Abstract
Seeds can activate a series of genes to avoid imbibition-associated stress during seed germination. However, the precise gene responses at the initial imbibition stage (Phase I) of seed germination are not yet fully understood in rice. In this study, a total of 1544 differentially expressed genes (DEGs) with at least 2-fold change were identified in 8 h imbibed seeds (Phase I) compared to dry seeds of rice using RNA-Seq approach. MapMan analysis revealed that the mainly signalling-, cell wall-, abiotic stress-, and antioxidant-related DEGs were associated with stress responses pathway involving in the initial imbibition of rice seed germination. Among them, the signalling-related DEGs were mainly receptor kinases, and the largest number of cell wall-related DEGs were expansins followed by pectinesterases and polygalacturonases. The abiotic stress-related DEGs were mainly cupin domain protein, methyltransferases and SPX domain protein, and the majority of antioxidant-related DEGs were glutathione S-transferases (GSTs) and peroxidases. Further qRT-PCR analysis revealed that the highest expressions of the majority of GST genes occurred at 8 h imbibition stage in rice, which caused the corresponding highest GST activity at that stage. GSTs might prevent the burst of H2O2 accumulation at the initial imbibition stage that contributes to the following successful seed germination. Our results provide further understanding of gene responses at the initial imbibition stage of seed germination in rice. The identified genes provide a foundation for future studies of seed germination in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed germination is a critical phase in plant life cycle, which determinates subsequent seedling establishment and crop yield (Han and Yang 2015). Imbibition is the initial step of seed germination. Typically, air-dried seeds have moisture contents in the range of 5–15%, whereas the germinating seeds have moisture contents about 75–100% (Bewley et al. 2013). If water uptake is intensively rapid into dry seeds, imbibition damage will occur in germinating seeds (Mccormac and Keefe 1990). Imbibition chilling injury will be taken more seriously when dry seeds are directly planted in cold soils below 15 °C in the early season of rice (Lou et al. 2007). With the popularity of rice direct-seeding methods in Asia countries (Wang et al. 2011), imbibition damage becomes an important issue for seed germination in rice. Understanding the genes responses in germinating seeds from dry state to imbibed state is helpful for the improvement of rice direct-seeding in future.
Receptor-like protein kinases (RLKs) localized on the plasma membrane and physically linked the cell wall to the cytoplasm that makes them ideal candidates for cell wall sensors (Steinwand and Kieber 2010). RLKs have been implicated in various signaling pathways, which play important roles in plant growth, development and stress responses (Morris and Walker 2003). For instance, the mutation of receptor like kinase 1 (RPK1) decreases ABA sensitivity during seed germination, seedling growth, and stomatal movement in Arabidopsis, whereas the overproduction of RPK1 increases stresses tolerance (Osakabe et al. 2005, 2010). ABA INSENSITIVE3 (ABI3)-activated lectin receptor-like kinase LecRK-b2 positively regulates ABA signalling during seed germination in Arabidopsis (Deng et al. 2009). In rice, LecRLKs have also been found to be involved in seed germination; knocking down the OslecRK gene depresses the expression of α-amylase genes and thereby decreases seed viability (Cheng et al. 2013). Many RLKs expressed at the late seed development stages are associated with embryo and endosperm dehydration and those are also regulated by abiotic stresses in rice (Gao and Xue 2012).
Cell wall is of critical importance for the cell shape, which provides mechanical strength to withstand the turgor pressure (Tenhaken 2015). Cell wall hydrolases appear to be involved in tissue weakening and an increased growth potential of the embryo during seed germination (Müller et al. 2013). There are several cell wall-modifying enzymes, including expansins, xyloglucanases, endo-beta mannanases, beta-1,3-glucanases, endotransglycosylases, pectin methylesterases, polygalacturonases and arabinogalactans glucosidases, involved in the wall modification during seed germination (Holdsworth et al. 2008; An and Lin 2011). In addition to maintaining structural integrity, cell wall provides environmental barrier to defend against stress (Houston et al. 2016). Cell wall is clearly affected by many abiotic stresses, and a common plant response is an increase of xyloglucan modifying enzymes and expansins (Tenhaken 2015). The expansins will influence wall stiffness during stress (Tenhaken 2015), and xyloglucan accompany stress responses (Le Gall et al. 2015). Wall-mediated responses to abiotic stresses involve in cell wall integrity sensing, plant hormone signaling, and generation of reactive oxygen species (ROS; Novaković et al. 2018).
In seeds, ROS are produced from embryogenesis to germination, not only in metabolically active states but also in quiescent dry states (Basbouss-Serhal et al. 2016; Oracz and Karpiński 2016). ROS play a dual role in seed germination, depending on the concentration, which can act both as damaging agents and as signals (Macovei et al. 2017). The high levels of ROS can reduce seed germination due to DNA damage, protein oxidation and lipid peroxidation (Mittler et al. 2004; Bailly et al. 2008). However, the moderate levels of ROS can enhance cell wall loosening and endosperm weakening, which is necessary for radicle protrusion during seed germination (Su et al. 2016). Therefore, the fine regulation of ROS concentration is required for successful germination. The enzymatic antioxidant defenses, including superoxide dismutases, glutathione peroxidases, peroxidases (PODs) and catalases, as well as the enzymes of the ascorbate–glutathione cycle, ascorbate peroxidases, glutathione reductases, monodehydroascorbate reductases and dehydroascorbate reductases, involve in the against deleterious effects of oxidative stress (Jimenez et al. 1997; Karmous et al. 2017). The involvement of ROS regulatory systems in stress responses of the initial imbibition of seed germination deserves investigation in rice.
