Abstract
Satsuma mandarin fruit (Citrus unshiu Mark.) photosynthesizes as comparable to leaf at about 100 days after full bloom (DAFB). In this study, translocation and accumulation of fruit-fixed photosynthate were investigated by using 14CO2. When fruit at 108 DAFB was exposed to 14CO2 for 48 h under 135 photosynthetic photon flux density (PPFD), 14C-sucrose, 14C-glucose and 14C-fructose were detected not only in flavedo but juice sac; more than 50 % of fruit assimilated 14C-sugars were present in juice sac. Thus, majority of rind-fixed photosynthate are infiltrated into juice sac and accumulated there within 48 h after assimilation. Although 14C-sucrose was predominant at flavedo where high SS (sucrose synthase) activity toward synthesis was present, the amount decreased gradually from the outside (flavedo) to the inside (juice sac) of fruit. In vascular bundle, strong SS toward cleavage and soluble acid invertase activities were involved, and 14C-fructose was predominant in juice sac. Accordingly, rind-fixed photosynthate is once converted to sucrose, the translocated sugar in Citrus, at flavedo by SS toward synthesis, and loaded on vascular bundle through symplastic and/or apoplastic movement in the albedo tissue. In the vascular bundle, sucrose may be degraded by SS toward cleavage and invertase, and resulting hexoses transported symplastically to the juice sac through juice stalk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar content is one of the most important characters for determining fruit quality, and the sugar usually depends on photosynthate assimilated at leaf. Shaded fruits or fruits bearing inside the canopy, however, are often impeded sugar accumulation compared with sunny fruits. Fruit bagging also causes negative effects on sugar increase in the fruit (Arakawa et al. 1994; Huang et al. 2009; Watanabe et al. 2011; Hiratsuka et al. 2012; Ryuhan et al. 2013), which is often conducted in Japanese commercial orchards to protect fruits from fungal, insect, and physical damages, and to develop coloration on fruits as well. These facts suggest the significance of light on fruit surface for accumulating sugars in the fruit, but no experimental proof has been made.
Fruit photosynthesis is known in several plant species including Citrus (Blanke and Lenz 1989; Blanke and Bower 1991; Blanke 1995; Hiratsuka et al. 2012), and the photosynthate largely contributes to crop production in Coffea (Lopez et al. 2000). However, almost no data is available on the role of fruit-fixed photosynthate in sugar increase in the fruit. Meanwhile, the transportation of leaf-fixed photosynthate is well known in Citrus; the photosynthate is once converted to translocated sugar, the sucrose, at leaf, then it is loaded on vascular bundle and transported to the fruit (Koch 1984; Lowell et al. 1989). Inside the fruit, thereafter, the sucrose is mainly loaded on dorsal vascular bundle, and moved to juice sac symplastically and/or apoplastically through non-vascular juice stalk (Koch 1984; Lowell et al. 1989; Koch and Avigne 1990). However, it is completely unclear how the fruit-fixed photosynthate is translocated to the inside of fruit.
The present study, therefore, aimed to determine whether the fruit-fixed photosynthate accumulates in juice sac, and the pathway of the photosynthate, from flavedo to juice sac, by using fruits exposed to 14CO2. Fruits were used at ~100 days after full bloom (DAFB), because Satsuma mandarin fruit photosynthesizes actively at this stage as reported earlier (Hiratsuka et al. 2012), perhaps due to normal stoma function on the fruit (Hiratsuka et al. 2015). Finally, sucrose-metabolizing enzyme activities were determined to understand the “translocated sugar” at each fruit tissue, because fruit-fixed photosynthate was proved to be once converted to sucrose at flavedo in above experiments.
Materials and methods
Plant materials
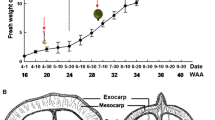
Three fully-grown trees of Satsuma mandarin (Citrus unshiu Marc., cv. Uenowase) were used at the Experimental Farm of Mie University, Tsu, Mie, Japan. In 2013, to know 14C-sugar accumulation into fruits at different developmental stages, i.e. small green fruit (70 DAFB, ~23 mm diameter), photosynthetically active fruit (98 DAFB, ~42 mm diameter) (Hiratsuka et al. 2012, 2015) and fully developed fruit (209 DAFB, ~60 mm diameter), three fruits were subjected to 14CO2 feeding study, respectively. In 2014, to understand detailed translocation manners of fruit-fixed photosynthate, fruits at 108 DAFB were subjected to 14CO2 feeding and enzyme activity determination, respectively. At 108 DAFB, fruits were ~1 month before the onset of coloration and their average diameter was 45 mm.
