Abstract
Soil salinity seriously restricts agricultural production. Halophytes adapt to saline environments through several strategies, including leaf or stem succulence. Succulence is associated with the increase in cell size or leaf thickness and high water content per unit of surface area, allowing salts to be diluted within the succulent leaves or stems. The proposed mechanisms of NaCl-induced plant tissue succulence include acidification and subsequent induction of cell wall elasticity, increased water uptake, cell turgor pressure, Na+ partitioning in vacuoles, abundance of plasma membrane aquaporins, cell wall formation and extensibility, as well as up-regulation of certain genes (e.g., XTH and CEB1) that control cell expansion and cell wall modification. However, the information on the mechanism of succulence activated by salinity is limited. In this paper, the possible mechanism of salinity-induced succulence, and the role of succulence in plant salt tolerance, are discussed. Understanding the mechanisms that activate succulence in halophytes opens up new opportunities for plant breeding to increase salt tolerance and improve crop productivity in saline soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinization is a serious problem worldwide due to its adverse effects on agricultural production (Rozema and Flowers 2008; Munns et al. 2020). It has been estimated that only about 1% of the land plant species can survive and reproduce in saline areas (Rozema and Flowers 2008). The physiological and molecular mechanisms of salinity tolerance in halophytes have been extensively studied (Shabala et al. 2014; Flowers and Colmer 2015; Munns et al. 2020), but it is worth noting that relatively little basic research into salt tolerance has been applied to crop plants (Munns and Gilliham 2015).
Succulent halophytes are plants that can dilute salt by storing large amounts of water within their leaves or stems, which is an important mechanism for their salt tolerance. This trait allows halophytes to achieve high water-use efficiencies and accumulate large amounts of salts, enabling them to extract water from substrates with extremely negative water potentials (Khan et al. 2000; Katschnig et al. 2013; Song and Wang 2015). Halophytes exhibit morpho-physiological adaptations that could be valuable in creating more resilient cash crops under extreme conditions such as high salinity (Rasheed et al. 2022). A particularly notable adaptation is their ability to store water in their living tissues, known as succulence. This enables them to mitigate the effects of drought or salinity stress and allows them to survive in semi-arid and arid regions (Lim et al. 2020).
Several species from the Amaranthaceae family demonstrate increased leaf or stem succulence as a strategy to overcome challenges such as heat and light-induced oxidative stress, enabling their survival in drought or saline environments (Griffiths, 2013). The morphological and physiological characteristics of succulence in halophytes have been known and studied for several decades. Halophytes have evolved in a unique environment, leading to a distinct type of succulence integrated into an eco-physiological syndrome that is different from other succulent groups. However, the molecular mechanisms regarding this process and its possible role in breeding salt-tolerant crops have not been well elucidated. Tissue succulence engineering can improve plant salt tolerance, as demonstrated by the overexpression of Populus euphratica PeXTH (a putative xyloglucan endotransglucosylase/hydrolase gene), which leads to leaf succulence development in transgenic tobacco plants (Han et al. 2013). Recently, Lim et al. (2020) reported that plant tissue succulence engineering improves salt tolerance in Arabidopsis. In this paper, the mechanisms of salinity-induced succulence in plants and the potential value of succulence as a target for breeding salt-tolerant plants are discussed.
The importance of understanding salt tolerance in plants
Excessive concentrations of Na+ in cultivated land worldwide seriously affect crop yield and quality (Zhang et al. 2019). Therefore, understanding ion sensing and transport in plants under salinity is beneficial for breeding salt-tolerant crops (Wu 2018).
In root epidermal cells, the primary pathways for Na+ uptake involve the glutamate receptor-like (GLRs) channels, cyclic nucleotide-gated (CNGCs) non-selective cation channels, and HKT2 high-affinity K+ transporters (Zhao et al. 2020). The salt overly sensitive (SOS) signaling is a key mechanism responsible for excluding Na+ from the cytoplasm in root epidermal cells (Halfter et al. 2000; Shi et al. 2000; Wu 2018). This mechanism is considered a milestone in understanding how plants maintaining Na+ homeostasis (Zhao et al. 2020). Vacuolar compartmentalization of Na+ is another important mechanism for maintaining Na+ homeostasis in plant salt tolerance. The Na+/H+ antiporter NHX1 in the tonoplast plays a central role in this process, with its activity controlled by the electrochemical H+-gradient across the tonoplast generated by V-H+-ATPase or V-H+-PPase (Song and Wang 2015; Zhao et al. 2020). Na+ compartmentalization in the vacuole prevents the cytoplasm from reaching toxic concentrations, reduces the water potential, and aids water absorption from saline soil (Flowers and Colmer 2015; Cui et al. 2021). Efficient control of tonoplast slow- (SV) and fast- (FV) activating ion channels is crucial for vacuolar Na+ sequestration, as these channels could allow Na+ to leak back into the cytoplasm (Zhao et al. 2020). The Na+ transporter HKT1 plays a role in retrieving Na+ from the xylem and may facilitate Na+ recirculation from shoots to roots (Zhu 2016), suggesting that the functions of SOS1 and HKT1 transporters may be interrelated in balancing Na+ efflux and xylem loading/unloading (Ismail and Horie 2017; Kotula et al. 2020).
