Abstract
Auxin has a central role in determining tomato fruit growth and development, and most of its action is mediated by gibberellins (GAs). The diageotropica (dgt) mutant of tomato exhibits many physiological responses that are related to a defective auxin sensitivity. In this paper we investigated the effects of the dgt mutation on tomato gibberellin biosynthesis regulation during fruit-set and early growth of pollinated fruits. In spite of an initial accumulation of active GAs in dgt ovaries, their content is significantly reduced at later stages. Indeed, at the beginning of rapid fruit growth, dgt fruits display a lower amount of GA1 and its direct catabolite GA8. Consistently, transcripts of GA 20-oxidase genes (GA20ox1, GA20ox2, GA20ox3) are low in the mutant. Moreover, low expression of genes encoding GA catabolism enzymes (GA 2β-hydroxylases) does not lead to an increase in the amount of active GAs, supporting the hypothesis that GA 20-oxidase genes downregulation might bottleneck the synthesis of active GAs in dgt. Interestingly, exogenous GA3 application has little effect on dgt ovaries. GA3-treated fruits of the mutant are smaller than those of its wild type as a result of fewer and smaller pericarp cells. Consistently, GA3 treatment in the dgt ovaries produces negligible effects on cell endoreduplication revealed by a lower nuclear DNA content in pericarp and locular tissue cells. The lack of DELLA-mediated constraint on GA signal in the double mutant dgt pro did not cause an increase in size and weight in pollinated fruits, suggesting that GA signalling is unable to overcome the inhibition of growth caused by the dgt mutation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In tomato (Solanum lycopersicum L.), the transition from a static ovary to a growing fruit is characterised by the succession of three phases. After fruit-set, ovary cells increase in number on account of a high mitotic activity that is followed by a stage of cell expansion (Gillaspy et al. 1993). Several positive and negative hormonal cues are involved in regulating fruit growth and development (McAtee et al. 2013). Lines of evidence have shown that the increase of auxin content is one of the earliest events that trigger fruit-set (Dorcey et al. 2009; Mariotti et al. 2011). Indeed, IAA is produced in fertilised ovules and subsequently transported towards outer tissues, such as placenta and pericarp, through the coordinated action of auxin efflux and influx carriers (Pattison et al. 2014; Sorce et al., 2017).
Auxin perception and signal transduction are initiated following the auxin-driven recruitment of the auxin signalling repressors Aux/IAAs by the nuclear-localised receptor TIR1/AFB. Following the degradation of Aux/IAAs via 26S proteasome, the repression on auxin responsive genes is released making their transcription possible (Salehin et al. 2015). Spontaneous fruit-set in tomato has been obtained by altering the expression of some auxin signalling components. Indeed, when the transcription of Aux/IAA9 (SlIAA9) is suppressed with an antisense construct or, as in the entire mutant, a truncated peptide version is encoded, spontaneous fruit initiation is triggered (Wang et al. 2005; 2009; Zhang et al. 2007; Mignolli et al. 2015). Other auxin signalling elements have emerged as new players involved in tomato fruit formation. Particularly, the Auxin Response Factors 7 (SlARF7), 9 (SlARF9) 5 (SlARF5) have been shown to modulate tomato ovary cells divisions and expansion (de Jong et al. 2011; 2015; Liu et al. 2018).
