Abstract
Delphinium flowers are highly sensitive to ethylene and its sepals abscise during senescence in association with an increase in 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO) activities and ethylene production in gynoecium and receptacle. Three ACS genes (DgACS1, DgACS2, and DgACS3) and three ACO genes (DgACO1, DgACO2, and DgACO3) of D. grandiflorum cv. Super Grand Blue were cloned. To investigate the contribution of these genes to ethylene production, their expression in the gynoecium and receptacle was analyzed during natural senescence and after ethylene exposure and pollination. Ethylene production in the receptacle significantly increased, whereas that in the gynoecium exhibited only a slight increase during natural senescence. The transcript levels of ACS and ACO in these organs, excluding those of DgACS2 in the receptacle, increased during senescence. Exposure to ethylene accelerated sepal abscission and increased ethylene production more markedly in the receptacle than in the gynoecium. DgACS1 transcript levels in the gynoecium and DgACS2 and DgACO3 transcript levels in the receptacle increased after ethylene exposure. Pollination accelerated sepal abscission and increased ethylene production in the gynoecium and receptacle. Additionally, it slightly affected ACS and ACO transcript levels in the gynoecium, whereas DgACO3 transcript levels in the receptacle markedly increased. These results reveal that ACS and ACO expression is differently regulated in the gynoecium and receptacle. Furthermore, some of these genes are upregulated by pollination only in the receptacle, indicating the significance of the receptacle in ethylene biosynthesis associated with sepal abscission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phytohormone ethylene plays an important role in various stages of plant growth and development, including senescence. Exposure to ethylene accelerates senescence in the flowers of many species, and the primary symptom of ethylene-induced senescence is petal wilting or petal (sepal) abscission (Woltering and van Doorn 1988). The petals of many ethylene-sensitive flowers, such as carnation (Nichols 1966) and Eustoma grandiflorum (Ichimura et al. 1998), wilt in response to ethylene exposure. In Digitalis (Stead and Moore 1983), Pelargonium (Hilioti et al. 2000), and Torenia flowers (Goto et al. 1999), petals abscise upon exposure to ethylene. In these flowers, ethylene production in the gynoecium or pistil increases with the progression of flower senescence. However, this finding is not observed in the petals (Stead and Moore 1983; Goto et al. 1999; Hilioti et al. 2000). Therefore, increased ethylene production in the gynoecium has been considered to cause petal abscission.
Pollination accelerates petal wilting in many ethylene-sensitive flowers, including carnation (Jones and Woodson 1997), petunia (Gillisen and Hoekstra 1984; Whitehead et al. 1984), and gentian (Shimizu-Yumoto and Ichimura 2012). It also induces petal abscission in Digitalis (Stead and Moore 1979, 1983), Pelargonium (Clark et al. 1997), and Torenia (Goto et al. 1999). Pollination induces climacteric-like increases in ethylene production, and ethylene inhibitors suppress pollination-induced senescence in many plants, including orchids (O’Neill et al. 1993; Porat et al. 1995), E. grandiflorum (Ichimura and Goto 2000), and Torenia (Goto et al. 1999), indicating that pollination-induced petal senescence is regulated by ethylene.
The ethylene biosynthesis pathway of higher plants has been established. 1-aminocyclopropane-1-carboxylic acid (ACC), the precursor of ethylene, is produced by the conversion of S-adenosyl-l-methionine by ACC synthase (ACS). ACC is converted into ethylene, carbon dioxide, and HCN by ACC oxidase (ACO). In plants, ACS is generally considered to be a rate-limiting enzyme in ethylene biosynthesis because ACO activity is constitutive in many species (Yang and Hoffman 1984; Kende 1993). However, ACO is important for ethylene biosynthesis in some examples, including fruit maturation (Nakatsuka et al. 1998) and flower senescence (Tang et al. 1994). In cut carnation flowers, the biosynthesis of ethylene is regulated by the activities of ACS and ACO (Woodson et al. 1992; Woltering et al. 1993; Lee et al. 1997). ACS and ACO are encoded by multi-gene families (Argueso et al. 2007; Lin et al. 2009), and these genes are differently regulated in response to developmental and environmental factors (Lin et al. 2009). Expression analyses of ACS and ACO in the floral organs of carnation (Jones and Woodson 1997, 1999; Jones 2003; Naiang et al. 2021; Norikoshi et al. 2022), petunia (Tang et al. 1994), tomato (Llop-Tous et al. 2000), and rose (Xue et al. 2008) have been performed. In carnation flowers, three ACS genes, namely, DcACS1, DcACS2, and DcACS3, have been cloned, and their expression is differentially regulated (Jones and Woodson 1999). In tomato flowers, SlACS2 is upregulated by pollination (Llop-Tous et al. 2000). However, few studies have analyzed ACS and ACO expression in flowers displaying petal abscission. In Pelargonium, ACS and ACO expression has only been semi-quantified using RNA gel blot analysis (Clark et al. 1997).
Delphinium has long spikes with flowers of various colors, such as white, blue, and purple. Delphinium consists of more than 300 species, and D. elatum, D. grandiflorum, and D. × belladonna, which is a hybrid of D. elatum and D. grandiflorum, are widely produced as ornamental plants. Instead of D. × belladonna, which was mainly produced approximately a decade ago, D. grandiflorum is widely produced for use as cut flowers. Delphinium flowers are sensitive to ethylene. Their sepals rapidly abscise upon ethylene exposure (Woltering and van Doorn 1988; Ichimura et al. 2000), which is accompanied by signs of programmed cell death (Yamada et al. 2007). In Delphinium, sepals and petals are directly connected to the receptacle and not the gynoecium. Thus, ethylene produced by the receptacle should directly induce sepal abscission (Ichimura et al. 2009). Ethylene production in the gynoecium and receptacle increases during flower senescence but that in other floral organs, including petals and sepals, does not increase (Ichimura et al. 2009). ACS and ACO activity in the gynoecium and receptacle increases during flower senescence (Ichimura et al. 2009). Although genes for ethylene receptors and signal transduction components have been identified in D. elatum (Kuroda et al. 2003, 2004) and D. × belladonna (Tanase and Ichimura 2006), ACS and ACO of Delphinium have not yet been cloned. Previously, Ichimura et al. (2009) highlighted the importance of the receptacle in sepal abscission during natural senescence. As observed in ethylene-sensitive flowers of many plants (van Doorn and Stead 1997), exogenous ethylene and pollination may accelerate sepal abscission in D. grandiflorum. Therefore, the quantification of ACS and ACO expression in the gynoecium and receptacle during natural senescence as well as after exposure to ethylene and pollination will elucidate the molecular mechanisms underlying ethylene biosynthesis in these organs associated with sepal abscission.