Although certain specific key proteins play crucial roles in maintaining Na+ homeostasis in plants under salt stress, the application of these fundamental research findings to crop plants has been limited (Munns and Gilliham 2015). Morphological adaptation to salt is important for salt resistance of halophytes. Root apoplastic barriers, such as Casparian bands (CBs) and suberin lamellae (SL), contribute to ion exclusion in some halophytes and crop plants (Cui et al. 2021). It has been estimated that salt avoidance involving ion exclusion by the roots is fundamental since roots exclude up to 95% of salt in soil solution (Munns and Gilliham 2015; Cui et al. 2021). An apoplastic exodermal barrier can decrease energy costs by 18% and 10% in the roots with two- and one-cortical-cell layer, respectively, indicating that energy savings may increase with more cortical layers (Munns et al. 2020). Overall, preventing apoplastic ion leakage from roots to shoots is a key mechanism of salt tolerance (Cui et al. 2021).
Various halophytes have evolved specialized structures to cope with elevated salinity levels. For example, Chenopodium quinoa features epidermal bladder cells that accumulate excessive Na+ in their vacuoles (Chen et al. 2018; Zhao et al. 2020). Similarly, Limonium bicolor possesses salt glands capable of secreting salts from plant tissues to the external environment (Yuan et al. 2016 a, b), which enable such recretohalophytes to adapt to high salinity (Chen et al. 2018; Zhao et al. 2020). Halophytes exhibit organ plasticity in response to salt stress, including leaf or stem specialization and root system adjustment. Leaf or stem succulence is another important morphological characteristic in certain highly salt tolerant halophytes and reflects the ability of plants adaptation to salt stress. Succulence, combined with cuticles and waxes on leaf surfaces, reduces the transpiration area and stomatal number to conserve water (Ma et al. 2019). Succulence is an essential evolutionary strategy for halophytes in saline environments, characterized by larger cells, reduced surface area per tissue volume, reduced stomata, high water content for a given surface area, smaller leaves, and thicker leaf cuticle (Jennings 1968; Gale 1975; Song and Wang 2015). Succulence is a key adaptation trait for dicotyledonous halophytes to regulate their internal ion concentrations. In other words, salt can be diluted within the succulent leaves or stems (Short and Colmer 1999). A study conducted on two succulent halophytes, Suaeda salsa and Salicornia europaea, concluded that the growth and tissue succulence of these plants were mostly influenced by variation in cell size (Ma et al. 2019). Plant tissue succulence engineering has been found to improve salt tolerance in tobacco and Arabidopsis (Han et al. 2013; Lim et al. 2020), indicating that tissue succulence could be a promising target for breeding salt-tolerant plants. Recent advances in understanding the mechanisms underlying salt stress tolerance in halophytes show promise for the development of salt-tolerant crops (Zhao et al. 2020).
The role of succulence in plant salt tolerance
Succulence is a typical morphological characteristic in certain highly salt-tolerant halophytes, especially in Amaranthaceae (e.g., Salicornioideae, Chenopodioideae and Suaedoideae) (Flowers et al. 1977; Khan et al. 2001; Rozema and Schat 2013). This adaptation is linked to an enlargement of cell size, a reduction in surface area per tissue volume, elevated water content per unit surface area, and increased leaf thickness (Black 1958; Aslam et al. 1986; Khan et al. 2005; Lim et al. 2020). It was regarded that succulence may be a component trait of the salt tolerance syndrome of certain halophytes (Rozema and Schat 2013). Jennings (1968) hypothesized that Na+-induced succulence might be a homeostatic cell mechanism that helps prevent ion toxicity. Salt-induced succulence can also optimize photosynthesis by reducing CO2 uptake resistance and enabling more gaseous exchange per leaf-unit area (Longstreth and Nobel 1979). Suaeda monoica showed both a greater increase in succulence and tolerance to high salinity than Atriplex spongiosa, suggesting that the degree of succulence may be related to their salt tolerance (Storey and Jones 1979). In Salicornia dolichostachya, its highest succulence (water content per unit leaf area) coincided with its growth optimum at 300 mM NaCl, suggesting that succulence may contribute to its stimulated growth (Katschnig et al. 2013).
In Carpobrotus rosii, a succulent halophyte, storage parenchyma cells act as a Na+ sink, facilitating efficient Na+ sequestration in leaf tissues. Meanwhile, it was revealed that Caprobrotus plants are capable of downregulating the activity of fast vacuolar cation channels (the channel is permeable for monovalent cations only) under salinity. Patch-clamp experiments have shown that this ability is more pronounced in storage parenchyma cells compared to mesophyll cells, providing a foundation for understanding the underlying mechanisms contributing to Na+ sequestration in the succulent leaf tissues (Zeng et al. 2018). Similarly, in the potential cash crop Chenopodium quinoa (Quinoa), which has leaves that display a certain degree of succulence, the negative control of fast- and slow-activating tonoplast channels reduces Na+ leakage from vacuoles in old leaves. This enables the efficient sequestration of Na+ to their vacuoles, allowing for an optimal photosynthetic performance even under saline conditions (Bonales-Alatorre et al. 2013). In the salt tolerant tree species P. euphratica, the development of leaf succulence due to an increase in cell number and cell volume leads to Na+ dilution (Ottow et al. 2005). Even in the salt sensitive crop Solanum lycopersicum (tomato), succulence has been regarded to be an important trait for salt tolerance and its introduction through breeding with close relatives such as S. pennellii, S. cheesmanii, or S. pimpinellifolium has been suggested to increase its salt tolerance (Cuartero 1992). Moreover, most modern crops are relatively sensitive to salinity; however, attempts to improve crop salt tolerance have met with limited success (Munns and Gilliham 2015). Messembryanthemum crystallinum, a succulent halophyte, belongs to the C3 photosynthetic pathway but has the ability to switch to crassulacean acid metabolism (CAM) under saline conditions. M. crystallinum plants can store water within leaf tissues and use water-filled epidermal bladder cells, representing modified trichomes, to accumulate Na+ and other osmotically active substances (Adams et al. 1998; Loconsole et al. 2019). Leaf beet (Beta vulgaris var. cicla) is an important vegetable resource with a degree of salt tolerance, adapts to salt stress through the development of leaf succulence. The leaf succulence of the species reaches its maximum value at the 0.7% NaCl. Thus, leaf water content and the normal progression of photosynthesis are maintained to a certain extent (He et al. 2022). Some reported NaCl-induced succulence in certain plant species and the possible mechanisms during the process of succulence were summarized in Table 1.