Based on the uptake of water, seed germination can be divided into three phases: a rapid uptake of water (Phase I), followed by a plateau phase of water uptake (Phase II), and the initiation of growth (Phase III; Wang et al. 2011). Physiological analyses indicate that imbibition damage is mainly associated with the membrane damage. If the membranes can revert from gel state to liquid crystalline state, seeds can avoid imbibition damage (Bewley et al. 2013). Recently several transcriptome and proteome analyses have been applied to elucidate the fundamental mechanism of seed germination in rice (Yang et al. 2007; Liu et al. 2015; Wang et al. 2015; Wei et al. 2015; Dametto et al. 2015). The degradation of seed maturation- and desiccation-associated proteins occurred at the early stage of Phase II (24 h imbibition), while that of storage proteins were mainly occurred at the late stage of Phase II (48 h imbibition) in rice (Yang et al. 2007). The imbibition response proteins, involving in energy metabolism, cell growth, cell defense and storage proteins, are activated at the middle stage of Phase II (33 h imbibition) in rice (Liu et al. 2015). Previous studies were mainly focused on Phase II of seed germination in rice, when seed cells switch from a quiescent state to a metabolically active state rapidly. However, scarcely research has been focused on the identification of gene responses at the initial imbibition stage (Phase I) of seed germination in rice.
It is generally accepted that the restoration of cellular integrity, the repair of mitochondrion and DNA damages, as well as the initiation of respiration and metabolic activities mainly take place during seed imbibition in rice (Yang et al. 2007; He et al. 2011; He and Yang 2013). Seeds would activate a number of genes to avoid imbibition damage during seed germination (Ventura et al. 2012; Macovei et al. 2017). However, very little is known about the RLKs-, cell wall- and ROS-related genes involving in the initial seed imbibition of rice. In this study, to determine the gene responses involving in the initial seed imbibition of rice, RNA-Seq was conducted in imbibed seeds after 8 h imbibition (Phase I). The signalling-, cell wall-, abiotic stress- and antioxidant-related regulators involving in stress responses pathway were highlighted in this study. Our results provide new information to elucidate the basis of gene responses during the initial imbibition stage of seed germination in rice, and lay a foundation for further studies of the early seed germination.
Materials and methods
Germination assay
The japonica Nipponbare was used for seed germination assay according to Cheng et al. (2017). Fifty seeds per replicate were imbibed in Petri dishes (d = 9 cm) with 10 mL distilled water in a growth chamber at 30 ± 1 °C for 72 h. The water content was conducted to determinate the stages of seed germination. Water content (g/g) = (W2 - W1)/W1, where W2 represents the total seed weight (including the weight of dry seed and imbibition water) after imbibition, and W1 represents the dry seed weight before imbibition. Three biological replications were performed.
RNA sequencing
Total RNA was extracted from approximately 80–100 mg powder of each sample (seeds after 0 and 8 h imbibition) using the Plant kit (Transgen, Beijing, China) according to the manufacturer’s instructions. The complementary DNA (cDNA) libraries were prepared by performing a series of procedures, including poly(A) enrichment, RNA fragmentation, cDNA synthesis, linker ligation, size selection and PCR amplification. Construction of cDNA libraries and HiSeq2500 sequencing were performed at Novogene Biotechnology Co., Ltd., Beijing, China. Three biological replications were performed.
Differentially expressed genes analysis
A quality control tool (FastQC) was performed to estimate the quality of raw reads, and the adapter sequences were trimmed and the low-quality reads were filtered according to He et al. (2019). The clean reads were mapped onto the Nipponbare reference genome (MSU Rice Genome Annotation Project Release 7) using Tophat version 2.0.12 (Kim et al. 2013). The changes of gene FPKM (fragments per kilo base of exon per million) were calculated between 0 and 8 h imbibed seeds. The differentially expressed genes (DEGs) with a P adj (P-adjusted) < 0.05 with more than 2-fold change were selected for further pathway analysis with MapMan (Thimm et al. 2004; Usadel et al. 2009).
Quantitative real-time PCR analysis
Total RNA was extracted from germinating seeds (0, 8, 12, 24, 36 and 48 h imbibition) of Nipponbare using the Plant kit (Transgen, Beijing, China), according to the manufacturer’s instructions. The first-strand cDNA was synthesized and qRT-PCR was carried out according to He et al. (2019). The PCR conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The rice OsActin gene was used as internal control. Primers used for qRT-PCR are listed in Table S1. Normalized transcript levels were calculated using the comparative CT method (Livak and Schmittgen 2001). Three biological replications were performed.