14CO2 feeding
Developmentally uniform three fruits were placed on wet filter paper in a transparent acryl box to make the stem cut surface contact with the wet paper, and 3.49 g NaHCO3 and 5.6 M Bq of NaH14CO3 (American Radiolabeled Chemicals Inc., USA) were put in a bottle equipped inside the box. Into the bottle, 80 % lactic acid was introduced to generate 14CO2, where the CO2 concentration was 500 ppm. Then, the box was irradiated with 135 photosynthetic photon flux density (PPFD) light supplied by halogen lamps (Sumita Optical Glass, Saitama, Japan), and incubated for 48 h at 25 °C. After incubation, fruits were brought out, washed with distilled water and stored at −80 °C until use.
Extraction of sugars and HPLC analysis
To compare differences in 14C-sugar accumulation into fruits between different developmental stages, juice was squeezed from juice sacs of 70, 98 and 209 DAFB fruits respectively, centrifuged at 8000×g for 5 min, and resulting supernatant was subjected to HPLC analysis. Before subjecting to HPLC analysis, sample solution was filtered through Millipore membrane (0.45 µm, Japan Millipore Co. Ltd., Japan). Meanwhile, to examine detailed translocation and accumulation manners of rind-fixed photosynthate, 14C-fed fruit at 108 DAFB was separated into flavedo, albedo, vascular bundle, segment epidermis and juice sac, respectively (Fig. 1). Then, the tissue was ground by mortar and pestle in 70 % EtOH made by 0.1 M phosphate buffer (pH 7.0), and boiled for 15 min. After the sample was centrifuged at 17,000×g for 5 min, the supernatant evaporated in vacuo and adjusted the volume with H2O. In juice sac sample, juice centrifuged was used directly as described above.
The HPLC equipment and analytical conditions were as follows; pump = LC-10AD (Shimadzu Co. Ltd., Japan), column = ZIC®-pHILIC PEEK (Shimadzu Co. Ltd., Japan), column temperature = 40 °C, eluent = 200 mM ammonium formate:acetonitrile = 25:75, flow rate = 2 ml min−1, detector = RID-10AD (Shimadzu Co. Ltd., Japan). The eluent was fractionated at 30 s intervals and 14C was counted in each fraction. Each sugar was identified by comparing its retention time with standard one. Experiments were repeated, at least, three times using three fruits and data were expressed as dpm g−1 fw or dpm fruit−1.
Estimation of 14C-sugar amount per fruit
Because sucrose, glucose and fructose were detected as major sugars in the sample (Supplementary Fig. 1), the sum of 14C-sugar per fruit was calculated by using data on the amount of 14C-sucrose, 14C-glucose, 14C-fructose and fresh weight of respective tissues. At 70, 98 and 209 DAFB in 2013, average fresh weight of juice sacs was 9.4, 47, and 91.3 g, respectively. At 108 DAFB in 2014, each tissue per fruit was as follows; flavedo = 11.4 g, albedo = 9.7 g, vascular bundle = 0.6 g, segment epidermis = 7.4 g, and juice sac = 65.0 g.
Determination of sucrose-metabolizing enzyme activity
Since 14C-sucrose was predominant at flavedo but decreased at the inside of fruit in above experiments, enzyme activities for both the synthesis and cleavage of sucrose were measured in respective tissues. Enzymes tested were sucrose synthase toward synthesis (SS-syn, EC: 2.4.1.13), sucrose phosphate synthase (SPS, EC: 2.4.1.14), sucrose synthase toward cleavage (SS-cleav, EC: 2.4.1.13), soluble acid invertase (INV-SA, EC: 3.2.1.26), insoluble acid invertase (INV-IA, EC: 3.2.1.26), soluble neutral invertase (INV-SN, EC: 3.2.1.26), and insoluble neutral invertase (INV-IN, EC: 3.2.1.26).