In the present study, we cloned ACS and ACO and determined their nucleotide sequences in D. grandiflorum. Furthermore, we investigated their expression in the gynoecium and receptacle during natural senescence, after exposure to ethylene, and after pollination in cut D. grandiflorum flowers.
Materials and methods
Plant materials
Delphinium grandiflorum cv. Super Grand Blue (Miyoshi, Tokyo, Japan) was grown in a greenhouse under natural day length conditions with temperatures ranging from 15 to 25 °C. On the day of flower opening, the anthers were removed, and individual flowers were covered with paper bags to prevent pollination. Flowers with peduncles of 3 cm in length were cut from the middle region of the flower spikes. D. grandiflorum flowers are protandrous, and the gynoecium matured approximately 4 days after emasculation. Next, the flowers were cut from the plant and transported to the laboratory. Cut peduncles were placed in a vessel with distilled water within 1 h of harvest and used for all subsequent experiments. Unless otherwise stated, the cut flowers were kept under a 12-h photoperiod at 23 °C, 70% relative humidity, and 10 µmol m− 2 s− 1 irradiance achieved using cool-white fluorescence lamps.
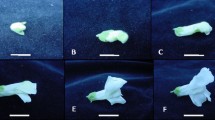
The morphology of D. grandiflorum flowers is shown in Fig. 1. The spike features approximately 10 flowers (Fig. 1A), and petals are lacking in the flower, unlike other Delphinium species (Fig. 1B). Its gynoecium is covered with stamens (Fig. 1C). The receptacle lies below the gynoecium, and the sepals are attached to the receptacle (Fig. 1D).
Ethylene treatment
The cut flowers placed in individual vessels containing distilled water were transferred to a 70-L transparent acrylic box fitted with a septum, through which ethylene was introduced to achieve a concentration of 10 µL L− 1. The box was kept at 23 °C under dark conditions. The flowers were removed from the box 4, 8, 12, 16, 20, and 24 h after ethylene exposure and used for ethylene production assessment and RNA extraction.
Pollination
The flowers were pollinated with fresh pollen collected from the same cultivar and kept under the aforementioned environmental conditions.
Observation of pollen tube growth
The gynoecia were removed from the pollinated flowers 6, 12, 24, or 48 h after pollination and immersed in FAA solution [formalin, 80% ethanol, acetic acid, 1:8:1 (v/v)]. Next, gynoecia were macerated in 8 M NaOH for 24 h, rinsed with tap water for 1 h, and stained with 0.1% aniline blue in 0.1 M K3PO4. The pollen tubes were observed microscopically under incident ultraviolet illumination.
Measurement of ethylene production
Some flowers experienced sepal abscission during natural senescence on day 5. Sepals were abscised in most of the flowers 24 h after ethylene treatment or pollination. Abscised sepals were not used for ethylene measurements. The flowers were individually placed 20-mL Erlenmeyer flasks (23.5 mL), whereas the gynoecia and receptacles were individually placed in test tubes (14.8 mL). The Erlenmeyer flasks and test tubes were sealed and maintained at 23 °C. One hour later, a 1-mL gas sample was withdrawn using a syringe and used to determine ethylene concentration using a gas-chromatograph (GC-14B, Shimadzu, Kyoto, Japan) equipped with an alumina column and a flame ionization detector. The results of ethylene production were expressed in terms of fresh weight.
RNA extraction
Frozen gynoecia and receptacles (0.1 g fresh weight) were powdered using liquid nitrogen and Shake Master Auto (Bio medical science, Tokyo, Japan). Next, 450 µL of RLT buffer containing 1% 2-mercaptoethanol was added to the obtained powder. The resulting mixture was incubated at 56 °C for 1 min. Total RNA was extracted using RNeasy Plant Mini kit (QIAGEN, Hilden, Germany) according to the manufacture’s instruction, with some modifications.
First strand cDNA and PCR amplification
Synthesis of cDNA was carried out using random hexamer primers and the Invitrogen SuperScript III first-strand synthesis system for RT-PCR (Thermo Fisher Scientific, Waltham, MA, USA). To clone the partial-length of the target cDNA, degenerate primer pairs were designed based on highly conserved regions of the ACS, ACO, or Actin genes: for ACS, 5ʹ-CARATGGGIYTIGCIGARAAYCA-3ʹ (forward) and 5ʹ-GYICCIARIGGRTTIGAIGGRTT- 3ʹ or 5ʹ- CAIAYICKRAACCAICCIGSYTC-3ʹ (reverse); for ACO, 5ʹ-GCITGYSAIAAYTGGGGGTT-3ʹ (forward) and 5ʹ-CCICCIGCRTCIGTRTGIGC-3ʹ (reverse); for Actin, 5ʹ-TGGGAYGAYATGGARAARATHTGG-3ʹ (forward) and 5ʹ-CCDATIGTDATIACYTGICC-3ʹ (reverse). PCR reactions were performed with Ex Taq (Takara bio, Kusatsu, Japan) and cycled 30 times at 94 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min. PCR products were separated electrophoretically onto 1% (w/v) agarose gel, stained with GelRadTM (Biotium, Fremont, CA, USA) and candidate bands were excised. The excised bands were purified with a Wizard Plus SV Minipreps DNA Purification System (Promega, San Luis Obispo, CA, USA) and subcloned into the pGEM-TeasyVecter (Promega) according to the manufacture’s instructions.