Evaluation of H2O2 level and antioxidant enzyme activities
The levels of H2O2 and the activities of PODs and glutathione S-transferases (GSTs) were measured using commercial assay kits following the manufacturer’s instructions (Suzhou Keming Bioengineering Company, Suzhou, Jiangsu, China). Approximately 0.1 g of each sample was rapidly frozen in liquid nitrogen and homogenized into a powder. 1 mL of acetone was added to the homogenate for the extraction of H2O2, and 1 mL of sodium phosphate buffer (50 mM, pH 7.0) were added to the homogenate for the extraction of POD and GST. The mixture was centrifuged at 8000 × g at 4 °C for 10 min. The absorbance of the supernatant was determined immediately for H2O2 content, POD and GST activity at 415 nm, 470 nm and 340 nm, respectively. The H2O2 content is expressed as µmol/g DW. One unit of POD activity was defined as an absorbance change of 0.005 units per minute at 470 nm. The POD activity (U/g DW) was calculated as 4000 × ΔA470/DW. The GST activity is expressed as nanomolar (nmol)/min/g DW. Three biological replications were performed.
Data analysis
Fisher's least significant difference (LSD) test was conducted to judge the significant differences of H2O2 level and antioxidant enzyme activities at the P < 0.05 level using SAS software (Cary, NC, USA).
Results
Differentially expressed genes involved in the initial seed imbibition
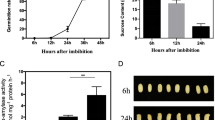
To determine the imbibition stage of seed germination in rice, the dynamic changes of water content in germinating seeds were analyzed during seed germination. Based on seed imbibition, the first 12 h of imbibition is associated with a rapid water uptake (Phase I), and the 36 h of imbibition is the beginning germination time-point when the radicle protrudes through seed coat (Phase III; Fig. 1). Meanwhile, the highest speed of imbibition was occurred after 8 h imbibition at Phase I stage in rice. Thus, to reveal the gene responses at the initial imbibition stage of seed germination in rice, the dry (0 h) and imbibed (8 h) seeds (at Phase I stage) were collected for RNA sequencing.
A total of 1544 DEGs with at least 2-fold change were identified in 8 h imbibed seeds compared with dry seeds in rice (Fig. 2a; Table S2), in which 1334 and 210 DEGs were up-regulated and down-regulated respectively. MapMan analysis revealed that these DEGs mainly belong to transcription factor- (183), large enzyme families- (135), signalling- (111), protein coding- (126), stress- (74), transport- (64), hormone- (61), development- (50) and cell wall-related genes (33) (Fig. 2b). Among them, further pathway investigation revealed that the majority of signalling-, cell wall-, abiotic stress- and antioxidant-related DEGs were involved in stress responses pathway during seed germination (Fig. 2c). The characteristics of these signalling-, cell wall-, abiotic stress- and antioxidant-related DEGs were further analyzed below.
Differentially expressed genes (DEGs) between dry seeds (0 h) and imbibed seeds (8 h) in rice. a Total, up- and down-regulated DEGs with at least 2-fold change, b functional classification of DEGs using MapMan analysis; the DEGs belong to transcription factor- (183), large enzyme families- (135), protein coding- (126), signalling- (111), stress- (74), transport- (64), hormone- (61), development- (50) and cell wall-related genes (33) and c the expression changes of signalling-, cell wall-, stress- and antioxidant-related DEGs associated with stress responses pathway involving in the initial seed imbibing. Red, up-regulation; gray, no change; blue, down-regulation. Values represent the fold changes of gene expression. (Color figure online)
Signalling-related DEGs involved in the initial seed imbibition
A total of 111 signalling-related DEGs were identified at the initial imbibition stage of seed germination in rice (Table S3). The majority of DEGs were receptor kinase-related genes (68) followed by calcium signalling-related genes (20) and light signalling-related genes (9) (Fig. 3a). Among them, several types of receptor kinase-related genes with higher fold changes were identified in 8 h imbibed seeds of rice, such as phytosulfokine receptor precursor (LOC_Os02g06200 and LOC_Os02g06250), OsWAK receptor-like protein kinase (LOC_Os09g38850 and LOC_Os09g38910), receptor kinase ORK10 (LOC_Os01g02300), Ser/Thr receptor-like kinase (LOC_Os01g02290 and LOC_Os04g34370), leucine-rich repeat (LRR) receptor protein kinase (LOC_Os04g52780), S-domain receptor-like protein kinase (LOC_Os11g10290) and receptor-like protein kinase precursor (LOC_Os10g22890, LOC_Os05g44930, LOC_Os07g05740, LOC_Os10g33040 and LOC_Os02g11930).
Expression pattern of signalling-related differentially expressed genes (DEGs) in embryo and endosperm during 24 h imbibition stage in rice using Genevestigator (http://www.genevestigator.com). a Genes involved in pathway and b gene expression pattern of receptor kinases. Red, up-regulation; gray, no change; blue, down-regulation. Values represent the fold changes of gene expression. (Color figure online)
The expression patterns of these receptor kinase-related DEGs in embryo and endosperm during seed germination were further conducted using Genevestigator (http://www.genevestigator.com; Fig. 3b). The similar expression patterns were observed in several genes. For example, the expressions of LOC_Os09g38850 and LOC_Os09g38910 corresponding to OsWAK receptor-like protein kinase, LOC_Os01g02290 and LOC_Os04g34370 corresponding to Ser/Thr receptor-like kinase, and LOC_Os01g02300, LOC_Os04g52780 and LOC_Os11g10290 corresponding to receptor kinase ORK10, LRR receptor protein kinase and S-domain receptor-like protein kinase, respectively, as well as LOC_Os02g11930 corresponding to receptor-like protein kinase were significantly induced in the embryo and endosperm during 24 h seed germination stage in rice. These receptor kinases might behave as signal transductors during seed imbibition in rice.