Developmentally uniform three fruits without 14CO2 treatment were used. Each fruit was separated into respective tissues as described above, and proteins were extracted according to the method of Kubo et al. (2001) with slight modifications. Briefly, the tissue was powdered in liquid nitrogen by using motor and pestle, and Polyclar-AT® (0.3 g g−1 fw, Sigma, USA) and extraction buffer (0.05 M Tris-HCl (pH 7.8) containing 150 mM NaCl, 1 mM CaCl2, 10 mM KCl, 10 mM l-cysteine, 1 mM ascorbic acid, 1 mM Na2-EDTA) were added. In juice sac preparations, 2 M Tris-HCl was used instead of 0.05 M buffer because of its high acidity. After stirring the homogenate for 15 min on ice, it was centrifuged at 16,000×g for 15 min and supernatant was obtained. To the resulting sediment, extraction buffer was added again, shaken vigorously, centrifuged, and the supernatant was combined with the first obtained one. Then, proteins in the supernatant were partially purified by sedimenting them between 20 and 100 % (NH4)2SO4 saturation, and subjected to measuring SS, SPS and soluble invertase activity, respectively. For determining insoluble invertase activity, sedimented debris was used.
Protein concentration was determined by the method of Bradford (1976) using BSA as a standard, and enzyme assay was carried out according to the methods of Kubo et al. (2001). Experiments were repeated, at least, three times using three fruits and data were expressed as unit (µmol mg−1protein h−1).
Statistical analysis
The experimental design was carefully set up based on several preliminary experiments and repeated 2 years, from 2013 to 2014. Because similar results were obtained in both years, data in 2014 were presented here, except for data on Supplementary Fig. 2 which were from results in 2013. Statistical analyses were performed for the data obtained by, at least, three replications in each experiment by using Excel (Version 12.3.2; Microsoft, Redmond, WA). Data were expressed means ± SES unless otherwise indicated.
Results
When tissue extract was separated by HPLC, sucrose, glucose and fructose were detected as major sugars (Supplementary Fig. 1), and fractions corresponding to respective sugars contained radioactivity. At gfw base, 14C-sugar accumulation into juice sacs was much larger in the young fruit (70 DAFB), especially in 14C-fructose, compared to developed ones (Supplementary Fig. 2A). At fruit base, however, respective 14C-sugar amounts tended to be larger in 98 DAFB fruit than those in 70 DAFB one (Supplementary Fig. 2B). In 209 DAFB fruit, the accumulation was strictly low at both gfw and fruit base. Although the degree of photosynthate accumulation altered considerably depending on developmental stages of the fruit, active accumulation was confirmed in the fruit at ~100 DAFB.
Comparing the amount of 14C-sugar among tissues from 108 DAFB fruit (Fig. 2A), 14C-sucrose showed large fluctuations; it was predominant at flavedo with ~3600 dpm g−1 fw but decreased gradually advancing toward the inside of fruit, ~200 dpm g−1 fw at juice sac. The 14C-glucose level was relatively constant in all tissues (~400 to ~600 dpm g−1 fw), though vascular bundle contained somewhat large amount. Although the 14C-fructose was predominant in the juice sac, almost constant radioactivity was detected in other tissues, ~400 to 600 dpm g−1 fw. When 14C-sugar amount per fruit was calculated based on fresh weight of each tissue, 14C-sucrose was also major in flavedo and 14C-fructose was in juice sac, respectively; 14C-sucrose occupied ~64 % of total radioactive sugars in flavedo and 14C-fructose ~67 % in juice sac (Fig. 2B).
The sum of 14C-sugars in each tissue is shown in Fig. 3A; ~4600 dpm g−1 fw sugars presented in flavedo, and the amount decreased gradually to ~1800 dpm g−1 fw with advancing toward the inside of fruit. When 14C-sugar amount was compared on fruit base, more than 50 % of fruit-assimilated sugars were infiltrated into the juice sac (Fig. 3B). Thus, rind-fixed photosynthate was transported to the inside of fruit and majority of them accumulated in juice sacs within 48 h after CO2 assimilation.