To clone the 3′ and 5′ ends of the target cDNA, RACE-PCR was performed using 3′-Full RACE Core Set (Takara bio) and CapFishing Full-Length cDNA Premix Kit (Seegene, Seoul, Korea). Specific primers were designed based on the sequence data obtained from the partial-length of the target cDNA (Table S1). PCR products were purified and sub-cloned into the pGEM-T Easy Vecter. The full length cDNA was cloned and the DNA sequence was confirmed using specific primers.
Determination of nucleotide sequence
DNA Sequencing was performed using the Applied Biosystems ABI Prism DNA sequencer (Model 377; Thermo Fisher Scientific) and Applied Biosystems Big Dye Terminator v.3.1 Cycle Sequencing Kit (Thermo Fisher Scientific), employed with both T7HT and M3RP universal primers. Sequence analysis was performed using ClustalW2 (European Bioinformatics Institute, http://www.ebi.ac.uk/Tools/msa/clustalw2/). Homology analysis was performed by comparing the sequenced clones with the existing translated DNA in the database of the National Center for Biotechnology Information BLAST algorithm (http://www.ncbi.nlm.nih.gov/nucleotide/). Phylogenetic analysis was performed using Phylogeny.fr (http://www.phylogeny.fr/).
Preparation of total RNA and real-time RT-PCR
The gynoecia and receptacles were collected from three individual flowers. Total RNA was isolated using RNeasy Plant Mini kit (QIAGEN). Genomic DNA included in the RNA sample was digested using the RNase-free DNase set. The quality and quantity of RNA were assessed using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). cDNA synthesis was performed with random hexamer primers using the Invitrogen SuperScript III first-strand system for RT-PCR. The following primers were used for real-time RT-PCR: for DgACS1, 5ʹ-TGGACAACGAGACGATGGAG-3ʹ (forward) and 5ʹ-GAAGCTGAGACGGAGGTTGTT-3ʹ (reverse); for DgACS2, 5ʹ-GACTCCTCATACTCCTATCCCACAG-3ʹ (forward) and 5ʹ-ACCTCCTGGTGTTTATTTCACAGTC-3ʹ (reverse); for DgACS3, 5ʹ-TTTTGCCAACATGAGCCAAC-3ʹ (forward) and 5ʹ-TTGTGGCATCTTCTTTTCTCCA-3ʹ (reverse); for DgACO1, 5ʹ-CCCAAATTTGTTTTCGAGGA-3ʹ (forward) and 5ʹ-TAATAAGCCGTGGCGATAGG-3ʹ (reverse); for DgACO2, 5ʹ-GGTGAAGTTTCAAGCCAAGG-3ʹ (forward) and 5ʹ-TTCTTCACTTGTTTGGGCACT-3ʹ (reverse); for DgACO3, 5ʹ-GGGTGAAGTTTCAAGCCAAG-3ʹ (forward) and 5ʹ-CAATTTGCTGACCCATCTGA-3ʹ (reverse); and for DgActin, 5ʹ-AGGGGATACATGTTCACAACCAC-3ʹ (forward) and 5ʹ-TTTCAAGCTCCTGCTCATAGTCC-3ʹ (reverse).
PCR reactions were performed using a Thermal Cycler Dice Real Time System (Takara bio), using SYBR Premix Ex Taq II (Perfect Real Time, Takara bio). Each reaction was performed using 0.8 µL aliquot of 20 µL cDNA solution derived from 1 µg of total RNA. The following program was used: initial polymerase activation at 95 °C for 30 s and; then 40 cycles at 95 °C for 5 s and 60 °C for 30 s. The specificity of PCR was checked using a heat dissociation protocol (from 95 to 60 °C) after the final cycle.
Absolute transcript levels were determined via the second derivative maximum method (Luu-The et al. 2005) using a dilution series of plasmid DNA containing the target sequence as external standards. To standardize the data, the ratio between the absolute transcript levels of the target and control genes was calculated for each sample.
Statistical analysis
Student’s t-test and Tukey–Kramer’s multiple range test were performed using SigmaStat software (v.12.5; Systat Software, San Jose, CA, USA).
Results
Cloning and nucleotide sequences of ACS
Three ACS genes were cloned and designated DgACS1, DgACS2 and DgACS3 and deposited in the DDBJ with accession numbers LC603027, LC603028, and LC603029, respectively. The cDNAs of these genes were 1901, 1739, and 1721 bp in length, respectively. The predicted proteins DgACS1, DgACS2, and DgACS3 consisted of 486, 485, and 444 amino acids, respectively. DgACS1 and DgACS2 had an amino acid homology of 80.9%, DgACS1 and DgACS3 had an amino acid homology of 55.8%, and DgACS2 and DgACS3 had an amino acid homology of 53.2%. Seven conserved domains were observed in the three genes (Fig. S1).
ACS has been categorized into three types based on its C-terminal sequences (Lin et al. 2009). Type 1 ACS is phosphorylated by mitogen-activated protein kinase and calcium-dependent protein kinase (CDPK), whereas type 2 ACS only has a CDPK site. Type 3 proteins lack these two phosphorylation sites. Based on these criteria, the predicted proteins DgACS1 and DgACS2 belong to type 1, whereas DgACS3 belongs to type 3 (Fig. S2).