How does NaCl induce succulence?
Succulent plants typically exhibit two types of anatomic arrangements, namely Allzellsukkulenz (or “all-cell succulent”) and Speichersukkulenz (or storage succulence). The first one implies a general increase in the size of all photosynthetically active cells. The second type is more common in perennial plants, where photosynthetic cells (chlorenchyma) and water-storage tissue (hydrenchyma) are distinct (Males 2017; Rawat et al. 2022). Succulence enables plants to enlarge their vacuoles, thus enhancing the capacity for vacuolar Na+ sequestration in halophytes (Shabala 2013). Na+ can induce an increase in succulence, and it has been proposed that Na+ may contribute to an increase in ATP synthesis (Jennings 1968). The plasma membrane proton pump (PM H+-ATPase) and tonoplast H+-ATPase (V-ATPase) are the main drivers for transporters that rely on protons. They are essential for maintaining Na+ homeostasis during plant salt tolerance (Song and Wang 2015). An electrochemical H+-gradient generated by the PM H+-ATPase energizes the Na+/H+ antiporter (SOS1) for Na+ efflux across the plasma membrane (Shi et al. 2000). Meanwhile, H+ generated by PM H+-ATPase may acidify and subsequently induce the elasticity of cell wall. An electrochemical H+-gradient generated by the V-ATPase energizes the tonoplast Na+/H+ antiporter (NHX) for the compartmentalization of Na+ in the vacuole (Bassil et al. 2012; Bassil and Blumwald 2014). In certain succulent halophytes, e.g., S. salsa, an increase in PM H+-ATPase activity and abundance was observed under salinity (Chen et al. 2010). Similarly, an up-regulation of the V-ATPase was also observed under salinity (Qiu et al. 2007). Furthermore, NaCl activated the PM H+-ATPase in Atriplex lentiformis and C. quinoa (Bose et al. 2015), and has been observed to activate the V-ATPase in Mesembryanfbemum crystallinum (Barkla et al. 1995). This suggests that the increased H+-ATPase under salinity may be important for salt-induced succulence and salt tolerance in these halophytes.
In two tomato cultivars and three wild relatives, the degree of succulence in different plant parts exhibited a positive correlation with the distribution of Na+ (Tal and Shannon 1983). In Atriplex amnicola, a higher Na+ concentration in older leaf tissue coincided with a greater degree of leaf succulence in older leaves (Aslam et al. 1986). These results support earlier suggestions that the development of succulence in plants exposed to high external NaCl concentrations is associated with an increase in turgor pressure, probably due to ion accumulation (Jennings 1976). This is supported by a study of the succulent halophyte Sarcocornia natalensis which suggested that its high salt tolerance primarily results from substantial inorganic ion accumulation, providing sufficient solutes for osmoregulation, increased water flux, and turgor-induced growth (Naidoo and Rughunanan 1990). Aslam et al. (1986) further suggested that such turgor effects could be attributed either to irreversible cell wall growth or to high elasticity of cell walls. Moreover, when S. salsa plants were treated with 100 mM of different salts, leaf succulence was induced by NaCl, and to some extent NaHCO3, but no obvious succulence appeared in leaves of seedlings treated with CaCl2 or KCl. This suggests that Na+ plays a key role in leaf succulence of S. salsa, indicating that the process of succulence may be Na+-specific rather than the osmotic effect of different salts (Qi et al. 2005). Similarly, in the succulent halophyte Sesuvium portulacastrum, the diameters of parenchyma and epidermal cells markedly increased after treatment with sodium-based ions. For example, when seedlings were treated with 200 mM NaCl, NaNO3, and Na-H, the diameter of epidermal cells was much higher than those in both the control and potassium-based treatments; a similar trend was observed with stem diameter (Wang et al. 2012). Interestingly, sodium-based ions increased shoot water content, while chloride or potassium-based ions significantly reduced it, indicating that Na+ was more effective than K+ and Cl− in promoting cell expansion, leaf succulence and shoot development in S. portulacastrum (Wang et al. 2012). These findings suggest that Na+ is an important factor for succulence in plants.