Gibberellins (GAs) represent a class of plant hormones that counts 136 different structures whose biosynthesis is summarised in Fig. S1. Physiologically, GAs exert major control in organ elongation and, in certain cases, in cell divisions. In addition, GAs regulate the physiological switch between vegetative to reproductive development, pollen fertility and seed germination (Hedden and Thomas, 2012). Together with auxin, GAs actively participate in fruit growth and development (Vriezen et al. 2008; Li et al. 2020; Shinozaki et al. 2020) since exogenous application of GA3 or silencing the GA signal repressor SlDELLA trigger parthenocarpic fruit formation, whereas the inhibition of GA biosynthesis strongly hinders fruit growth (Serrani et al. 2007a, 2008; Martí et al. 2007; Carrera et al. 2012). The fact that GA metabolism and signal are modulated in response to auxin indicates that tomato fruit formation actually depends on auxin and GA crosstalk (Serrani et al. 2008; Tang et al. 2015). Indeed, genes involved in GA biosynthesis (GA 20-oxidases) and in GA catabolism (GA 2β-hydroxylases) are, respectively, induced and repressed in auxin-treated ovaries or in auxin signalling repressor mutants (Serrani et al. 2008; Mariotti et al. 2011; Mignolli et al. 2015). Similarly, in SlARF7 and SlARF5 RNA interference lines of tomato, GA responsive genes are upregulated, indicating a control of GA signalling by auxin (De Jong et al. 2011; Liu et al. 2018). Although it has been suggested that auxin acts upstream of GAs in regulating tomato fruit-set, recent pieces of evidence indicated that GAs are able to modulate auxin signalling (Hu et al. 2018; Mignolli et al. 2019).
Since its first physiological description, the tomato diageotropica (dgt) mutant has been considered an auxin insensitive mutant (Zobel 1973). Along with several auxin-related responses such as root and shoot gravitropism, hypocotyl elongation, apical dominance and lateral root formation, also primary metabolism, such as photosynthesis and respiration, is controlled by DGT (Kelly and Bradford 1986; Coenen et al. 2002; Batista-Silva et al. 2019). The DGT locus encodes a cyclophilin (LeCYP1) that travels via phloem from shoot to roots as a signalling molecule (Oh et al. 2006; Spiegelman et al. 2017). The dgt mutation has also been shown to negatively affect tomato fruit formation since it reduces seed number, fruit set rate and fruit size (Balbi and Lomax 2003). Mignolli et al. (2012) have found out that auxin signalling transduction is dramatically impaired in auxin-treated dgt fruits, confirming the positive function of DGT in auxin signal transduction in fruit. Furthermore, the DGT gene seems to be involved in mediating the auxin-induced GA biosynthesis, acting as a positive regulator (Mignolli et al. 2019).
The objective difficulty in blocking the auxin signal or biosynthesis with a pharmacological approach (Fukui and Hayashi 2018), makes the dgt mutant a valid tool to dissect the role of auxin in tomato. To the best of our knowledge, no information is available about the effect of a reduced auxin perception on GA metabolism in fruits. Analysis of endogenous GAs and GA metabolism gene expression have allowed us to demonstrate that DGT plays a role as a positive modulator of GA biosynthesis. In addition, since neither exogenous application of GA3 nor a constitutive GA signal are capable of restoring a normal fruit phenotype in dgt, we suggest that DGT might also be implicated in processes that override the role of GAs in fruit growth and development.
Materials and methods
Plant material and ovary treatments
Seeds of tomato (Solanum lycopersicum L.) cv. Ailsa Craig (AC) were obtained from the Tomato Genetics Resource Center (University of California, Davis, CA, USA). Seeds of diageotropica (dgt) mutant, backcrossed into AC genetic background, were donated by Dr. C. Coenen (Allegheny College, Meadville, PA, USA). Double mutant dgt pro was obtained as described by Mignolli et al. (2019). Some phenotypical characteristics of dgt pro are reported in supplemental material (Fig. S1 and Table S1).
Plants were grown under greenhouse conditions as reported by Mignolli et al. (2012). Only four flowers per truss were left in order to limit fruit competition. Flowers were emasculated at pre-anthesis (one day before anthesis) which is equivalent to 0 DAP (days after pollination) in order to prevent self-pollination. Once emasculated, flowers were manually pollinated using pollen from AC plants for both genotypes. Ovaries/fruits were collected at different time points from pollination (from 0, to 8 DAP). Samples were immediately stored at − 70 °C up to analyses.
Similar to Serrani et al. (2007b), treatments with the gibberellin biosynthesis inhibitor LAB198999 (3,5-dioxo-4-butyryl-cyclohexane carboxylic acid ethyl ester; BASF) were performed by applying on 2 DAP ovaries 10 μl of 2 mM LAB198999 dissolved in 1% ethanol and 0.1% Tween 20 solution. An equal volume of solvent was used as mock. Gibberellin application was carried out on emasculated flowers at pre-anthesis with 10 µl of 0.2 μg μl−1 GA3 (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 1% ethanol and 0.1% of Tween 20 solution. Equal volume of solvent was used as mock treatment.