Cloning and nucleotide sequences of ACO
Three ACO genes were cloned and designated DgACO1, DgACO2 and DgACO3, and deposited in the DDBJ with accession numbers LC603030, LC603031, and LC603032, respectively. The cDNAs of these genes were 1182, 1159, and 1072 bp in length, respectively. The predicted protein DgACO1 consisted of 317 amino acids, DgACO2 consisted of 317 amino acids, and DgACO3 consisted of 319 amino acids (Fig. S3). DgACO1 and DgACO2 as well as DgACO1 and DgACO3 had amino acid homologies of 78.6%, whereas DgACO2 and DgACO3 had an amino acid homology of 94%. Twelve amino acids, which are known to be conserved in ACO proteins (Wang and Woodson 1991; Do et al. 2005; Momonoi et al. 2007), were found to be conserved in DgACO1, DgACO2, and DgACO3.
The phylogenetic analysis revealed that ACO proteins were clustered into three groups (Houben and Van de Poel 2019). The amino acid sequences of the three genes were highly similar, and they clustered into type 1 (Fig. S4).
Ethylene production and ACS and ACO transcript levels in the gynoecium and receptacle during natural senescence
The time until sepal abscission in all the tested flowers was 5 days. Ethylene production in the whole flowers was almost constant during the first 3 days and significantly increased on day 4 (Fig. 2). Ethylene production in the gynoecium was relatively high on day 0, decreased over the first 3 days and slightly increased on day 4. Ethylene production in the receptacle decreased during the first 3 days and significantly increased on day 4.
In the gynoecium, DgACS1 transcript level, which was higher than that of the other two ACS transcript levels at harvest, decreased during the first 3 days and increased thereafter (Fig. 3). DgACS2 transcript level in the gynoecium gradually increased during senescence, and DgACS3 transcript level markedly increased from day 3, peaking on day 5. In the receptacle, DgACS2 transcript level was the highest on day 0 among the three transcript levels; however, its level decreased during senescence. DgACS1 transcript level decreased during the first 3 days and increased on day 4. DgACS3 transcript level peaked on day 4.
In the gynoecium, DgACO1 transcript level, which was higher than the other two ACO transcript levels on day 0, increased during senescence (Fig. 4). DgACO2 and DgACO3 transcript levels in the gynoecium were almost constant during the first 4 days and increased thereafter. In the receptacle, DgACO1 transcript level increased during the first 4 days and decreased thereafter. DgACO2 transcript level was almost constant during the first 3 days, increased thereafter, and peaked on day 5. DgACO3 transcript level increased starting from day 1 and peaked on day 4.
Ethylene production and ACS and ACO transcript levels in the gynoecium and receptacle after exposure to ethylene
Exposure to ethylene accelerated sepal abscission; the sepals began to abscise 20 h after ethylene exposure and were abscised in most of the flowers 24 h after exposure. In the control and ethylene-treated whole flowers, there were no significant differences in terms of ethylene production at most time points. There was a greater increase in ethylene production in the receptacle than in the gynoecium after 8 h of ethylene exposure (Fig. 5).
In the gynoecium, exposure to ethylene significantly increased DgACS1 and DgACS2 transcript levels at several time points (Fig. 6). However, ethylene exposure had only a slight effect on DgACS3 transcript level. In the receptacle, DgACS2 transcript level was the highest among the three ACS transcript levels and was higher in ethylene-treated flowers than in control flowers at all time points. DgACS1 transcript level did not noticeably increase in ethylene-treated flowers compared with control flowers. DgACS3 transcript level decreased after ethylene treatment at all time points and was the lowest among the three transcript levels.
In the gynoecium, DgACO1 transcript level was higher than that of the other two transcript levels; however, ethylene exposure only slightly increased it (Fig. 7). Ethylene exposure did not increase DgACO2 and DgACO3 transcript levels. In the receptacle, DgACO1 transcript level significantly increased after 12 h of ethylene exposure. After ethylene treatment, DgACO2 transcript level did not increase whereas DgACO3 transcript level markedly increased.
Effect of pollination on sepal abscission and pollen tube growth
Pollination accelerated sepal abscission. The sepals began to abscise at 12 h and were abscised in all the flowers 24 h after pollination. Pollen tube growth in the gynoecia was observed via fluorescent microscopy. Many pollen tubes reached the ovary 6 h after pollination (Fig. S5).
Ethylene production and ACS and ACO transcript levels in the gynoecium and receptacle in unpollinated and pollinated flowers
Ethylene production was low in the unpollinated whole flowers throughout the experimental period. It was also low in the gynoecium and receptacle of the unpollinated flowers. Pollination increased ethylene production in the whole flowers. Similarly, pollination markedly increased ethylene production in the gynoecium, and two ethylene peaks were observed after pollination (Fig. 8). Pollination increased ethylene production in the receptacle, with production peaking at 8 h after pollination.
In the gynoecium, ACS transcript levels did not significantly increase after pollination (Fig. 9). In the receptacle, DgACS2 transcript level increased after pollination, but DgACS1 and DgACS3 transcript levels did not.
In the gynoecium, the three ACO transcript levels did not significantly increased after pollination (Fig. 10). In the receptacle, DgACO1 transcript level was significantly higher in the pollinated flowers than in the unpollinated flowers at 4, 8, and 12 h after pollination. Although DgACO2 transcript level slightly changed after pollination, DgACO3 transcript level markedly increased after pollination.
Discussion
Delphinium flowers are highly sensitive to ethylene, and their sepals abscise during senescence (Ichimura et al. 2009). In this study, we isolated three ACS and three ACO genes from D. grandiflorum and analyzed their expression in the gynoecium and receptacle during natural senescence and after ethylene exposure and pollination. ACS and ACO expression were regulated differently in the gynoecium and receptacle.
Ethylene production and ACS and ACO expression during natural senescence
Ethylene production in the gynoecium and receptacle was relatively high on day 0 (Fig. 2). This result somewhat differed from that obtained for D. × belladonna in which ethylene production in the gynoecium and receptacle was relatively low at harvest (Ichimura et al. 2009). In our study, the flowers were emasculated on the day of flower opening and harvested approximately 4 days later. Emasculation increased ethylene production in the gynoecium and receptacle, and it peaked 3 days later (Ichimura et al. 2009). It is known that wounding increases ethylene production (Yang and Hoffman 1984). Therefore, high levels of ethylene production at day 0 may be attributed to wounding possibly caused by emasculation.