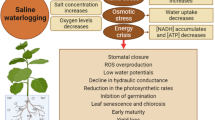
Plasma membrane aquaporins (AQPs) facilitate water movement across biomembranes and play a crucial role in controlling trans-cellular water transport. In S. salsa, NaCl increases the abundance of transcripts for a plasma-membrane AQP homologous to AtPIP2;7. Meanwhile, immunoblot analyses of PIP AQPs in plasma membrane-enriched fractions of S. salsa leaves reveal a significant increase under salinity (Qi et al. 2009). This indicates that the increase of AQP activity under salinity may due to both the increased transcript and protein levels, which correlates with the observed increase in leaf succulence of S. salsa under salinity (Qi et al. 2009). The mechanisms of NaCl-induced succulence are postulated in Fig. 1. In brief, during this process, NaCl-induced H+ generated by PM H+-ATPase may acidify and subsequently induce the elasticity of cell wall. The up-regulation of V-ATPase by NaCl can generate more H+ which energizes NHX for Na+ compartmentalization in vacuoles. Additionally, NaCl can increase the abundance of the transcripts and protein amounts for PIP, which may correlate with the increase in succulence.
The postulated mechanisms that may be involved in the process of NaCl-induced plant tissue succulence. During this process, the role of NaCl may be Na+-specific. (1) H+ generated by plasma membrane (PM) H+-ATPase may acidize and subsequently induce the elasticity of cell wall; (2) increased Na+ generated by tonoplast Na+/H+ antiporter (NHX) can decrease osmotic potential and increased water uptake; (3) cell turgor pressure may increase due to water uptake; (4) the up-regulation of tonoplast H+-ATPase (V-ATPase) by NaCl can generate more H+ which energizes NHX for Na+ compartmentalization in vacuolar; (5) NaCl increased the abundance of the transcripts and protein amount for plasma-membrane aquaporins (PIP), which may correlate with the increase of succulence (high water content); (6) NaCl increase the expression of CEB1, which controls cell expansion and affects the expression of genes encoding cell wall modification proteins and enzymes, and aquaporins, (7) NaCl upregulates the expression of XTH, possibly increased cell wall formation and cell wall extensibility, leading to increased leaf succulence;. The red font indicates the up-regulation or increment
The evolutionary developmental and genetic control of succulence
Succulent plants were initially described by Johann Bauhin as ‘thick-leaved and juicy herbs’ (Rowley 1997). Eggli and Nyffeler subsequently described succulence as a plant’s capacity to store ‘utilizable’ water, allowing it to sustain physiological activity during short periods of limited water supply (Eggli and Nyffeler 2009). Succulence exists in any vegetative organ, including leaves and stems, roots, bulbs or tubers of geophytes, orchid pseudobulbs, and the parenchymatous rays of pachycaul trees (Eggli and Nyffeler 2009; Nyffeler and Eggli 2010).
Succulence begins at the cellular level with the development of a central vacuole for storing water and other substances, which was an important event in land plant evolution (Becker 2007; Grace 2019). For example, in some plants, the vacuoles may occupy 90% or more of the cell volume (Gibson 1982; Von Willert et al. 1992).
The primary role of succulence is storing water in living cells for later use, facilitating water homeostasis (Griffiths and Males 2017). In some succulence plants, apoplastic mucilage or pectic compounds may also contribute significantly to water storage, as seen in cacti and Asteraceae (Nobel et al. 1992). Succulent plants have a low surface area to volume ratio (SA:V), which maximizes water storage while minimizing transpirational water loss (Griffiths and Males 2017). Adaptations such as thick cuticles, low stomatal density, high stomatal sensitivity to environmental stimuli, low hydraulic conductances and anatomically-reduced vasculature are in line with their conservative water-use strategies (Heyduk 2021).
The genetic regulation of succulent leaf development primarily involves the control of cell size, determination of vascular patterning, and intercellular water transport (Heyduk 2021). Cell size is an essential determinant of succulence and is mainly controlled by cell cycle and cell division timing (Kalve et al. 2014; Griffiths and Males 2017; Conklin et al. 2019). Target of rapamycin (TOR), a master cell cycle regulator in plants (Ahmad et al. 2019), and ribosomal S6 kinases (S6Ks) promote cell expansion through an auxin-TOR signaling cascades (Meyuhas 2008). Retinoblastoma related (RBR) and E2F transcription factors (E2FA and E2FB) form S6Ks-RBR-E2FA /EF2B complexes that regulate entry into the endocycle and rates of cell proliferation via the TOR signaling cascade (Sozzani et al. 2006; Borghi et al. 2010; Henriques et al. 2010; Magyar et al. 2012), indicating that this pathway may contribute to leaf succulence development. Auxin-related genes, such as auxin related gene involved in organ size (ARGOS), aintegumenta (ANT), and organ size related 1 (OSR1), can increase cell sizes and are important in controlling cell proliferation (Hu et al. 2003, 2006; Feng et al. 2011). In succulent leaves, vascular patterning and intercellular water movement principles ensure hydraulic connectivity (Heyduk 2021). The initiation of 3D leaf venation patterns in thicker leaves may result from auxin’s ability to move through multiple planes.
Auxin biosynthesis and transport play an essential role in vascular patterning (Kleine-Vehn et al. 2009). Pin-formed1 (PIN1) can direct auxin to be formed in procambial cells, aiding in the formation of vascular tissue (Wenzel et al. 2010). Vasculature complexity and connectivity (VCC) control the development of veins in cotyledons (Yanagisawa et al. 2021). VCC and pinoid (PID) regulate PIN1 polarity, which is required for controlling vasculature development (Yanagisawa et al. 2021). All of these factors may affect the formation of tissue succulence and could be potential targets for breeding salt-tolerant plants (Table 2). However, further research is needed to determine whether these factors are involved in the process of tissue succulence and how salinity affects their function.
