of Tomato Plants through Foliar Flavonoid application in relation to enhanced growth. Genes (Basel) 14:2208. https://doi.org/10.3390/genes14122208
Moreno DA, Carvajal M, López-Berenguer C, García-Viguera C (2006) Chemical and biological characterisation of nutraceutical compounds of broccoli. J Pharm Biomed Anal 41:1508–1522. https://doi.org/10.1016/j.jpba.2006.04.003
Muries B, Faize M, Carvajal M, Martínez-Ballesta MDC (2011) Identification and differential induction of the expression of aquaporins by salinity in broccoli plants. Mol Biosyst 7:1322–1335. https://doi.org/10.1039/c0mb00285b
Nicolas-Espinosa J, Garcia-Ibañez P, Lopez-Zaplana A et al (2023) Confronting secondary metabolites with Water Uptake and Transport in Plants under Abiotic Stress. Int J Mol Sci 24:2826. https://doi.org/10.3390/ijms24032826
Nicolas-Espinosa J, Yepes-Molina L, Martinez-Bernal F et al (2024) Deciphering the effect of salinity and boron stress on broccoli plants reveals that membranes phytosterols and PIP aquaporins facilitate stress adaptation. Plant Sci 338:111923. https://doi.org/10.1016/J.PLANTSCI.2023.111923
Okumura H (2021) Application of phenolic compounds in plants for green chemical materials. Curr Opin Green Sustain Chem 27:100418. https://doi.org/10.1016/j.cogsc.2020.100418
Pascale S, De, Maggio A, Barbieri G (2005) Soil salinization affects growth, yield and mineral composition of cauliflower and broccoli. Eur J Agron 23:254–264. https://doi.org/10.1016/j.eja.2004.11.007
Pollastri S, Tattini M (2011) Flavonols: old compounds for old roles. Ann Bot 108:1225–1233. https://doi.org/10.1093/aob/mcr234
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing, Vienna
Ray A, Kundu S, Mohapatra SS et al (2024) An insight into the role of phenolics in Abiotic Stress Tolerance in plants: current perspective for sustainable environment. J Pure Appl Microbiol 18:64–79. https://doi.org/10.22207/JPAM.18.1.09
Reyes Jara AM, Gómez Lobato ME, Civello PM, Martínez GA (2022) Phenylalanine ammonia lyase is more relevant than Chalcone synthase and chalcone isomerase in the biosynthesis of flavonoids during postharvest senescence of broccoli. J Food Biochem 46:e14054. https://doi.org/10.1111/jfbc.14054
Roberts MR (2007) Does GABA Act as a Signal in plants? Hints from Molecular studies. Plant Signal Behav 2:408–409. https://doi.org/10.4161/psb.2.5.4335
Selinski J, Scheibe R (2019) Malate valves: old shuttles with new perspectives. Plant Biol 21:21–30. https://doi.org/10.1111/plb.12869
Sharma A, Kohli SK, Khanna K et al (2023) Salicylic acid: a phenolic molecule with multiple roles in salt-stressed plants. J Plant Growth Regul 42:4581–4605. https://doi.org/10.1007/s00344-022-10902-z
Shukla P, Kumar A, Kumar R (2023) Climate change and stress mitigation strategy in plants. Front Plant Sci 14:1291905. https://doi.org/10.3389/fpls.2023.1291905
Singh DP, Beloy J, McInerney JK, Day L (2012) Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota L). Food Chem 132:1161–1170. https://doi.org/10.1016/j.foodchem.2011.11.045
Sohail M, Wills RBH, Bowyer MC, Pristijono P (2021a) Beneficial impact of exogenous arginine, cysteine and methionine on postharvest senescence of broccoli. Food Chem 338:128055. https://doi.org/10.1016/j.foodchem.2020.128055
Sohail M, Wills R, Bowyer M, Pristijono P (2021b) Multiple amino acids inhibit Postharvest Senescence of Broccoli. Horticulturae 7:71. https://doi.org/10.3390/horticulturae7040071
Tanase C, Bujor O-C, Popa VI (2019) Phenolic natural compounds and their influence on physiological processes in plants. Polyphenols in plants. Elsevier, pp 45–58
Tariq A, Zeng F, Graciano G et al (2023) Regulation of metabolites by nutrients in plants. In: Pratap Singh V, Siddiqui MH (eds) Plant Ionomics: sensing, Signaling, and Regulation. John Wiley & Sons Ltd., pp 1–18
Tian P, Lu X, Bao J et al (2021) Transcriptomics analysis of genes induced by melatonin related to glucosinolates synthesis in broccoli hairy roots. Plant Signal Behav 16:1952742. https://doi.org/10.1080/15592324.2021.1952742
Trovato M, Funck D, Forlani G et al (2021) Amino acids in plants: regulation and functions in development and stress defense. Front Plant Sci 12:772810. https://doi.org/10.3389/fpls.2021.772810
Turk MA, Tawaha AM (2003) Allelopathic effect of black mustard (Brassica nigra L) on germination and growth of wild oat (Avena fatua L). Crop Prot 22:673–677. https://doi.org/10.1016/S0261-2194(02)00241-7
Wang S, Li Y, He L et al (2022) Natural variance at the interface of plant primary and specialized metabolism. Curr Opin Plant Biol 67:102201. https://doi.org/10.1016/j.pbi.2022.102201
Xu L, Geelen D (2018) Developing Biostimulants from Agro-food and Industrial By-Products. Front Plant Sci 9:1567. https://doi.org/10.3389/fpls.2018.01567
Zhang Y, Hu Z, Zhu M et al (2015) Anthocyanin Accumulation and Molecular Analysis of Correlated Genes in Purple Kohlrabi (Brassica oleracea var. gongylodes L). J Agric Food Chem 63:4160–4169. https://doi.org/10.1021/acs.jafc.5b00473
Acknowledgements
The authors acknowledge Sol de Levante S.L.U Company for allowing the use of the farm to treat the broccoli plants.
Funding
This research was funded by Spanish Ministerio de Ciencia e Innovación (CPP2021-008588) and by a grant for Albaladejo-Marico, L. from the 22328/FPI/23. Fundación Séneca. Cofinanced by the Cooperative Agrícola Levante Sur. Región de Murcia (Spain). This research formed part of the AGROALNEXT programme and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by Fundación Séneca with funding from Comunidad Autónoma Región de Murcia (CARM).
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lorena Albaladejo-Maricó, Lucia Yepes-Molina and Micaela Carvajal. The first draft of the manuscript was written by Lorena Albaladejo-Maricó, Lucia Yepes-Molina and Micaela Carvajal. All authors commented different versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albaladejo-Marico, L., Yepes-Molina, L. & Carvajal, M. Alteration of nutrient uptake and secondary metabolism connection by foliar application of citrus flavonoids to broccoli plants. Plant Growth Regul (2024). https://doi.org/10.1007/s10725-024-01204-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10725-024-01204-3