In the gynoecium of carnation, DcACS2 and DcACO1 transcript levels increase during senescence (ten Have and Woltering 1997; Jones and Woodson 1999). Similarly, SlACS1A transcript levels increased during senescence in tomato flowers (Llop-Tous et al. 2000). In petals displaying abscission, changes in ACS and ACO transcript levels in the gynoecium during natural senescence have only been reported for Pelargonium; however, no substantial increase in their transcript levels was observed (Clark et al. 1997). In the present study, based on the observed expression levels and trends, DgACS3 and DgACO1 were found to produce the highest levels of transcripts (Figs. 3 and 4), and the expression of these genes appears to contribute to increased ethylene production in the gynoecium.
To date, the expression of ACS and ACO in the receptacle has only been investigated in carnation (Jones and Woodson 1999) and rose (Xue et al. 2008); however, no obvious change in these transcript levels was observed during senescence. In our study, increases were observed in the expression of DgACS1, DgACS3, and the three ACO genes during senescence (Fig. 4). Thus, the expression of these ACS and ACO genes appears to contribute to ethylene production in the receptacle.
Cell wall hydrolases, including cellulases and pectinases, are believed to be involved in petal abscission (Brown 1997; van Doorn and Stead 1997). Petal abscission in rose is associated with enhanced expression of genes encoding expansin, pectate lyase, and xyloglucan endotransglucosylase/hydrolase (Sane et al. 2007; Singh et al. 2011ab). Sepals are directly connected to the receptacle. Therefore, an increase in ethylene production mediated by the expression of ACS and ACO in the receptacle may increase the expression of these genes, leading to sepal abscission.
In the receptacle, the expression of DgACS2, which produced the highest transcript levels on day 0, decreased during senescence (Fig. 3). In the D. × belladonna receptacle, wounding caused by emasculation induced a climacteric-like increase in ethylene production, which reached a peak 3 days later (Ichimura et al. 2009). Therefore, the high levels of DgACS2 expression observed in this study may be a result of emasculation.
It is unclear whether ethylene produced in the gynoecium affects ethylene production in the receptacle; additional research is needed to clarify this relationship.
ACS and ACO gene expression following ethylene exposure
Exposure to ethylene accelerated sepal abscission, which is consistent with previous findings (Ichimura et al. 2000, 2009). In some ethylene-sensitive flowers, including carnation (Jones 2003; Norikoshi et al. 2022) and Tweedia caerulea (Pun et al. 2013), exogenous ethylene induces autocatalytic ethylene production. However, ethylene exposure did not increase ethylene production in the whole flower but significantly increased ethylene production in the receptacle (Fig. 5). Ethylene production might decrease in the sepals and filaments following ethylene exposure, resulting in different trends of ethylene production in the whole flower and receptacle. In the whole flowers, high ethylene production was observed 24 h after ethylene exposure, when the sepals were abscised. The total fresh weight of the gynoecium and receptacle was one-tenth of that of the whole flower, which includes the sepals and filaments. The observed increase in ethylene production in whole flower after sepal abscission can be explained by the fact that ethylene production in the sepals is very low (Ichimura et al. 2009).
The expression of DgACS1 and DgACS2 in the gynoecium was increased after ethylene exposure (Figs. 6 and 7), suggesting that the expression of these genes may have contributed to the slight increase in ethylene production in this organ in response to ethylene exposure. The expression of ACS in carnation (Jones and Woodson 1997) and tomato (Llop-Tous et al. 2000), and that of ACO in petunia (Tang et al. 1994) increased in response to ethylene.
ACS and ACO expression in the receptacle following ethylene exposure has been reported in carnation (Jones and Woodson 1999) and rose (Xue et al. 2008). In rose, RhACS2 expression slightly increased after ethylene exposure (Xue et al. 2008). In this study, the expression of DgACS2 and DgACO3 markedly increased after ethylene exposure (Figs. 6 and 7), suggesting that the expression of these genes may contribute to increased ethylene production in the receptacle.
In carnation, exogenous ethylene markedly accelerates petal senescence, irrespective of treatment with aminoethoxyvinylglycine, an inhibitor of ethylene biosynthesis (Pun et al. 2016). This finding suggests that exogenous ethylene directly induces petal senescence. However, pollination accelerated sepal abscission more than high-concentration ethylene exposure did. Therefore, endogenous ethylene production in the receptacle induced by exogenous ethylene exposure may be involved in sepal abscission.
ACS and ACO expression in pollinated flowers
As reported in many plants, including Digitalis (Stead and Moore 1979), Pelargonium (Clark et al. 1997) and Torenia (Goto et al. 1999), pollination accelerated sepal abscission in D. grandiflorum. Although a marked increase in ethylene production in the gynoecium was observed (Fig. 8), the expression of ACS and ACO in the gynoecium did not significantly increase after pollination (Fig. 10). In Delphinium gynoecium, ACO activity at anthesis is relatively high (Ichimura et al. 2009). The pollen of some plants, including petunia, contains large amounts of ACC (Whitehead et al. 1983). In petunia, an early increase in ethylene production is associated with the ACC content of the pollen (Singh et al. 1992). We confirmed that pollen tubes reached the ovary 6 h after pollination (Fig. S5). Thus, the marked increase in ethylene production in the gynoecium may be attributed to pollen-borne ACC, which is converted to ethylene via ACO activity. Alternatively, other ACS and ACO genes may be specifically induced by pollination, although these types of ACS and ACO genes have not yet been identified in other plant species.