Cell wall-related DEGs involved in the initial seed imbibition
A total of 33 cell wall-related DEGs, involving in cell wall proteins (7), cell wall degradation (6), cell wall modification (8) and cell wall pectin esterases (5), were identified at the initial imbibition stage of seed germination in rice (Fig. 4a; Table S4). The majority of DEGs were up-regulated at the initial imbibition stage; 3 and 30 DEGs were down-regulated and up-regulated, respectively. Among them, the majority of DEGs were expansins (6) followed by pectinesterases (5) and polygalacturonases (4). By comparison, the transcript of pectinacetylesterase domain protein (LOC_Os04g51340) was most significantly up-regulated at the initial imbibition stage in rice.
Expression pattern of cell wall-related differentially expressed genes (DEGs) in embryo and endosperm during 24 h imbibition stage in rice using Genevestigator (http://www.genevestigator.com). a Genes involved in pathway and b gene expression pattern. Red, up-regulation; gray, no change; blue, down-regulation. Values represent the fold changes of gene expression. (Color figure online)
The expression patterns of these DEGs in embryo and endosperm during seed germination were further conducted using Genevestigator (http://www.genevestigator.com; Fig. 4b). The higher expressions were observed in embryo than in endosperm in the majority of cell wall-related DEGs (LOC_Os11g44950 not available) in rice. The majority of cell wall-related DEGs (except LOC_Os06g10920, LOC_Os05g08770 and LOC_Os08g34900) were significantly induced during seed germination. By comparison, the similar expression patterns were observed in several genes. For example, LOC_Os05g48890 and LOC_Os04g51340 encoding fasciclin domain protein and pectinacetylesterase domain protein, respectively, were continuously expressed during seed germination. The expressions of LOC_Os04g42620, LOC_Os03g01260 and LOC_Os01g21034 encoding uncharacterized protein, expansin precursor and pectinacetylesterase domain protein, respectively, were increased gradually during seed germination. These expressed genes might play important roles in the early seed germination of rice.
Abiotic stress-related DEGs involved in the initial seed imbibition
A total of 23 abiotic stress-related DEGs, involving in drought/salt (5), heat (4), cold (2), touch/wounding (2) and abiotic (3) stresses, were identified at the initial imbibition stage of seed germination in rice (Fig. 5a; Table S5). By comparison, the majority of DEGs were up-regulated at the initial imbibition stage; 6 and 17 DEGs were down-regulated and up-regulated respectively. The largest numbers of abiotic stress-related DEGs were cupin domain protein- (3), methyltransferase- (3), and SPX domain protein-related (3) genes identified at the initial imbibition stage in rice. The transcript of heat shock protein DnaJ (LOC_Os08g35160) was most significantly up-regulated; however the transcript of cupin domain protein (LOC_Os03g59010) was most significantly down-regulated at the initial imbibition stage in rice.
Expression pattern of abiotic stress-related differentially expressed genes (DEGs) in embryo and endosperm during 24 h imbibition stage in rice using Genevestigator (http://www.genevestigator.com). a Genes involved in pathway and b gene expression pattern. Red, up-regulation; gray, no change; blue, down-regulation. Values represent the fold changes of gene expression. (Color figure online)
The expression patterns of these DEGs in embryo and endosperm during seed germination were further explored using Genevestigator (http://www.genevestigator.com; Fig. 5b). The similar expression patterns were observed that LOC_Os09g24900, LOC_Os10g33720 and LOC_Os01g66110 corresponding to methyltransferases, and LOC_Os08g44850, LOC_Os03g18600 and LOC_Os07g47620 corresponding to C2 domain protein, cyclase/dehydrase family protein, and universal stress protein domain protein, respectively, were significantly induced in the embryo and endosperm during seed germination in rice. However, the expression of LOC_Os08g08960 corresponding to cupin domain protein was significantly reduced in rice. Expressions of these abiotic stress-related genes might contribute to stress tolerance during seed imbibition in rice.
Antioxidant-related DEGs involved in the initial seed imbibition
A total of 35 antioxidant-related DEGs, including ascorbates and glutathiones (5), redox hemes (4), glutaredoxins (3), PODs (10) and GSTs (12), were identified at the initial imbibition stage in rice (Table 1; Fig. 6a). By comparison, the majority of DEGs were up-regulated in the initial imbibition stage; 2 and 33 DEGs were down-regulated and up-regulated respectively. Of them, the majority of DEGs were GST-related genes followed by POD-related genes. The transcript of POD (LOC_Os06g35480) was most significantly up-regulated at the initial imbibition stage of seed germination in rice.