Quantification of endogenous GAs
Endogenous GAs were determined in pollinated ovaries and fruits of AC and dgt from 0 to 8 DAP. Extraction, purification and GAs determination through GC–MS/MS were performed according to Mariotti et al. (2011).
Histology and ploidy level determination
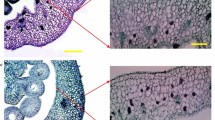
Histological analysis was carried out on fruits treated with GA3 or a mock solution, according to Gonzalez and Cristóbal (1997). Fruits of AC and dgt were sampled after 4 days from the treatment, immediately fixed in a formaldeyde:ethanol:acetic acid (4:50:5 v/v) and dehydrated in xylene:ethanol solution series. Transversal sections 10 µm thick were stained with safranin-fast green. Sections were observed with a Leica DM LB2 microscope and microphotographs were taken with a Leica ICC50HD digital camera (Leica, Wetzlar, Germany).
Nuclear DNA content was assessed in pericarp and locular tissue of GA3-treated fruits after 10 days from treatment according to Mignolli et al. (2012). Mean of C value (MCV) was calculated according to Serrani et al. (2007a).
RNA extraction and gene expression analysis
Total RNA extraction, purification and conversion into cDNA from ovaries and fruits were performed according to Mignolli et al (2012). Expression analysis of GA metabolism genes was carried out using TaqMan Universal PCR Master Mix (Applied Biosystems) by using specific probes and primers as reported by Mariotti et al. (2011). Transcript levels of all genes were normalised with the expression of SlEF1α. Each sample derived from a pool of at least five fruits. Gene accession numbers and primer sequences were reported in Mignolli et al. (2015).
Statistical analysis
Analysis of variance (one-way ANOVA, Tuckey post-test) and Student t-test were performed using the software GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
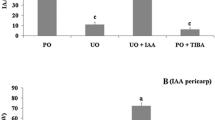
Pollinated fruits of dgt grew less and had lower fresh weight compared with pollinated ovaries of AC. From 6 to 8 DAP, fruit weight increased 7.5 times in AC but only 3.2 times in dgt (Fig. 1a). In order to assess the role of GAs in dgt fruit growth, we treated pollinated ovaries with the inhibitor LAB198999, which is known to block the last steps of GA biosynthesis (Rademacher 2000). While LAB198999 application determined a significant reduction of fruit weight in AC (more than 2.5 times), no statistically significant difference was observed between mock- and LAB-treated dgt fruits (Fig. 1b).
Weight of fruits of AC and dgt collected after 8 days from manual pollination (a). Each point represents a mean ± SEM of 20 to 90 ovaries/fruits. Asterisks indicate significant differences between AC and dgt (Student’s t-test, P < 0.05). Effect of the GA biosynthesis inhibitor LAB198999 on pollinated AC and dgt fruit growth (b). Manual pollination was carried out at pre-anthesis stage and application of LAB198999 or mock solution was performed 2 days later. Fruits were harvested at 8 days from pollination (or 6 days from mock/LAB198999 treatment). Data are means of 20 fruits ± SEM. Different letters indicate statistical differences according to one-way ANOVA Tuckey post-test (P < 0.05). Endogenous GA content in pollinated AC and dgt fruits (c–h). Samples were collected from 1 day before anthesis (0 DAP) to 8 days after pollination. Analyses were carried out through GC–MS/MS as reported in Materials and Methods. Data are means ± SEM (n = 3). Asterisks indicate significant differences between AC and dgt (Student’s t-test, P < 0.05)
To establish whether the dgt mutation alters GA metabolism, concentrations of GAs from the early 13-hydroxilation pathway (GA19, GA20, GA1, GA3, GA8 and GA29), which is the most representative pathway in tomato (Fos et al. 2000; Serrani et al. 2007a, b; Garcia-Hurtado et al. 2012), were quantified. After an initial increase, GA19 content steadily declined in dgt. However, levels of GA19 from 4 to 8 DAP were higher in dgt than in AC (Fig. 1c). Levels of GA20 did not change substantially in dgt in comparison to AC (Fig. 1d). In both genotypes, they decreased at 2 DAP to rise again at 4 DAP. Singularly, the content of GA1 and GA3 in pre-anthesis ovaries and at 2 DAP were higher in dgt than in AC. However, levels of GA1 in dgt were lower than in AC fruits at 8 DAP (Fig. 1e, f). Levels of GA8 (the GA1 catabolite) were high during the first 2 days from pollination in dgt but, at 6 to 8 DAP, its levels were 2 and 4 times lower than in AC (Fig. 1g). The content of GA29 (the GA20 inactive form) increased in both genotypes after pollination; however, a sharp decrease after 4 DAP was observed in AC. The amount of GA29 did not decrease and was significantly higher than in AC in fruits of dgt at 6 and 8 DAP (Fig. 1h).