The coordination of senescence processes in pollinated flowers involves interorgan signaling (Jones and Woodson 1997). Two transmission factors for this signaling have been proposed: ethylene (Woltering et al. 1995) and ACC (Reid et al. 1984). The increase in ethylene production in the receptacle after pollination was associated with an increase in the expression of DgACO1 and DgACO3 (Figs. 8 and 10). These results suggest that pollination generates some transmission factor that induces ethylene production in the receptacles because the receptacle lies below the gynoecium. From these findings, we propose that ethylene produced in the gynoecium in response to pollination increases the expression of DgACO1 and DgACO3, resulting in increased ethylene production in the receptacle.
Significance of the receptacle and possible model for sepal abscission
The significance of the gynoecium in petal senescence has been emphasized for petal wilting in petunia (Whitehead et al. 1984) and carnation (Shibuya et al. 2000) and for petal abscission in Digitalis (Stead and Moore 1983) and Pelargonium (Clark et al. 1997). In Digitalis (Stead and Moore 1983) and Pelargonium (Clark et al. 1997), pollination markedly accelerates petal abscission. In our study, however, pollination did not significantly increase the expression of ACS and ACO in the gynoecium.
Changes in the expression of ACS and ACO in the receptacle have been reported in carnation (Jones and Woodson 1999) and rose (Xue et al. 2008). However, the significance of this organ in petal wilting has only been mentioned by Hsieh and Sacalis (1986) who reported ACC levels in the receptacle during senescence in cut carnation. Although the significance of the receptacle in sepal abscission has been described for D. × belladonna flowers, the activity of ACS and ACO during natural senescence has only been investigated (Ichimura et al. 2009). In our study, ethylene exposure increased ethylene production more markedly in the receptacle than in the gynoecium, and this finding was associated with marked increases in the expression of some ACS and ACO genes in the receptacle. This suggests that the responsiveness of the receptacle to ethylene exposure is higher than in that of the gynoecium. Moreover, the expression of ACS and ACO after pollination increased only in the receptacle. Therefore, the receptacle is likely to be the primary organ that regulates ethylene biosynthesis through the expression of ACS and ACO, leading to sepal abscission in D. grandiflorum.
Thus, we propose a model for sepal abscission based on these results (Fig. 11). During natural flower senescence, increases in the expression of DgACS1, DgACS3, DgACO1, and DgACO3 are responsible for ethylene production in the receptacle, leading to sepal abscission. However, whether an increase in ethylene production in the gynoecium affects ethylene biosynthesis remains unclear. Furthermore, exposure to ethylene increases the expression of DgACS2 and DgACO3 in the receptacle, leading to sepal abscission. Therefore, the receptacle may directly respond to exogenous ethylene. Pollination increases ethylene production in the gynoecium without inducing ACS and ACO expression. Therefore, increase in ethylene production in the gynoecium may lead to increases the expression of DgACS2 and DgACO3 in the receptacle, resulting in sepal abscission.
Conclusions
Three ACS genes (DgACS1, DgACS2, and DgACS3) and three ACO genes (DgACO1, DgACO2, and DgACO3) were cloned. The expression of ACS and ACO in the gynoecium and receptacle increased during senescence, with the exception of DgACS2 expression in the receptacle. Exposure to ethylene increased ethylene production more markedly in the receptacle than in the gynoecium and induced sepal abscission. Additionally, the expression of DgACS1 in the gynoecium and that of DgACS2 and DgACO3 in the receptacle increased after ethylene exposure. Pollination increased ethylene production in the gynoecium and receptacle and markedly accelerated sepal abscission. However, pollination only slightly affected the expression of ACS and ACO in the gynoecium, whereas the expression of DgACO3 in the receptacle was markedly increased. In addition to the fact that the sepals are directly connected to the receptacle, we conclude that the receptacle is the primary organ that regulates ethylene biosynthesis associated with sepal abscission.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACO:
-
1-Aminocyclopropane-1-carboxylic acid oxidase
- ACS:
-
1-Aminocyclopropane-1-carboxylic acid synthase
References
Argueso CT, Hansen M, Kieber JJ (2007) Regulation of ethylene biosynthesis. J Plant Growth Regul 26:92–105. https://doi.org/10.1007/s00344-007-0013-5
Brown KM (1997) Ethylene and abscission. Physiol Plant 100:567–576. https://doi.org/10.1111/j.1399-3054.1997.tb03062.x
Clark DG, Richards C, Hilioti Z, Lind-Iversen S, Brown K (1997) Effect of pollination on accumulation of ACC synthase and ACC oxidase transcripts, ethylene production and flower petal abscission in geranium (Pelargonium × hortorum L.H. Bailey). Plant Mol Biol 34:855–865. https://doi.org/10.1023/A:1005877809905
Do YY, Thay TS, Chang TW, Huang PL (2005) Molecular cloning and characterization of a novel 1-aminocyclopropane-1-carboxylate oxidase gene involved in ripening of banana fruits. J Agric Food Chem 53:8239–8247. https://doi.org/10.1021/jf051224+
Gillisen LJW, Hoekstra FA (1984) Pollination-induced corolla wilting in Petunia hybrida rapid transfer through the style of a wilting-inducing substance. Plant Physiol 75:496–498. https://doi.org/10.1104/pp.75.2.496