Activities of sucrose-metabolizing enzymes were conspicuous in flavedo and vascular bundle (Fig. 4). At the flavedo, higher activities of SS toward synthesis (~2.5U) and SPS (~1U) were found compared to other tissues (<0.4U) except for vascular bundle (~3U SS-syn and ~1.5U SPS) (Fig. 4A). Meanwhile, though extremely higher activities of SS toward cleavage (~6U) and soluble acid invertase (~3U) were detected in vascular bundle, other tissues showed considerably lower activities of sucrose cleavage (<1U) (Fig. 4B). When total units of activities for sucrose synthesis and cleavage were calculated at each tissue, activity for the synthesis (~3.5U) was largely superior to degradative one (~1.5U) in flavedo, whereas cleavage action (~10.5U) far surpassed the synthesis one (~4.5U) in vascular bundle (Fig. 4C). Thus, sucrose synthesis and cleavage occurred simultaneously at each fruit tissue, and sucrose seemed to be synthesized at flavedo by SS toward synthesis and SPS using rind-assimilated hexoses, and it degraded gradually on the way to juice sac, especially at vascular bundle by SS toward cleavage and soluble acid invertase.
Sucrose-metabolizing enzyme activities in each tissue of the fruit. A Activity toward synthesis. B Activity toward cleavage. SS-syn sucrose synthase toward synthesis, SPS sucrose phosphate synthase, SS-cleav sucrose synthase toward cleavage, INV-SA soluble acid invertase, INV-IA insoluble acid invertase, INV-SN soluble neutral invertase, INV-IN insoluble neutral invertase. C Total activity for sucrose synthesis and cleavage. Activity toward synthesis = SS-syn + SPS, Activity toward cleavage = SS-cleav + INV-SA + INV-IA + INV-SN + INV-IN
Discussion
When accumulation of rind-fixed photosynthate was compared among juice sacs from young, middle and fully matured mandarin fruits, 14C-sugar amount was conspicuous in young fruit on gfw base, but larger in middle fruit on fruit base. Although young fruit tissue may function very actively on photosynthate accumulation, middle fruit rind seemed to contribute much larger to CO2 assimilation and sugar increase in the fruit, which may be due to both the increase in number of intact stomata on the fruit (Hiratsuka et al. 2015) and the fruit surface area. Detailed analyses of 14C-sugar behavior in the fruit, therefore, were conducted using fruits at ~100 DAFB in this study.
More than 50 % of rind-fixed photosynthate were accumulated as sugars in juice sac of 108 DAFB fruit within 48 h after the CO2 assimilation (Fig. 3B), implies that fruit-assimilated carbon functions on sugar increase in the fruit. Therefore, sugar decrease by fruit bagging is, at least in part, due to inhibition of fruit photosynthesis. Actually, fruit shading caused ~0.3 % sugar reduction in Satsuma mandarin at harvest (Hiratsuka et al. 2012), and ~1.0 % reduction in Japanese pear (Ryuhan et al. 2013). Thus, fruit photosynthate considerably contributes to sugar increase in the fruit, though contribution degree seems to be different largely among plant species. However, since we examined fruits at ~45 days before maturation in this study, it should be confirmed whether photosynthate fixed by young fruit contributes to the sugar content at harvest.
Majority of fruit-fixed photosynthate were present as hexoses in the juice sac, especially as fructose (Fig. 2A), though major accumulated sugar is sucrose in Satsuma mandarin (Kubo et al. 1996, 2001). Since young mandarin fruit usually contains sucrose, glucose, and fructose almost equally, and sucrose concentration increases with fruit maturation (Kubo et al. 1996, 2001), the fructose may be converted to sucrose at later ripening stages in the juice sac. Actually, activity for sucrose synthesis was very low in the juice sac (Fig. 4), but it is activated thereafter with fruit maturation (Kubo et al. 2001). Alternatively, rind-fixed photosynthate may mainly contribute to hexose accumulation. However, it is unexplainable that young fruit accumulated more 14C-sucrose than middle or mature fruit (Supplementary Fig. 2); vascular bundle of young fruit might have much less ability for sucrose cleavage in Satsuma mandarin.