Real time PCR analysis was performed in order to establish whether the dgt mutation alters the expression of GA metabolism genes. The expression of SlGA20ox1 and SlGA20ox3 was similar in both genotypes from 0 to 4 DAP but, following, the expression of both genes in dgt failed to increase. On the contrary, the expression of SlGA20ox1and SlGA20ox3 in AC abruptly increased after 8 and 6 DAP, respectively (Fig. 2a, c). SlGA20ox2 gene showed a two-fold increase in AC fruits between 2 and 6 DAP but its expression was barely detectable in dgt fruits throughout the 8 days (Fig. 2b). In AC and dgt, SlGA3ox1 and SlGA3ox2 transcripts peaked in ovaries at pre-anthesis stage but plummeted thereafter. Both genes were relatively more expressed in dgt at 0, 2 e 4 DAP but reached a similar level to AC at 6 DAP. In AC, the expression of SlGA3ox1 and SlGA3ox2 from 2 to 8 DAP was kept extremely low (Fig. 2d, e). Among the genes encoding GA 2β-hydroxylases, the expression pattern of SlGA2ox1 in dgt was slightly higher than AC after 2 and 4 DAP (Fig. 2f). On the contrary, while SlGA2ox2 transcript levels were higher in dgt ovaries at pre-anthesis stage and sharply decreased after pollination, they remained higher than in AC from 2 to 6 DAP (Fig. 2g). SlGA2ox3, SlGA2ox4, and SlGA2ox5 were downregulated soon after pollination in both genotypes; yet, an upsurge in their expression was observed in AC at 6 and 8 DAP (Fig. 2h–j).
GA biosynthesis genes expression in developing AC and dgt fruits (a–e). Relative expression of GA 20-oxidase (a–c) and GA 3β-hydroxylase (d, e) gene family were measured in ovaries and fruits from 0 to 8 DAP. Relative expression of GA catabolism genes, GA 2β-hydroxylases (f–j). Transcript levels were normalized to the SlEF1α expression. Value at 0 DAP in AC was set to 1 for each gene, and all other values were calculated relative to this. Data are mean ± SD (n = 3). Asterisks indicate significant differences between AC and dgt (Student’s t-test, P < 0.05)
With the aim of testing dgt ovary responsiveness to active GAs, we treated emasculated unpollinated ovaries with an optimal dose of GA3 and we measured fruit weight and some cellular parameters. GA3 treatment triggered fruit growth in both genotypes, albeit dgt fruits were smaller and weighted less than AC ones (Fig. 3a–e). Histological observations showed that GA3-treated fruits of dgt had thinner pericarps as a result of fewer cell layers and reduced cell size (Fig. 3f–h). In order to assess whether the dgt mutation also altered ploidy level in GA3-treated fruits, flow cytometry analysis of pericarp and locular tissue was performed. The effect of GA3 on cell endoreduplication in dgt was less marked than in AC (Fig. 3i–l) since no 32 C nuclei were detected in dgt pericarp and the MCV values in pericarp and locular tissues were significantly lower than in AC (Fig. 3j, l).