Goto R, Aida R, Shibata M, Ichimura K (1999) Role of ethylene on flower senescence of Torenia. J Jpn Soc Hort Sci 68:263–268. https://doi.org/10.2503/jjshs.68.263
Hilioti Z, Richards C, Brown KM (2000) Regulation of pollination-induced ethylene and its role in petal abscission of Pelargonium × hortorum. Physiol Plant 109:322–332. https://doi.org/10.1034/j.1399-3054.2000.100314.x
Houben M, Van de Poel B (2019) 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): the enzyme that makes the plant hormone ethylene. Front Plant Sci 10:695. https://doi.org/10.3389/fpls.2019.00695
Hsieh YC, Sacalis J (1986) Levels of ACC in various floral portions during aging of cut carnations. J Am Soc Hort Sci 111:942–944
Ichimura K, Goto R (2000) Acceleration of senescence by pollination of cut ‘Asuka-no-nami’ Eustoma flowers. J Jpn Soc Hort Sci 69:166–170. https://doi.org/10.2503/jjshs.69.166
Ichimura K, Shimamura M, Hisamatsu T (1998) Role of ethylene in senescence of cut Eustoma flowers. Postharvest Biol Technol 14:193–198. https://doi.org/10.1016/S0925-5214(98)00039-8
Ichimura K, Kohata K, Goto R (2000) Soluble carbohydrate in Delphinium and their influence on sepal abscission in cut flowers. Physiol Plant 108:307–313. https://doi.org/10.1034/j.1399-3054.2000.108003307.x
Ichimura K, Shimizu-Yumoto H, Goto R (2009) Ethylene production by the gynoecium and receptacle is associated with sepal abscission in cut Delphinium flowers. Postharvest Biol Technol 52:267–272. https://doi.org/10.1016/j.postharvbio.2008.12.008
Jackson MB, Campbell DJ (1975) Movement of ethylene from roots to shoots, a factor in the responses of tomato plants to waterlogged soil conditions. New Phytol 74:397–406. https://doi.org/10.1111/j.1469-8137.1975.tb01350.x
Jones ML (2003) Ethylene biosynthetic genes are differentially regulated by ethylene and ACC in carnation styles. Plant Growth Regul 40:129–138. https://doi.org/10.1023/A:1024241006254
Jones ML, Woodson WR (1997) Pollination-induced ethylene in carnation. Role of stylar ethylene in corolla senescence. Plant Physiol 115:205–212. https://doi.org/10.1104/pp.115.1.205
Jones ML, Woodson WR (1999) Differential expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in carnation. Plant Physiol 119:755–764. https://doi.org/10.1104/pp.119.2.755
Kende H (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Mol Biol 44:283–307. https://doi.org/10.1146/annurev.pp.44.060193.001435
Kuroda S, Hakata M, Hirose Y, Shiraishi M, Abe S (2003) Ethylene production and enhanced transcription of an ethylene receptor gene, ERS1, in Delphinium during abscission of florets. Plant Physiol Biochem 41:812–820. https://doi.org/10.1016/S0981-9428(03)00115-3
Kuroda S, Hirose Y, Shiraishi M, Davies E, Abe S (2004) Co-expression of an ethylene receptor gene, ERS1, and ethylene signaling regulator gene, CTR1, in Delphinium during abscission of florets. Plant Physiol Biochem 42:745–751. https://doi.org/10.1016/j.plaphy.2004.07.006
Lee MM, Lee SH, Park KY (1997) Effects of spermine on ethylene biosynthesis in cut carnation (Dianthus caryophyllus L.) flowers during senescence. J Plant Physiol 151:68–73. https://doi.org/10.1016/S0176-1617(97)80038-7
Lin Z, Zhong S, Grierson D (2009) Recent advances in ethylene research. J Exp Bot 60:3311–3336. https://doi.org/10.1093/jxb/erp204
Llop-Tous I, Barry CS, Grierson D (2000) Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol 123:971–978. https://doi.org/10.1104/pp.123.3.971
Luu-The V, Paquet N, Calvo E, Cumps J (2005) Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques 38:287–293. https://doi.org/10.2144/05382RR05
Momonoi K, Shoji K, Yoshida K (2007) Cloning and characterization of ACC oxidase genes from tulip. Plant Biotechnol 24:241–246. https://doi.org/10.5511/plantbiotechnology.24.241
Naing AH, Soe MT, Kyu SY, Kim CK (2021) Nano-silver controls transcriptional regulation of ethylene- and senescence-associated genes during senescence in cut carnations. Sci Hortic 287:110280. https://doi.org/10.1016/j.scienta.2021.110280
Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A (1998) Differential expression and internal feedback regulation of 1-aminocyclopropene-1-carboxylate synthase, 1-aminocyclopropene-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118:1295–1305. https://doi.org/10.1104/pp.118.4.1295
Nichols R (1966) Ethylene production during senescence of flowers. J Hort Sci 41:279–290. https://doi.org/10.1080/00221589.1966.11514176
Norikoshi R, Niki T, Ichimura K (2022) Differential regulation of two 1-aminocyclopropane-1-carboxylate oxidase (ACO) genes, including the additionally cloned DcACO2, during senescence in carnation flowers. Postharvest Biol Technol 183:111752. https://doi.org/10.1016/j.postharvbio.2021.111752
O’Neill SD, Nadeau JA, Zhang XS, Bui AQ, Halevy AH (1993) Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell 5:419–432. https://doi.org/10.1105/tpc.5.4.419
Porat R, Halevy AH, Serek M, Borochov A (1995) An increase in ethylene sensitivity following pollination in the initial event triggering an increase in ethylene production and enhanced senescence of Phalaenopsis orchid flowers. Physiol Plant 93:778–784. https://doi.org/10.1111/j.1399-3054.1995.tb05131.x
Pun UK, Niki T, Ichimura K (2013) Ethanol reduces sensitivity to ethylene and delays petal senescence in cut Tweedia caerulea flowers. Plant Growth Regul 69:125–130. https://doi.org/10.1007/s10725-012-9755-6
Pun UK, Yamada T, Azuma M, Tanase K, Yoshioka S, Shimizu-Yumoto H, Satoh S, Ichimura K (2016) Effect of sucrose on sensitivity to ethylene and enzyme activities and gene expression involved in ethylene biosynthesis in cut carnations. Postharvest Biol Technol 121:151–158. https://doi.org/10.1016/j.postharvbio.2016.08.001