Expression pattern of antioxidant-related differentially expressed genes (DEGs) in embryo and endosperm during 24 h imbibition stage in rice using Genevestigator (http://www.genevestigator.com). a Genes involved in pathway and b gene expression pattern. Red, up-regulation; gray, no change; blue, down-regulation. Values represent the fold changes of gene expression
The expression patterns of these genes in embryo and endosperm during seed germination were further conducted using Genevestigator (http://www.genevestigator.com; Fig. 6b). The higher expressions of antioxidant-related DEGs (LOC_Os03g13150, LOC_Os10g22070 and LOC_Os10g38600 not available) were observed in embryo than in endosperm during seed germination. Particularly, the continuously higher expressions in embryo were observed in three redox.heme- and eight misc.peroxidases-related genes during seed germination. The similar expression patterns were also observed in several genes during seed germination. For example, the highest expressions of LOC_Os01g27210 and LOC_Os09g20220 encoding GSTs were observed at 4 h imbibition stage, and after that stage those expressions were decreased gradually during seed germination. These antioxidant-related genes might contribute to the increase of imbibition-associated stress tolerance in rice.
Glutathione S-transferases involved in the initial seed imbibition
Based on above results, the GST- and POD-related genes are important stress response regulators at the initial imbibition stage of seed germination in rice. To validate RNA sequencing results and to explore the roles of antioxidant-related DEGs, the expressions of GSTs- and PODs-related genes were conducted during seed germination in rice. The consistent change trends were observed in the majority of GSTs- and PODs-related DEGs between RNA-Seq and qRT-PCR approaches; their expressions were significantly induced in germinating seeds compared to dry seeds (Figs. 7, 8). Interestingly, the higher expressions were observed at the late (36 h and/or 48 h) imbibition stage in the majority of PODs-related DEGs (Fig. 7), while the higher expressions of the majority of GST genes were observed at the initial (8 h) imbibition stage (Fig. 8).
Meanwhile, physiological analysis indicated that the activities of PODs were slightly changed at the initial (0 to 12 h) imbibition stage, after that its activities were significantly increased with seed germination (12 to 48 h) in rice (Fig. 9a). However, the activities of GSTs were firstly (0 to 8 h) increased and then decreased (8 to 48 h) during seed germination in rice (Fig. 9b). The highest activities of PODs and GSTs were observed at the late (48 h) and initial (8 h) imbibition stage, respectively, which coincided with the gene expression patterns during seed germination. The levels of H2O2 were firstly increased and then decreased at the initial (0 to 8 h) imbibition stage in rice, after that its levels were significantly increased with seed germination (12 to 48 h; Fig. 9c). A significant reduction of H2O2 level was observed at 8 h imbibition stage, which coincided with the higher gene expressions and enzyme activities of GSTs at that time. These results suggested that GSTs might play important roles for preventing H2O2 accumulation at the initial imbibition stage in rice.
Discussion
When mature dry seeds are placed in water, seeds activate a series of mechanisms to avoid imbibition damage. In this study, to reveal the gene responses involved in the initial seed imbibition, the DEGs were analyzed in imbibed seeds (0 vs. 8 h) in rice. Previously, the genes associated with desiccation, storage reserve, cell wall biosynthesis, cell defense and rescue, as well as proteolysis have been identified as the important regulators of seed germination in rice (Wei et al. 2015; Liu et al. 2016). In this study, the characteristics of signalling-, cell wall-, abiotic stress- and antioxidant-related DEGs involving in stress responses pathway were focused at the initial imbibition stage of seed germination in rice.
In plants, RLKs were firstly implicated in the regulation of development, pathogen responses and recognition events (Vaid et al. 2013). The greater number of rice RLKs is mostly involved in pathogen responses (Morillo and Tax 2006). However, lectin receptor-like kinases (LecRLKs) play important roles in seed germination of rice (Cheng et al. 2013), and in ABA signalling during seed germination of Arabidopsis (Deng et al. 2009). In this study, several types of receptor kinase involved in the early germination were identified in rice. For instance, the expressions of OsFLS2 encoding a RLK with an extracellular LRR, two genes encoding Ser/Thr RLK, and one gene encoding S-domain RLK were significantly induced in the early germination in rice. It has reported that OsFLS2 mediated the perception of bacterial flagellins in rice (Wang et al. 2015), whereas the functions of other two types of RLKs are understood limitedly in rice. Meanwhile, two wall-associated kinases (WAKs) OsWAK91 and OsWAK92 were identified in this study. The rice OsWAK91 and OsWAK92 have been shown to be positive regulators of blast resistance, in which OsWAK91 is required for H2O2 production and sufficient to enhance defense gene expression during infection (Delteil et al. 2016). RLKs are located at the plasma membrane (Steinwand and Kieber 2010), and their roles in development include the recognition, the regulation of cell division and cell expansion, and differentiation (Morillo and Tax 2006). We proposed that the RLKs identified here play important roles in the early germination by recognizing internal and environmental signals and activating downstream signalling cascades. More research is required to unravel the molecular mechanisms of RLKs regulating seed germination in rice.