Microphotographs of transversal sections of pericarps of mock- and GA3-treated fruits of AC (a, c) and dgt (b, d). Fruits were emasculated at pre-anthesis and treated either with a mock solution or with 2 µg GA3. Fruits were collected after 4 days from the treatment. Fruit weight (e), number of pericarp cell layers (f), pericarp thickness (g) and pericarp cell size (h) in fruit of AC and dgt treated with mock or 2 µg ovary−1 GA3. Each bar represents the mean of 4 biological replicates ± SEM. Different letters indicate statistical differences according to one-way ANOVA Tuckey post-test (P < 0.05). Ploidy level in GA3-treated fruits of AC (i, j) and dgt (k, l). Nuclear DNA content was analysed in pericarp (i, k) and in locular tissue (j, l) of fruits after 10 days from GA3 treatment. Mean of C value (MCV) was calculated from measurements performed on a pool of 5 fruit in two independent experiments ± SEM
PROCERA (PRO) is the tomato DELLA protein and its mutation pro, confers a constitutive activation of the GA signal (Jasinski et al. 2008). We sought to determine whether the dgt fruit phenotype could be reverted by the pro mutation. Analysis of some vegetative traits of the double mutant dgt pro indicates that plant height and mean internode length were only partially rescued while stem diameter, number of leaves, leaf area, leaf perimeter and leaf pigments content did not differ from the dgt parent (Table S1). Notably, leaf shape of dgt pro displayed the reduced lobing of the main leaflets typical of pro leaf phenotype (Fig. S2). As far as fruit phenotype is concerned, 30-day-old pollinated fruits of the double mutant have similar ellipsoid shape as in pro but both size and weight are statistically identical to dgt (Fig. 4).
Pollinated AC (a), dgt (b), pro (c) and dgt pro (d) fruits collected after 30 days from manual pollination. Weight of fruits after 30 days after pollination (e). Data are means of 20–30 fruits ± SEM. Different letters indicate statistical differences according to one-way ANOVA Tuckey post-test (P < 0.05)
Discussion
Fruit growth and development in tomato depend on a tightly regulated interplay between auxin and GAs (Koshioka et al. 1994; Serrani et al. 2007b, 2008; Mariotti et al. 2011; Hu et al. 2018; Mignolli et al. 2019). Our data confirmed that the dgt lesion dramatically alters fruit growth and development (Fig. 2a; Balbi and Lomax 2003; Mignolli et al. 2012). We tested the hypothesis that the reduced auxin sensitivity in dgt adversely affects GA biosynthesis and hence fruit growth. However, when ovaries of dgt were treated with the GA biosynthesis inhibitor LAB198999, fruit growth was practically unaffected (Fig. 1b). Since GA biosynthesis inhibition has stronger effects on parthenocarpic mutants whose fruits growth largely depend on GAs (Fos et al. 2000, 2001; Olimpieri et al. 2007), our findings could indicate that, in pollinated dgt fruits, GAs are either synthesised in low concentrations or/and that they have little effect on fruit growth. Surprisingly, the early accumulation of high amounts of GA1 and GA3 in dgt ovaries (Fig. 1e, f) results neither in spontaneous fruit set nor in steeper growth rate (Fig. 1a), indicating that the dgt ovary might not sense this initial peak of active GAs. Consistently, exogenous GA3 produces smaller fruits with fewer cells in the pericarp in dgt (Fig. 3a–h) compared with AC. Since fruit size is highly correlated with pericarp cell nuclear DNA content (Chevalier et al. 2011), smaller cell area in GA3-treated dgt fruits reflects their lower ploidy levels (Fig. 3i–l).