Reid MS, Fujino DW, Hoffman NE, Whitehead CS (1984) 1-Aminocyclopropane-1-carboxylic acid (ACC): the transmitted stimulus in pollinated flowers? J.Plant Growth Regul 3:189–196. https://doi.org/10.1007/BF02042003
Sane AP, Kaushal S, Tripathi K, Nath P (2007) Petal abscission in rose (Rosa bourboniana var Gruss an Teplitz) is associated with the enhance expression of an alpha expansin gene, RbEXPA1. Plant Sci 172:481–487. https://doi.org/10.1016/j.plantsci.2006.10.005
Shibuya K, Yoshioka T, Hashiba T, Satoh S (2000) Role of the gynoecium in natural senescence of carnation (Dianthus caryophyllus L.) flowers. J Exp Bot 51:2067–2073. https://doi.org/10.1093/jexbot/51.353.2067
Shimizu-Yumoto H, Ichimura K (2012) Effects of ethylene, pollination, and ethylene inhibitor treatments on flower senescence of gentians. Postharvest Biol Technol 63:111–115. https://doi.org/10.1016/j.postharvbio.2011.08.009
Singh A, Evensen KB, Kao Th (1992) Ethylene synthesis and floral senescence following compatible and incompatible pollinations in Petunia inflata. Plant Physiol 99:38–45
Singh AP, Pandy SP, Pandy S, Nath P, Sane AP (2011a) Transcriptional activation of a pectate lyase gene, RbPel1, during petal abscission in rose. Postharvest Biol Technol 60:143–148. https://doi.org/10.1016/j.postharvbio.2010.12.014
Singh AP, Tripathi SK, Nath P, Sane AP (2011b) Petal abscission in rose is associated with the differential expression of two ethylene-sensitive xyloglucan endotransglucosylase/hydrolase genes, RbXTH1, and RbXTH2. J Exp Bot 62:5091–5103 https://doi.org/10.1093/jxb/err209
Stead AD, Moore KG (1979) Studies on flower longevity in Digitalis. Pollination induced corolla abscission in Digitalis flowers. Planta 146:409–414. https://doi.org/10.1007/BF00380853
Stead AD, Moore KG (1983) Studies on flower longevity in Digitalis. The role of ethylene in corolla abscission. Planta 157:15–21 https://doi.org/10.1007/BF00394535
Tanase K, Ichimura K (2006) Expression of ethylene receptors Dl-ERS1-3 and Dl-ERS2, and ethylene response during flower senescence in Delphinium. J Plant Physiol 163:1159–1166. https://doi.org/10.1016/j.jplph.2005.12.003
Tang X, Gomes AMTR, Bhatia A, Woodson WR (1994) Pistil specific and ethylene-regulated expression of 1-aminocyclopropene-1-carboxylate oxidase genes on petunia flower. Plant Cell 6:1227–1239. https://doi.org/10.1105/tpc.6.9.1227
ten Have A, Woltering EJ (1997) Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Mol Biol 34:89–97. https://doi.org/10.1023/A:1005894703444
van Doorn WG, Stead AD (1997) Abscission of flowers and flower parts. J Exp Bot 48:821–837. https://doi.org/10.1093/jxb/48.4.821
Wang H, Woodson WR (1991) A flower senescence-related mRNA from carnation shares sequence similarity with fruit ripening-related mRNAs involved in ethylene biosynthesis. Plant Physiol 96:1000–1001. https://doi.org/10.1104/pp.96.3.1000
Whitehead CS, Fujino DW, Reid MS (1983) Identification of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), in pollen. Sci Hortic 21:291–297. https://doi.org/10.1016/0304-4238(83)90103-6
Whitehead CS, Halevy AH, Reid MS (1984) Roles of ethylene and 1-aminocyclopropane-1-carboxylic acid in pollination and wound-induced senescence of Petunia hybrida flowers. Physiol Plant 61:643–648. https://doi.org/10.1111/j.1399-3054.1984.tb05184.x
Woltering EJ, van Doorn WG (1988) Role of ethylene in senescence of petals-morphological and taxonomical relationships. J Exp Bot 39:1605–1616. https://doi.org/10.1093/jxb/39.11.1605
Woltering EJ, Somhorst D, de Beer CA (1993) Roles of ethylene production and sensitivity in senescence of carnation flower (Dianthus caryophyllus) cultivars White Sim, Chinera and Epomeo. J Plant Physiol 141:329–335. https://doi.org/10.1016/S0176-1617(11)81743-8
Woltering EJ, Somhorst D, van der Veer P (1995) The role of ethylene in interorgan signaling during flower senescence. Plant Physiol 109:1219–1225. https://doi.org/10.1104/pp.109.4.1219
Woodson WR, Park KY, Drory A, Larsen PB, Wang H (1992) Expression of ethylene biosynthetic pathway transcripts in senescing carnation flowers. Plant Physiol 99:526–532. https://doi.org/10.1104/pp.99.2.526
Xue J, Li Y, Tan H, Yang F, Ma N, Gao J (2008) Expression of ethylene biosynthetic and receptor genes in rose floral tissues during ethylene-enhanced flower opening. J Exp Bot 59:2161–2169. https://doi.org/10.1093/jxb/ern078
Yamada T, Ichimura K, van Doorn WG (2007) Relationship between petal abscission and programmed cell death in Prunus yedoensis and Delphinium belladonna. Planta 226:1195–1205. https://doi.org/10.1007/s00425-007-0566-3
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol 35:155–189. https://doi.org/10.1146/annurev.pp.35.060184.001103
Zeroni M, Jerie PH, Hall MA (1977) Studies on the movement and distribution of ethylene in Vicia faba L. Planta 134:119–125. https://doi.org/10.1007/BF00384960
Author information
Authors and Affiliations
Contributions
MO, KS and KI conceived and designed the research. MO and MA performed physiological experiments. MO and TN performed molecular experiments. KI wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Vijay Pratap Singh
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okamoto, M., Niki, T., Azuma, M. et al. Expression of ethylene biosynthesis genes in the gynoecium and receptacle associated with sepal abscission during senescence in Delphinium grandiflorum. Plant Growth Regul 97, 593–609 (2022). https://doi.org/10.1007/s10725-022-00822-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00822-z