It is well known that the synthesis, degradation and modification of cell wall play important roles on seed germination (Endo et al. 2012). In this study, the DEGs, involving in cell wall proteins, cell wall degradation, cell wall modification, and cell wall pectinesterases, were identified at the initial imbibition stage of seed germination in rice. Of them, the largest number of DEGs was cell wall modification-related genes (expansins) followed by cell wall pectinesterases-related genes (pectinesterases), and cell wall degradation-related genes (polygalacturonases). Expansins are proteins that have previously been shown to loosen and modify the plant cell wall during growth and adaptation to stress (Cosgrove 2005). Pectins are often modified in plants exposed to drought stress, possibly because pectins form hydrated gels which limit the damage to cells (Leucci et al. 2008). Polygalacturonase is one of the hydrolases responsible for cell wall pectin degradation, which involved in biotic and abiotic stress in plants. For example, overexpression of OsBURP16, encoding the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice (Liu et al. 2014). We speculated that the upregulated genes of expansins, pectinesterases and polygalacturonases identified here will contribute to imbibition-associated stress tolerance at the initial imbibition stage of seed germination in rice. The precise contributions of cell wall-related genes to imbibition-associated stress tolerance are deserved further investigation in rice.
The initial imbibition is critical for tolerance to imbibition-associated stress (Bewley et al. 2013). In this study, the abiotic stress-related DEGs, mainly cupin domain protein- and SPX domain protein-related genes, were identified at the initial imbibition stage in rice. Our previous study indicated that two cupin domain proteins (LOC_Os03g57960 and LOC_Os03g21790) involved in seed imbibition under salt stress in rice (Xu et al. 2017). Here other three cupin domain proteins (LOC_Os03g59010, LOC_Os03g48750 and LOC_Os08g08960) involving in the initial imbibition were identified under normal condition in rice. Of them, overexpression of LOC_Os03g48750 (OsOXO1) can improve the resistance to rice blast and bacterial blight (Zhang et al. 2013); the cupin domain protein gene LOC_Os08g08960 (OsGLP8-2) as rice germin-like proteins was involved in the early stress responses (Davidson et al. 2010). In rice, the SPX domain proteins OsSPX1 (LOC_Os06g40120) and OsSPX2 (LOC_Os02g10780) were involved in the Pi-sensing process (Wang et al. 2014), and down-regulation of OsSPX1 caused transgenetic rice high sensitivity to cold and oxidative stresses at seedling stage (Wang et al. 2013). Interestingly, these two SPX domain proteins OsSPX1 and OsSPX2 were also identified in the initial imbibition stage of rice in this study. We speculated that these above abiotic stress-related DEGs might play important roles in imbibition-associated stress tolerance at the initial imbibition stage of seed germination in rice.
Antioxidant enzymes, including catalases, superoxide dismutases, PODs and enzymes in the ascorbate–glutathione cycle, are the most active and efficient protective mechanism against ROS (Halliwell and Gutteridge 2007). Genes for glutathione peroxidases, redox metabolism and superoxide dismutases were reported to be activated during seed germination in barley (Sreenivasulu et al. 2008). In this study, we found that antioxidant-related genes, mainly GSTs and PODs, were significantly induced at the initial imbibition stage of seed germination in rice. Particularly, the significantly higher gene expression and enzyme activity of GSTs were observed at the initial (8 h) imbibition stage of seed germination in rice. GSTs are a ubiquitous superfamily of multifunctional enzymes involved in cellular detoxification of a wide variety of endobiotic and xenobiotic substrates by conjugating the tripeptide (γ-Glu-Cys-Gly) glutathione (Dixon et al. 2002). Some GSTs consist of the ROS-producing and -scavenging network genes which are essential for ROS maintenance and regulation (Chen et al. 2012; Yang et al. 2016). In this study, the reduction of H2O2 was observed at 8 h imbibition stage presumably due to the scavenging actions of GSTs in rice.
The oxidative damage due to the accumulation of ROS has been widely documented during seed germination (Ventura et al. 2012; Li et al. 2017; Macovei et al. 2017). ROS can also act as signalling molecules and trigger the activation of transcription factors, as well as the regulation of gene expression, cellular elongation and endosperm weakening during seed germination (Bailly et al. 2008; Kumar et al. 2015; Macovei et al. 2017). In this study, the maintaining of a proper H2O2 level by GSTs at the initial imbibition stage will contribute to the following successful seed germination in rice. Previously, GSTs have been proposed to afford protection under various stress conditions by detoxifying endogenous plant toxins that accumulate due to increased oxidative stress (Chen et al. 2012; Xu et al. 2018). In rice, overexpression of GSTs (OsGSTU30) can improve heavy metal and drought stress tolerance (Srivastava et al. 2019). The potential regulatory function of GSTs on oxidative stress tolerance at the initial imbibition stage of seed germination requires further confirmation in future.
In summary, an integrated RNA-Seq and physiological study was employed to reveal gene responses at the initial imbibition stage (Phase I) of seed germination in rice in this study. Here the signalling-, cell wall-, abiotic stress-, and antioxidant-related genes that associated with stress responses pathway involving in the initial seed imbibition were highlighted. Our results provided new information on the understanding gene responses in the early seed germination of rice. The functions of these genes need to be further studied for understanding the molecular mechanisms of seed germination in the future.