Our data showed that DGT is actively involved in regulating GA metabolism in pollinated tomato fruits. Despite the early upregulation of GA 3β-hydroxylase genes after pollination, which may account for the early high content of GA1 and GA3, the overall GA biosynthesis in dgt seems to be limited by the low induction of GA 20-oxidases (Fig. 2a–e). Growing evidence reveals a role for GA 20-oxidases as one of the rate limiting steps in active GA synthesis in tomato fruits (Serrani et al. 2007b; Mariotti et al. 2011; García-Hurtado et al. 2012). In fact, some authors indicated that a concerted action of GA 20-oxidases is necessary to regulate the growth of tomato fruits (Xiao et al. 2006; Olimpieri et al. 2007; 2010). Although no significant change in GA20 content was detected in dgt (Fig. 1d), its precursor GA19, was not depleted as fast as in AC (Fig. 1c), which indicates a slower conversion rate into GA20. In addition, SlGA20ox1 and SlGA20ox3 are not upregulated in dgt at 6 and 8 DAP, and SlGA20ox2 shows no expression whatsoever (Fig. 2a–c). The presence of an auxin-responsive element in the tomato GA20ox1 gene promoter (Martí et al. 2010) and the little induction of GA20ox1 in auxin-treated dgt fruits (Mignolli et al. 2019) support the idea that DGT positively regulates the expression of GA 20-oxidases genes.
Along with GA biosynthesis, endogenous GAs homeostasis is controlled by GA catabolic processes (Thomas et al. 1999). Unlike AC, dgt fruits accumulate significantly lower quantities of GA8 but higher amounts of GA29 at 6 and 8 DAP (Fig. 1g, h). Being GA8 the record of the earlier GA1 (Coles et al. 1999) and GA29 the inactive form of GA20, we can infer that the GA flux through GA1 could be diminished in the mutant at the beginning of the rapid fruit growth phase. Since the moderate induction of SlGA2ox3, SlGA2ox4 and SlGA2ox5 (Fig. 2h–j) in dgt at 6 to 8 DAP does not contribute to increase the level of active GAs, we believe that this could be the effect of a feedforward regulation in response to a relatively low active GA1 supply. Indeed, in Arabidopsis, active GAs upregulated the expression of AtGA2ox1 and AtGA2ox2, whereas their deficiency promoted the transcription of PsGA2ox1 and PsGA2ox2 in pea shoots according to a positive feedforward control (Thomas et al. 1999; Elliott et al. 2001).
In conclusion, our data indicate that the dgt mutation slows down active GAs biosynthesis in pollinated fruits by preventing the upregulation of the GA 20-oxidases and, at the same time, limits the effect of active GAs (i.e. GA3) on fruit growth. However, it seems unlikely that these findings may account for the reduced fruit size in the mutant. If the phenotype of the dgt fruit was the result of an attenuated GA signalling, we would have expected them to grow more in presence of a constitutively active GA signalling. In fact, the lack of the protein DELLA in the double mutant dgt pro does not result in bigger fruits than in dgt (Fig. 4). This leads us to think that, regardless of the abundance of active GAs or GA responsiveness, the GA-induced response in tomato ovaries may only be partial in the dgt mutant. Interestingly, fruit respiration and sugar metabolism are severely affected in dgt resulting in impaired cell growth (Batista-Silva et al. 2019; 2022). It is therefore conceivable that primary metabolism constraint in dgt overrules the effect of active GAs or GA signalling on fruit growth.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Balbi V, Lomax TL (2003) Regulation of early tomato fruit development by the diageotropica gene. Plant Physiol 131:186–197
Batista-Silva W, Medeiros DB, Rodrigues-Salvador A, Daloso DM, Omena-Garcia RP, Oliveira FS, Pino LE, Pereira Peres LE, Nunes-Nesi A, Fernie AR, Zsögön A, Araújo WL (2019) Modulation of auxin signalling through DIAGETROPICA and ENTIRE differentially affects tomato plant growth via changes in photosynthetic and mitochondrial metabolism. Plant Cell Environ 42:448–465