Succulence engineering can improve plant salt tolerance
Despite the few advances in the understanding of genetic and ontogenetic mechanisms associated with succulence, little is known about the molecular regulation of salt-induced succulence. Research on succulence has primarily focused on its genetic regulation in leaves through the modulation of cell size, vascular patterning, and water transport between cells, pathways related to succulence as described by Heyduk (2021). Cell wall loosening and rearrangement rely on wall-modifying enzymes, such as expansin, xyloglucan endotransglucosylase, and β-1, 4-glucanase (Cosgrove 2005). Among them, xyloglucan endotransglucosylase/hydrolases (XTHs) play a key role in cell wall extensibility. In B. vulgaris ssp. maritima, salt treatments influenced the gene expression such as XTH, expansin, or glucan endo-1,3-beta-D-glucosidase, potentially leading to increased cell wall formation and extensibility, and therefore, greater leaf succulence (Skorupa et al. 2016). Tobacco plants overexpressing P. euphratica PeXTH exhibited higher water content per unit area (36%) and a higher ratio of fresh weight to dry weight (39%), characteristics of leaf succulence, compared to wild-type tobacco (Han et al. 2013). The anatomical changes in PeXTH-transgenic plants promoted the leaf water-retaining capacity, lowering salt concentration in the succulent tissues and mesophyll cells, due to densely packed palisade parenchyma cells and reduced intercellular air spaces (Han et al. 2013). Moreover, the decrease in intercellular air space also resulting in a 47–78% increase in net photosynthesis (Han et al. 2013). These results indicate that PeXTH overexpression enhanced salt tolerance in tobacco plants by promoting leaf succulence development, i.e., the increased succulence has a positive effect on salt dilution and net photosynthesis in PeXTH-transgenic plants (Table 2).
A basic helix-loop-helix (bHLH) transcription factor from Vitis vinifera has been shown to promote cell expansion in developing fruit (Nicolas et al. 2013). When the V. vinifera cell expansion bHLH (VvCEB1) gene was overexpressed in Arabidopsis, it resulted in larger cells, leaves, and increased biomass accumulation (Lim et al. 2018). Lim et al. (2020) further reported that tissue succulence was engineered in Arabidopsis by overexpressing a codon-optimized helix-loop-helix transcription factor (VvCEB1opt) from wine grape, which is responsible for the cell expansion phase of berry development (Lim et al. 2020).
Cell size and the degree of succulence increased, and intercellular air space decreased in VvCEB1opt-overexpressing Arabidopsis lines compared to the wild type (WT), while water-use efficiency (WUE) increased due to reduced stomatal conductance and density, which may contribute to the attenuation of water-deficit stress (Lim et al. 2020). Furthermore, the overexpressed lines demonstrated greater salinity tolerance, attributed to decreased salinity uptake and dilution of internal Na+ and Cl−, compared to WT Arabidopsis plants (Lim et al. 2020). This means the overexpressed Arabidopsis lines can better adapt to both osmotic stress and ion toxicity due to high salinity, compared to WT Arabidopsis. These findings provide valuable information for understanding the role of succulence in plant salt tolerance.
Efficient sequestration of cytotoxic Na+ in vacuoles is regarded as a critical feature of halophytes, as Na+ accumulation in vacuoles can help regulate the osmotic balance and alleviate Na+ toxicity in the cytoplasm (Song and Wang 2015). Halophytic succulence is due to ion accumulation in enlarged vacuoles, sequestering toxic ions away from the cytoplasm (Lim et al. 2020). The larger cells observed in the VcCEB1opt overexpression lines were considered to offer increased vacuolar storage capacity for sequestering toxic ions under salinity stress (Lim et al. 2020). The higher degree of succulence in transgenic plants provides a high capacity for the dilution of salt compared to WT plants (Han et al. 2013; Lim et al. 2020). Meanwhile, high WUE enable transgenic plants to attenuate osmotic stress resulting from salinity (Lim et al. 2020). Some reported genes and their putative function related to succulence were summarized in Table 2. These studies provide theoretical and practical examples of the viability of engineering increased succulence in plants and support succulence as an important trait in the breeding of salt-tolerant plants.
Conclusions
Soil salinization has become a serious problem worldwide, limiting the development of agroforestry. Breeding salt-tolerant crops is a promising avenue for sustainable agricultural development in saline lands. Although leaf or stem succulence may help mitigate salt stress, and it has been largely overlooked in agricultural research and crop improvement efforts. This is probably because it is considered a halophytic trait predominantly found in natural environments and is rarely used in agricultural production.
A more detailed understanding of how NaCl-induced succulence works is needed, including the identification of the key genes involved and their mechanisms. Molecular approaches could then be used to engineer crops to produce limited layers of succulent cells, potentially enhancing their ability to tolerate salt. Modern biotechnology offers various tools and techniques for gene screening and function studies. For example, whole genome sequencing, proteomics, transcriptomics, metabolomics, molecular markers, gene-tagging methodologies and bioinformatic analysis have been instrumental in identifying important genes and their functions. Whole genome sequences for crops such as Chenopodium quinoa and Beta vulgaris are already available (Jarvis et al. 2017; Dohm et al. 2014). However, the genetic transformation techniques for succulent halophytes such as Suaeda and Salicornia are not well established, hampering gene functional analyses. To overcome this limitation, future research should focus on developing transformation techniques and employing tools such as CRISPR/Cas9, CRISPR/Cpf1, prime and base editing, dCas9 epigenetic changes, and other transgene-free genome editing approaches. These tools can be used to screen for genes involved in succulence beyond the already reported XTH and CEB1 genes. Undertaking such efforts is essential for understanding the roles of these genes in succulence and for breeding salt-tolerant crops.