Data availability
The RNA sequencing data have been submitted to the Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra/) under accession number PRJNA544406.
References
An YQ, Lin L (2011) Transcriptional regulatory programs underlying barley germination and regulatory functions of gibberellin and abscisic acid. BMC Plant Biol 11:105
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Basbouss-Serhal I, Leymarie J, Bailly C (2016) Fluctuation of Arabidopsis seed dormancy with relative humidity and temperature during dry storage. J Exp Bot 67:119–130
Bewley JD, Bradford KJ, Hilhorst HW, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy, 3rd edn. Springer, Heidelberg, pp 145–147
Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP (2012) Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol 158:340–351
Cheng X, Wu Y, Guo J, Du B, Chen R, Zhu L, He G (2013) A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J 76:687–698
Cheng J, Wang L, Zeng P, He Y, Zhou R, Zhang H, Wang Z (2017) Identification of genes involved in rice seed priming in the early imbibition stage. Plant Biol 19:61–69
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Dametto A, Sperotto RA, Adamski JM, Blasi ÉA, Cargnelutti D, de Oliveira LF, Ricachenevsky FK, Fregonezi JN, Mariath JE, da Cruz RP, Margis R, Fett JP (2015) Cold tolerance in rice germinating seeds revealed by deep RNAseq analysis of contrasting indica genotypes. Plant Sci 238:1–12
Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB (2016) Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol 16:17
Deng K, Wang Q, Zeng J, Guo X, Zhao X, Tang D, Liu X (2009) A lectin receptor kinase positively regulates ABA response during seed germination and is involved in salt and osmotic stress response. J Plant Biol 52:493–500
Davidson RM, Manosalva PM, Snelling J, Bruce M, Leung H, Leach JE (2010) Rice germin-like proteins: allelic diversity and relationships to early stress responses. Rice 3:43–55
Dixon DP, Lapthorn A, Edwards R (2002) Plant glutathione transferases. Genome Biol 3:REVIEWS3004
Endo A, Tatematsu K, Hanada K, Duermeyer L, Okamoto M, Yonekura-Sakakibara K, Saito K, Toyoda T, Kawakami N, Kamiya Y, Seki M, Nambara E (2012) Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynthesis and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds. Plant Cell Physiol 53:16–27
Gao LL, Xue HW (2012) Global analysis of expression profiles of rice receptor-like kinase genes. Mol Plant 5:143–153
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. J Free Radic Biol Med 1:331–332
Han C, Yang P (2015) Studies on the molecular mechanisms of seed germination. Proteomics 15:1671–1679
He D, Yang P (2013) Proteomics of rice seed germination. Front Plant Sci 4:246
He D, Han C, Yao J, Shen S, Yang P (2011) Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics 11:2693–2713
He Y, Cheng J, He Y, Yang B, Cheng Y, Yang C, Zhang H, Wang Z (2019) Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotechnol J 17:322–337
Holdsworth MJ, Bentsink L, Soppe WJ (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. N Phytol 179:33–54
Houston K, Tucker MR, Chowdhury J, Shirley N, Little A (2016) The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front Plant Sci 7:984
Jimenez A, Hernandez JA, Del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Karmous I, Trevisan R, El Ferjani E, Chaoui A, Sheehan D (2017) Redox biology response in germinating Phaseolus vulgaris seeds exposed to copper: evidence for differential redox buffering in seedlings and cotyledon. PLoS ONE 12:e0184396
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36
Kumar JSP, Prasad RS, Banerjee R, Thammineni C (2015) Seed birth to death: dual actions of reactive oxygen species in seed physiology. Ann Bot 116:663–668
Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4:112–166
Leucci MR, Lenucci MS, Piro G, Dalessandro G (2008) Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J Plant Physiol 165:1168–1180
Li WY, Chen BX, Chen ZJ, Gao YT, Chen Z, Liu J (2017) Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. Int J Mol Sci 18:110
Liu H, Ma Y, Chen N, Guo S, Liu H, Guo X, Chong K, Xu Y (2014) Overexpression of stress-inducible OsBURP16, the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant Cell Environ 37:1144–1158
Liu SJ, Xu HH, Wang WQ, Li N, Wang WP, Møller IM, Song SQ (2015) A proteomic analysis of rice seed germination as affected by high temperature and ABA treatment. Physiol Plant 154:142–161
Liu SJ, Xu HH, Wang WQ, Li N, Wang WP, Lu Z, Møller IM, Song SQ (2016) Identification of embryo proteins associated with seed germination and seedling establishment in germinating rice seeds. J Plant Physiol 196–197:79–92
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2– DDC(T) method. Methods 25:402–408
Lou Q, Chen L, Sun Z, Xing Y, Li J, Xu X, Mei H, Luo L (2007) A major QTL associated with cold tolerance at seedling stage in rice (Oryza sativa L.). Euphytica 158:87–94
Macovei A, Pagano A, Leonetti P, Carbonera D, Balestrazzi A, Araujo SS (2017) Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: implications on seed technology traits. Plant Cell Rep 36:669–688