Batista-Silva W, Carvalho de Oliveira A, Martins AO, Siqueira JA, Rodrigues-Salvador A, Omena-Garcia RP, Medeiros DB, Peres LEP, Ribeiro DM, Zsögön A, Fernie AR, Nunes-Nesi A, Araújo WL (2022) Reduced auxin signalling through the cyclophilin gene DIAGEOTROPICA impacts tomato fruit development and metabolism during ripening. J Exp Bot 73:4113–4128
Carrera E, Ruiz-Rivero O, Peres LEP, Atares A, Garcia-Martinez JL (2012) Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol 160:1581–1596
Chevalier C, Nafati M, Mathieu-Rivet E, Bourdon M, Frangne N, Cheniclet C et al (2011) Elucidating the functional role of endoreduplication in tomato fruit development. Ann Bot 107:1159–1169
Coenen C, Bierfreund N, Lüthen H, Neuhaus G (2002) Developmental regulation of H+-ATPase-dependent auxin responses in the diageotropica mutant of tomato (Lycopersicon esculentum). Physiol Plantarum 114:461–471
Coles JP, Phillips AL, Crocker SJ, García-Lepe R, Mervyn JL, Hedden P (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J 17:547–556
de Jong M, Wolters-Arts M, García-Martínez JL, Mariani C, Vriezen WH (2011) The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J Exp Bot 62:617–626
de Jong M, Wolters-Arts M, Schimmel BCJ, Stultiens CLM, de Groot PFM, Powers SJ et al (2015) Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development. J Exp Bot 66:3405–3416
Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332
Elliott RC, Ross JJ, Smith JJ, Lester DR, Reid JB (2001) Feed-forward regulation of gibberellin deactivation in pea. J Plant Growth Regul 20:87–94
Fos M, Nuez F, García-Martínez JL (2000) The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol 122:471–480
Fos M, Proaño K, Nuez F, Garcia-Martinez JL (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plantarum 111:545–550
Fukui K, Hayashi KI (2018) Manipulation and sensing of auxin metabolism, transport and signaling. Plant Cell Physiol 59:1500–1510
García-Hurtado N, Carrera E, Ruiz-Rivero O, López-Gresa MP, Hedden P, Gong F, García-Martínez JL (2012) The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J Exp Bot 63:5803–5813
Gillaspy G, Ben-David H, Gruissem W (1993) Fruit: a developmental perspective. Plant Cell 5:1439–1451
González AM, Cristóbal CL (1997) Anatomía y ontogenia de semillas de Helicteres lhotzkyana (Sterculiaceae). Bonplandia 9:287–294
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444:11–25
Hu J, Israeli A, Ori N, Sun TP (2018) The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell 30:1710–1728
Jasinski S, Tattersall A, Piazza P, Hay A, Martinez-Garcia JF, Schmitz G et al (2008) PROCERA encodes a DELLA protein that mediates control of dissected leaf form in tomato. Plant J 56:603–612
Kelly MO, Bradford KJ (1986) Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol 82:713–717
Koshioka M, Nishijima T, Yamazaki H, Liu Y, Nonaka M, Mander LN (1994) Analysis of gibberellins in growing fruits of Lycopersicon esculentum after pollination or treatment with 4- chlorophenoxyacetic acid. J Hortic Sci 69:171–179
Li Y, Sun M, Xiang H, Meng S, Wang B, Qi M, Li T (2020) Transcriptome analysis reveals regulatory effects of exogenous gibberellin on locule number in tomato. Plant Growth Regul 91:407–417
Liu S, Zhang Y, Feng Q, Qin L, Pan C, Lamin-Samu AT et al (2018) Tomato AUXIN RESPONSE FACTOR 5 regulates fruit set and development via the mediation of auxin and gibberellin signaling. Sci Rep. https://doi.org/10.1038/s41598-018-21315-y
Mariotti L, Picciarelli P, Lombardi L, Ceccarelli N (2011) Fruit-set and early fruit growth in tomato are associated with increase in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J Plant Growth Regul 30:405–415
Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A (2007) Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J 52:865–876
Martí E, Carrera E, Ruiz-Rivero O, García-Martínez JL (2010) Hormonal regulation of tomato gibberellin 20-oxidase1 expressed in Arabidopsis. J Plant Physiol 167:1188–1196
McAtee P, Karim S, Schaffer R, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci. https://doi.org/10.3389/fpls.2013.00079
Mignolli F, Mariotti L, Lombardi L, Vidoz ML, Ceccarelli N, Picciarelli P (2012) Tomato fruit development in the auxin-resistant dgt mutant is induced by pollination but not by auxin treatment. J Plant Physiol 169:1165–1172