References
Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138:171–190
Ahmad Z, Magyar Z, Bögre L, Papdi C (2019) Cell cycle control by the target of rapamycin signalling pathway in plants. J Exp Bot 70:2275–2284
Aslam Z, Jeschke WD, Barrett-Lennard EG, Setter TL, Greenway H (1986) Effects of external NaCl on the growth of Atriplex amnicola and the ion relations and carbohydrate status of the leaves. Plant Cell Environ 9:571–580
Barkla BJ, Zingarelli L, Blumwald E, Smith J (1995) Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol 109:549–556
Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol 22:1–6
Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63:5727–5740
Becker B (2007) Function and evolution of the vacuolar compartment in green algae and land plants (Viridiplantae). Int Rev Cytol 264:1–24
Benzarti M, Rejeb KB, Messedi D, Mna AB, Hessini K, Ksontini M, Abdelly C, Debez A (2014) Effect of high salinity on Atriplex portulacoides: growth, leaf water relations and solute accumulation in relation with osmotic adjustment. S Afr J Bot 95:70–77
Black RF (1958) Effect of sodium chloride on leaf succulence and area of Atriplex Hastata L. Vol-6. Aust J Bot 6:306–321
Bonales-Alatorre E, Shabala S, Chen ZH, Pottosin I (2013) Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa. Plant Physiol 162:940–952
Borghi L, Gutzat R, Futterer J, Laizet Y, Hennig L, Gruissem W (2010) Arabidopsis RETINOBLASTOMA-RELATED is required for stem cell maintenance, cell differentiation, and lateral organ production. Plant Cell 22:1792–1811
Bose J, Rodrigo-Moreno A, Lai D, Xie Y, Shen W, Shabala S (2015) Rapid regulation of the plasma membrane H-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann Bot 115:481–494
Chen M, Song J, Wang BS (2010) NaCl increases the activity of the plasma membrane H+-ATPase in C3 halophyte Suaeda salsa callus. Acta Physiol Plant 32:27–36
Chen M, Yang Z, Liu J, Zhu TT, Wei XC, Fan H, Wang BS (2018) Adaptation mechanism of salt excluders under saline conditions and its applications. Int J Mol Sci 19:3668
Conklin PA, Strable J, Li S, Scanlon MJ (2019) On the mechanisms of development in monocot and eudicot leaves. New Phytol 221:706–724
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Bio 6:850–861
Cuartero J (1992) Selection of donors for salt-tolerance in tomato using physiological traits. New Phytol 121:63–69
Cui B, Liu RR, Flowers TJ, Song J (2021) Casparian bands and suberin lamellae: key targets for breeding salt tolerant crops? Environ Exp Bot 191:104600
Debez A, Saadaoui D, Ramani B, Ouerghi Z, Koyro HW, Huchzermeyer B, Abdelly C (2006) Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ Exp Bot 57:285–295
Dohm JC, Minoche AE, Holtgräwe D, Capella-Gutiérrez S, Zakrzewski F, Tafer H, Rupp O, Sörensen TR, Stracke R, Reinhardt R, Goesmann A, Kraft T, Schulz B, Stadler PF, Schmidt T, Gabaldón T, Lehrach H, Weisshaar B, Himmelbauer H (2014) The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 505:546–549
Eggli U, Nyffeler R (2009) Living under temporarily arid conditions - succulence as an adaptive strategy. Bradleya 27:13–36
Feng GP, Qin ZX, Yan JZ, Zhang XR, Hu YX (2011) Arabidopsis ORGAN SIZE RELATED1 regulates organ growth and final organ size in orchestration with ARGOS and ARL. New Phytol 191: 635–646
Flowers TJ, Colmer TD (2015) Plant salt tolerance: adaptations in halophytes. Ann Bot 115:327–331
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Ann Rev Plant Physiol 28:89–121
Gale J (1975) Water balance and gas exchange of plants undersaline conditions. Plants in saline environments. Springer, Berlin/Heidelberg, Germany, pp 168–185
Gibson AC (1982) The anatomy of succulence. Rockville: American Society of Plant Physiologists, Rockville, p 17
Grace OM (2019) Succulent plant diversity as natural capital. Plants People Planet 1:336–345
Griffiths H (2013) Plant venation: from succulence to succulents. Curr Biol 23:R340–R341.