The pathway of leaf-fixed photosynthate into Citrus fruit is relatively well understood (Koch 1984; Lowell et al. 1989). Glucose, the first product of leaf photosynthesis, is once converted to sucrose as a translocated sugar at leaf, and the sucrose is transported to fruit through phloem. Then, it enters mainly into dorsal vascular bundle in the fruit, where the sucrose is partially degraded by SS toward cleavage and invertase. The resulting hexoses are moved to juice sac through non-vascular juice stalk (Koch and Avigne 1990), where the hexoses are converted to sucrose by SS toward synthesis (Kubo et al. 2001). Meanwhile, the route of fruit-fixed photosynthate is completely unclear. Judging from mandarin fruit structure (Fig. 1) and 14C-sugar distribution among fruit tissues (Figs. 2, 3), photosynthate in flavedo may apoplastically move to the inside of fruit through albedo and load on dorsal vascular bundle. Then, it is transported to juice sac apoplastically through juice stalk and accumulated there as the same way with leaf photosynthate.
Although rind-fixed 14C-sugars were detected in the segment epidermis (Figs. 2, 3), it is obscure whether they come via vascular bundle or directly from albedo. Since the leaf 14C-photosynthate is also detectable not only in segment epidermis but albedo of the grapefruit (C. paradise Macf.) (Lowell et al. 1989), the rind photosynthates seem to load on the dorsal vascular bundle first, then a part of them may be provided to segment epidermis to deposit and/or utilize them by its tissue cell.
The amount of 14C-sucrose was predominant at flavedo (Fig. 2), suggests that the hexose produced by fruit photosynthesis would be turned to sucrose there. The high enzyme activity for sucrose synthesis at flavedo (Fig. 4) supports this hypothesis, and this sugar processing is very similar with that of leaf photosynthate (Koch 1984; Lowell et al. 1989); the flavedo functions just like a leaf. During the sucrose transportation to the inside of fruit, more than 60 % of them were degraded at both albedo and vascular bundle (Fig. 2) by SS toward cleavage and soluble acid invertase (Fig. 4B, C). This degradation process of sucrose is also similar to that in leaf photosynthate (Lowell et al. 1989). Meanwhile, each fruit tissue showed both activities for the sucrose synthesis and cleavage simultaneously (Fig. 4). Especially in vascular bundle, though remarkably higher activity for sucrose synthesis (SS-syn + SPS = ~4.5U) was detected, activity toward cleavage (SS-cleav + invertases = ~10.5U) far surpassed the synthesis one (Fig. 4C), may result in severe sucrose degradation there. Thus, reactions for sucrose synthesis and cleavage occur concomitantly at each fruit tissue; the cleavage action may account for constructing and nourishing respective tissue cells. On the other hand, since activities of SS and SPS are known to be controlled by their upstream enzymes such as protein phosphatase and UDP-glucose pyrophosphorylase (Huber and Huber 1996; Coleman et al. 2006), these enzymes may be activated first in the flavedo of Satsuma mandarin. Gene expression of SS (Zhao et al. 2016) and possible occurrence of SS isozymes (Suzuki et al. 1996) may also be involved in SS activation at flavedo. Taken together, the translocated sugar in fruit-fixed photosynthate seems to be sucrose at the outside of Satsuma mandarin fruit but hexoses at the inside, which is controlled precisely by SS toward synthesis, SPS, SS toward cleavage and invertases at each fruit tissue.
The mechanism of fruit photosynthesis is considerably complex; it may be intermediate status between C3, C4, CAM and shade leaf photosynthesis (Blanke and Lenz 1989; Hiratsuka et al. 2015). In addition, since the photosynthetic mechanism may alter during fruit development (Blanke and Lenz 1989; Hiratsuka et al. 2012), detailed research is necessary to understand the translocated sugar and role of fruit-fixed photosynthate in sugar increase in the fruit. Meanwhile, 14C feeding experiments were conducted using detached fruits in this study because of strictly legal limitation of radioisotope use in Japanese orchards. It should be examined whether the same reaction occurs in fruits on the tree.
In conclusion, majority of fruit-assimilated photosynthate are accumulated as sugars in juice sac of Satsuma mandarin. The photosynthate may be once converted to sucrose at flavedo by both SS toward synthesis and SPS, then, the sucrose moved to the inside of fruit with gradual degradation by SS toward cleavage and invertase, and finally accumulated mainly as hexoses in the juice sac.