Mignolli F, Vidoz ML, Mariotti L, Lombardi L, Picciarelli P (2015) Induction of gibberellin 20-oxidases and repression of gibberellin 2β-hydroxylases in unfertilized ovaries of entire tomato mutant, leads to accumulation of active gibberellins and parthenocarpic fruit formation. Plant Growth Regul 75:415–425
Mignolli F, Vidoz ML, Picciarelli P, Mariotti L (2019) Gibberellins modulate auxin responses during tomato (Solanum lycopersicum L.) fruit development. Physiol Plantarum 165:768–779
Oh K, Ivanchenko MG, White TJ, Lomax TL (2006) The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signalling. Planta 224:133–144
Olimpieri I, Siligato F, Caccia R, Mariotti L, Ceccarelli N, Soressi GP et al (2007) Tomato fruit-set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta 226:877–888
Olimpieri I, Caccia R, Picarella ME, Pucci A, Santangelo E, Soressi GP et al (2010) Constitutive co-suppression of the GA 20-oxidase1 gene in tomato leads to severe defects in vegetative and reproductive development. Plant Sci 180:496–503
Pattison RJ, Csukasi F, Catalá C (2014) Mechanisms regulating auxin action during fruit development. Physiol Plantarum 151:62–72
Rademacher W (2000) Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol 51:501–531
Salehin M, Bagchi R, Estelle M (2015) SCFTIR1/AFB-Based auxin perception: mechanism and role in plant growth and development. Plant Cell 27:9–19
Serrani JC, Fos M, García-Martínez AA, JL, (2007a) Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of tomato. J Plant Growth Regul 26:211–221
Serrani JC, Sanjuán R, Ruiz-Rivero O, Fos M, García-Martínez JL (2007b) Gibberellin regulation of fruit set and growth in tomato. Plant Physiol 145:246–257
Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56:922–934
Shinozaki Y, Beauvoit BP, Takahara M, Hao S, Ezura K, Andrieu MH et al (2020) Fruit setting rewires central metabolism via gibberellin cascades. PNAS 117:23970–23981
Sorce C, Montanaro G, Bottega S, Spanó C (2017) Indole-3-acetic acid metabolism and growth in young kiwifruit berry. Plant Growth Regul 82:505–512
Spiegelman Z, Omer S, Mansfeld BN, Wolf S (2017) Function of Cyclophilin1 as a long-distance signal molecule in the phloem of tomato plants. J Exp Bot 68:953–964
Tang N, Deng W, Hu G, Hu N, Li Z (2015) Transcriptome profiling reveals the regulatory mechanism underlying pollination dependent and parthenocarpic fruit set mainly mediated by auxin and gibberellin. PLoS ONE. https://doi.org/10.1371/journal.pone.0125355
Thomas SG, Hedden PAL, P, (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. PNAS 96:4698–4703
Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177:60–76
Wang P, Heitman J (2005) The cyclophilins. Genome Biol 6:226–231
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F et al (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Xiao J, Li H, Zhang J, Chen R, Zhang Y, Ouyang B et al (2006) Dissection of GA 20-oxidase members affecting tomato morphology by RNAi-mediated silencing. Plant Growth Regul 50:179–189
Zhang J, Cheng R, Xiao J, Quian C, Wang T, Li H, Ouyang B, Ye Z (2007) A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. J Plant Res 120:671–678
Zobel RW (1973) Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol 52:385–389
Acknowledgements
We would like to thank Jean Pierre Solani and Anatoli Gety for their technical assistance.
Funding
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (Grant number PICT 2018-02960).
Author information
Authors and Affiliations
Contributions
FM and MLV conceived the experiments. LM performed endogenous gibberellins analyses while FM carried out gene expression and histological analyses. FM and MLV wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Vijay Pratap Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mignolli, F., Mariotti, L. & Vidoz, M.L. Reduced gibberellin biosynthesis and response in fruits of the auxin insensitive diageotropica tomato mutant. Plant Growth Regul 99, 505–513 (2023). https://doi.org/10.1007/s10725-022-00921-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00921-x