Griffiths H, Males J (2017) Succulent plants. Curr Biol: CB 27:890–896
Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97:3735–3740
Han YS, Wei W, Sun J, Ding MQ, Zhao R, Deng SR, Wang FF, Hu Y, Wang Y, Lu YJ, Du LP, Hu ZM, Diekmann H, Shen X, Polle A, Chen SL (2013) Populus Euphratica XTH overexpression enhances salinity tolerance by the development of leaf succulence in transgenic tobacco plants. J Exp Bot 64:4225–4238
Harvey D, Hall JL, Flowers TJ, Kent B (1981) Quantitative ion localization within Suaeda maritima leaf mesophyll cells. Planta 151:555–560
He H, Zhou W, Lü H, Liang B (2022) Growth, leaf morphological and physiological adaptability of leaf beet (Beta vulgaris var. cicla) to salt stress: a soil culture experiment. Agron 12:1393
Henriques R, Magyar Z, Monardes A, Khan S, Zalejski C, Orellana J, Szabados L, de la Torre C, Koncz C, Bögre L (2010) Arabidopsis S6 kinase mutants display chromosome instability and altered RBR1-E2F pathway activity. Embo J 29:2979–2993
Heyduk K (2021) The genetic control of succulent leaf development. Curr Opin Plant Biol 59:101978
Hu YX, Xie Q, Chua NH (2003) The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15:1951–1961
Hu YX, Poh HM, Chua NH (2006) The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J 47:1–9
Ismail AM, Horie T (2017) Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Ann Rev Plant Biol 68:405–434
Jarvis DE, Ho YS, Lightfoot DJ, Schmöckel SM, Li B, Borm TJA, Ohyanagi H, Mineta K, Michell CT, Saber N, Kharbatia NM, Rupper RR, Sharp AR, Dally N, Boughton BA, Woo YH, Gao G, Schijlen EGWM, Guo X, Momin AA, Negrão S, Al-Babili S, Gehring C, Roessner U, Jung C, Murphy K, Arold ST, Gojobori T, van Linden CGvd EN, Jellen EN, Maughan PJ, Tester M (2017) The genome of Chenopodium quinoa. Nature 542:307–312
Jennings DH (1968) Halophytes, succulence and sodium in plants-a unified theory. New Phytol 67:899–911
Jennings DH (1976) The effects of sodium on higher plants. Biol Rev 51:453–486
Kalve S, De Vos D, Beemster GT (2014) Leaf development: a cellular perspective. Front Plant Sci 5:362
Katschnig D, Broekman R, Rozema J (2013) Salt tolerance in the halophyte Salicornia Dolichostachya Moss: growth, morphology and physiology. Environ Exp Bot 92:32–42
Khan MA, Ungar IA, Showalter AM (2000) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J Arid Environ 45:73–84
Khan MA, Gul B, Weber DJ (2001) Effect of salinity on the growth and ion content of Salicornia rubra. Commun Soil Sci Plan 32:2965–2977
Khan MA, Ungar IA, Showalter AM (2005) Salt stimulation and tolerance in an intertidal stem-succulent halophyte. J Plant Nutr 28:1365–1374
Kleine-Vehn J, Huang F, Naramoto S, Zhang J, Michniewicz M, Offringa R, Friml J (2009) PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 21:3839–3849
Kotula L, Garcia Caparros P, Zörb C, Colmer TD, Flowers TJ (2020) Improving crop salt tolerance using transgenic approaches: an update and physiological analysis. Plant Cell Environ 43:2932–2956
Lim SD, Yim WC, Liu D, Hu R, Yang X, Cushman JC (2018) A Vitis vinifera basic helix–loop–helix transcription factor enhances plant cell size, vegetative biomass and reproductive yield. Plant Biotechnol J 16:1595–1615
Lim SD, Mayer JA, Yim WC, Cushman JC (2020) Plant tissue succulence engineering improves water-use efficiency, water-deficit stress attenuation and salinity tolerance in Arabidopsis. Plant J 103:1049–1072
Loconsole D, Amador B, Cristiano G, De Lucia B (2019) Halophyte common ice plants: a future solution to arable land salinization. Sustainability 11:6076
Longstreth DJ, Nobel PS (1979) Salinity effects on leaf anatomy: consequences for photosynthesis. Plant Physiol 63:700–703
Ma FL, Barrett-Lennard EG, Tian CY (2019) Changes in cell size and tissue hydration (‘succulence’) cause curvilinear growth responses to salinity and watering treatments in euhalophytes. Environ Exp Bot 159:87–94
Magyar Z, Horváth B, Khan S, Mohammed B, Henriques R, De Veylder L, Bakó L, Scheres B, Bögre L (2012) Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO J 31:1480–1493
Males J (2017) Secrets of succulence. J Exp Bot 68:2121–2134
Meyuhas O (2008) Physiological roles of ribosomal protein S6: one of its kind. Int Rev Cell Mol Biol 268:1–37
Munns R, Gilliham M (2015) Salinity tolerance of crops - what is the cost? New Phytol 208:668–673
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla BJ, Bose J, Byrt CS, Chen Z, Foster KJ (2020) Energy costs of salt tolerance in crop plants. New Phytol 225:1072–1190
Naidoo G, Rughunanan R (1990) Salt tolerance in the succulent, coastal halophyte, Sarcocornia natalensis. J Exp Bot 41:497–502
Nicolas P, Lecourieux D, Gomes E, Delrot S, Lecourieux F (2013) The grape berry-specific basic helix-loop-helix transcription factor VvCEB1 affects cell size. J Exp Bot 64:991–1003
Nobel PS, Cavelier J, Andrade JL (1992) Mucilage in cacti: its apoplastic capacitance, associated solutes, and influence on tissue 5. J Exp Bot 43:641–648
Nyffeler R, Eggli U (2010) An up-to-date familial and suprafamilial classification of succulent plants. Bradleya 28:125–144
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M, Kangasjärvi J, Jiang X, Polle A (2005) Populus Euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139:1762–1772
Qi CH, Han N, Wang B (2005) Effect of different salt treatments on succulence of Suaeda salsa seedlings. Chin Bull Bot 22:175–182