Mccormac AC, Keefe PD (1990) Cauliflower (Brassica oleracea L.) seed vigour: imbibition effects. J Exp Bot 41:893–899
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Morillo SA, Tax FE (2006) Functional analysis of receptor-like kinases in monocots and dicots. Curr Opin Plant Biol 9:460–469
Morris ER, Walker JC (2003) Receptor-like protein kinases: the keys to response. Curr Opin Plant Biol 6:339–342
Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR (2013) Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol 161:305–316
Novaković L, Guo T, Bacic A, Sampathkumar A, Johnson KL (2018) Hitting the wall-sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants (Basel) 7:E89
Oracz K, Karpiński S (2016) Phytohormones signaling pathways and ROS involvement in seed germination. Front Plant Sci 7:864
Osakabe Y, Maruyama K, Seki M, Satou M, Shinozaki K, Yamaguchi-Shinozaki K (2005) Leucine-rich repeat receptor-like kinase 1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis Plant Cell 17:1105–1119
Osakabe Y, Mizuno S, Tanaka H, Maruyama K, Osakabe K, Todaka D, Fujita Y, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis J Biol Chem 285:9190–9201
Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M, Graner A, Wobus U (2008) Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol 146:1738–1758
Srivastava D, Verma G, Chauhan AS, Pande V, Chakrabarty D (2019) Rice (Oryza sativa L.) tau class glutathione S-transferase (OsGSTU30) overexpression in Arabidopsis thaliana modulates a regulatory network leading to heavy metal and drought stress tolerance. Metallomics 11:375–389
Steinwand BJ, Kieber JJ (2010) The role of receptor-like kinases in regulating cell wall function. Plant Physiol 153:479–484
Su L, Lan Q, Pritchard HW, Hua X, Wang X (2016) Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol Biochem 109:406–415
Tenhaken R (2015) Cell wall remodeling under abiotic stress. Front Plant Sci 5:771
Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M (2009) A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant Cell Environ 32:1211–1229
Vaid N, Macovei A, Tuteja N (2013) Knights in action: lectin receptor-like kinases in plant development and stress responses. Mol Plant 6:1405–1418
Ventura L, Donà M, Macovei A, Carbonera D, Buttafava A, Mondoni A, Rossi G, Balestrazzi A (2012) Understanding the molecular pathways associated with seed vigor. Plant Physiol Biochem 60:196–206
Wang C, Wei Q, Zhang K, Wang L, Liu F, Zhao L, Tan Y, Di C, Yan H, Yu J, Sun C, Chen WJ, Xu W, Su Z (2013) Down-regulation of OsSPX1 causes high sensitivity to cold and oxidative stresses in rice seedlings. PLoS ONE 8:e81849
Wang WQ, Liu SJ, Song SQ, Moller IM (2015) Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol Biochem 86:1–15
Wang Z, Ruan W, Shi J, Zhang L, Xiang D, Yang C, Li C, Wu Z, Liu Y, Yu Y, Shou H, Mo X, Mao C, Wu P (2014) Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc Natl Acad Sci USA 111:14953–14958
Wang Z, Wang J, Bao Y, Wu Y, Zhang H (2011) Quantitative trait loci controlling rice seed germination under salt stress. Euphytica 178:297–307
Wei T, He Z, Tan X, Liu X, Yuan X, Luo Y, Hu S (2015) An integrated RNA-Seq and network study reveals a complex regulation process of rice embryo during seed germination. Biochem Biophys Res Commun 464:176–181
Xu J, Zheng AQ, Xing XJ, Chen L, Fu XY, Peng RH, Tian YS, Yao QH (2018) Transgenic Arabidopsis plants expressing grape glutathione S-transferase gene (VvGSTF13) show enhanced tolerance to abiotic stress. Biochemistry 83:755–765
Xu E, Chen M, He H, Zhan C, Cheng Y, Zhang H, Wang Z (2017) Proteomic analysis reveals proteins involved in seed imbibition under salt stress in rice. Front Plant Sci 7:2006
Yang G, Xu Z, Peng S, Sun Y, Jia C, Zhai M (2016) In planta characterization of a tau class glutathione S-transferase gene from Juglans regia (JrGSTTau1) involved in chilling tolerance. Plant Cell Rep 35:681–692
Yang P, Li X, Wang X, Chen H, Chen F, Shen S (2007) Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 7:3358–3368
Zhang XY, Nie ZH, Wang WJ, Leung DW, Xu DG, Chen BL, Chen Z, Zeng LX, Liu EE (2013) Relationship between disease resistance and rice oxalate oxidases in transgenic rice. PLoS ONE 8:e78348
Acknowledgements
This work was supported by the National Key Research and Development Plan (Grant No. 2018YFD0100901), the Guangdong Province Key Research and Development Program (Grant No. 2018B020202012), the National Natural Science Foundation of China (Grant No. 31771889), the Guangdong Province Key Laboratory of Plant Molecular Breeding (Grant No. GPKLPMB201903), and the Major Scientific Research Projects of General Colleges and Universities of Guangdong Province (Grant No. 2017KTSCX024).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, J., He, Y., Li, X. et al. An integrated RNA-Seq and physiological study reveals gene responses involving in the initial imbibition of seed germination in rice. Plant Growth Regul 90, 249–263 (2020). https://doi.org/10.1007/s10725-019-00567-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00567-2