Qi CH, Chen M, Song J, Wang BS (2009) Increase in aquaporin activity is involved in leaf succulence of the euhalophyte Suaeda salsa, under salinity. Plant Sci 176:200–205
Qiu NW, Chen M, Guo JR, Bao HY, Wang BS (2007) Coordinate up-regulation of V-H+-ATPase and vacuolar Na+/H+ antiporter as a response to NaCl treatment in a C3 halophyte Suaeda salsa. Plant Sci 172:1218–1225
Rasheed A, Koyro H-W, Hameed A, Gul B (2022) Physiological responses of the xero-halophyte Salsola drummondii to seasonal alterations of environmental conditions in a salt desert. Ecol Res 37:738–752
Rawat N, Wungrampha S, Singla-Pareek SL, Yu M, Shabala S, Pareek A (2022) Rewilding staple crops for the lost halophytism: toward sustainability and profitability of agricultural production systems. Mol Plant 15:45–64
Rowley GD (1997) A history of succulent plants. Strawberry Press Bibliography, pp 391–400
Rozema F, Flowers T (2008) Crops for a salinized world. Science 322:1478–1480
Rozema J, Schat H (2013) Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ Exp Bot 92:83–95
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot 112:1209–1221
Shabala S, Bose J, Hedrich R (2014) Salt bladders: do they matter? Trends Plant Sci 19:687–691
Shennan C, Hunt R, Macrobbie EAC (1987) Salt tolerance in Aster tripolium L. I. The effect of salinity on growth. Plant Cell Environ 10:59–65
Shi HZ, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901
Short DC, Colmer TD (1999) Salt tolerance in the halophyte Halosarcia Pergranulata subsp. Pergranulata. Ann Bot 3:207–213
Skorupa M, Gołębiewski M, Domagalski K, Kurnik K, Abu Nahia K, Złoch M, Tretyn A, Tyburski J (2016) Transcriptomic profiling of the salt stress response in excised leaves of the halophyte Beta vulgaris ssp. maritima. Plant Sci 243:56–70
Sobrado MA (2005) Leaf characteristics and gas exchange of the mangrove Laguncularia racemosa as affected by salinity. Photosynthetica 43:217–221
Song J, Wang BS (2015) Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot 115:541–553
Sozzani R, Maggio C, Varotto S, Canova S, Bergounioux C, Albani D, Cella R (2006) Interplay between Arabidopsis activating factors E2Fb and E2Fa in cell cycle progression and development. Plant Physiol 140:1355–1366
Storey R, Jones RGW (1979) Responses of Atriplex spongiosa and Suaeda monoica to salinity. Plant Physiol 63:156–162
Tal M, Shannon MC (1983) Salt tolerance in the wild relatives of the cultivated tomato: responses of Lycopersicon esculentum, L. Cheesmanii, L. Peruvianum, Solanum pennellii and F1 hybrids to high salinity. Aust J Plant Physiol 10:109–117
Von Willert DJ, Eller BM, Werger M, Brinckmann E, Ihlenfeldt HD (1992) Life strategies of succulents in deserts: with special reference to the namib desert. Cambridge University Press, Cambridge, England
Wang DY, Wang HY, Han B, Wang B, Guo AP, Zheng D, Liu CJ, Chang LL, Peng M, Wang XC (2012) Sodium instead of potassium and chloride is an important macronutrient to improve leaf succulence and shoot development for halophyte Sesuvium portulacastrum. Plant Physiol Bioch 51:53–62
Wenzel CL, Schuetz M, Yu Q, Mattsson J (2010) Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J 49:387–398
Wu HH (2018) Plant salt tolerance and Na+ sensing and transport. Crop J 6:215–225
Xi JJ, Chen HY, Bai WP, Yang RC, Yang PZ, Chen RJ, Hu TM, Wang SM (2018) Sodium-related adaptations to drought: new insights from the xerophyte plant Zygophyllum Xanthoxylum. Front Plant Sci 9:1678
Yanagisawa M, Poitout A, Otegui MS (2021) Arabidopsis vascular complexity and connectivity controls PIN-FORMED1 dynamics and lateral vein patterning during embryogenesis. Development 148:dev197210
Yuan F, Leng BY, Wang BS (2016a) Progress in studying salt secretion from the salt glands in recretohalophytes: how do plants secrete salt? Front Plant Sci 7:977
Yuan F, Lyu MJ, Leng BY, Zhu XG, Wang BS (2016b) The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Front Plant Sci 91:241–256
Zeng FR, Shabala S, Maksimović JD, Maksimović V, Bonales-Alatorre E, Shabala L, Yu M, Zhang GP, Živanović BD (2018) Revealing mechanisms of salinity tissue tolerance in succulent halophytes: a case study for Carpobrotus Rossi. Plant Cell Environ 41:2654–2667
Zhang M, Liang XY, Wang LM, Cao YB, Song WB, Shi JP, Lai JS, Jiang CFA (2019) A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat Plants 5:1297–1308
Zhao CZ, Zhang H, Song CP, Zhu JK, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. Innovation 1:69–109
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Funding
The work is supported by Tianshan elite program-Youth science and technology top talent program (2022TSYCCX0039), the National Natural Science Research Foundation of China (32171499), and Open Fund of Shandong Provincial Key Laboratory of Plant Stress, China.
Author information
Authors and Affiliations
Contributions
R-R.L., and T.W. wrote the paper. Q.L, L.W., and J.S. conceived the idea and led the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Communicated by Jiayin Pang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Wang, T., Li, Q. et al. The role of tissue succulence in plant salt tolerance: an overview. Plant Growth Regul 103, 283–292 (2024). https://doi.org/10.1007/s10725-024-01122-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-024-01122-4