References
Arakawa O, Uematsu N, Nakajima H (1994) Effect of bagging on fruit quality in apples. Bull Fac Agric Hirosaki Univ 57:25–32 (in Japanese with English summary)
Blanke MM (1995) Regulation of respiration in apple, avocado and citrus orange fruit. Acta Hortic 398:139–146
Blanke MM, Bower JP (1991) Small fruit problem in Citrus trees. Trees 5:239–243
Blanke MM, Lenz F (1989) Fruit photosynthesis. Plant Cell Environ 12:31–46
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Coleman HD, Ellis DD, Gilbert M, Mansfield SD (2006) Up-regulation of sucrose synthase and UDP-glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotech J 4:87–101
Hiratsuka S, Yokoyama Y, Nishimura H, Miyazaki T, Nada K (2012) Fruit photosynthesis and phosphoenolpyruvate carboxylase activity as affected by lightproof fruit bagging in Satsuma mandarin. J Amer Soc Hortic Sci 137:215–220
Hiratsuka S, Suzuki M, Nishimura H, Nada K (2015) Fruit photosynthesis in Satsuma mandarin. Plant Sci 241:65–69
Huang C, Yu B, Teng Y, Su J, Shu Q, Cheng Z, Zeng L (2009) Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci Hortic 121:149–158
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Ann Rev Plant Physiol Plant Mol Biol 47:431–444
Koch KE (1984) The path of photosynthate translocation into citrus fruit. Plant Cell Environ 7:647–653
Koch KE, Avigne WT (1990) Post-phloem, nonvascular transfer in Citrus. Kinetics, metabolism, and sugar gradients. Plant Physiol 93:1405–1416
Kubo T, Maegawa M, Hiratsuka S (1996) Relationship between rind surface morphology and sugar concentration in the juice of Satsuma mandarin fruit. J Japan Soc Hortic Sci 65:447–453 (in Japanese with English summary)
Kubo T, Hohjo I, Hiratsuka S (2001) Sucrose accumulation and its related enzyme activities in the juice sacs of Satsuma mandarin fruit from trees with different crop loads. Sci Hortic 91:215–225
Lopez Y, Riano N, Mosquera P, Cadavid A, Arcila J (2000) Activities of phosphoenolpyruvate carboxylase and ribulose-1, 5-bisphosphate carboxylase/oxygenase in leaves and fruit pericarp tissue of different coffee (Coffea sp.) genotypes. Photosynthetica 38:215–220
Lowell CA, Tomlinson PT, Koch KE (1989) Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing Citrus fruit. Plant Physiol 90:1394–1402
Ryuhan T, Hiratsuka S, Nada K (2013) Effect of lightproof fruit bagging on fruit growth and quality in Japanese pear. Hortic Res 12 (suppl. 2):88 (in Japanese)
Suzuki A, Kanayama Y, Yamaki S (1996) Occurrence of two sucrose synthase isozymes during maturation of Japanese pear fruit. J Amer Soc Hortic Sci 121:943–947
Watanabe M, Oyamada T, Suzuki A, Murakami M, Sugaya S, Komori S, Arakawa O (2011) Comparison of sugar accumulation characteristics in ‘Haruka’ and ‘Fuji’ apple fruits. Hortic Res 10:565–571 (in Japanese with English summary)
Zhao C, Hua LN, Liu XF, Li YZ, Shen YY (2016) Sucrose synthase FaSS1 plays an important role in the regulation of strawberry fruit ripening. Plant Growth Regul. doi:10.1007/s10725-016-0189-4
Acknowledgments
The authors thank to Mr. T. Kurosawa of Mie University for his help in radioisotope operation. This work was supported in part by the Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 19658013 and 23658029).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10725_2016_204_MOESM2_ESM.pptx
Supplementary Fig. 2. Comparison of 14C-sugar accumulation among juice sacs from developmentally different fruits. Juice sacs were sampled after exposing fruits to 14CO2 under 135 PPFD for 48 h. A: Radioactivity on fresh weight base. B: Radioactivity on fruit base. Vertical bars indicate SE. (PPTX 56 KB)
Rights and permissions
About this article
Cite this article
Hiratsuka, S., Nakayama, S., Tamura, S. et al. Translocation and accumulation of fruit-fixed photosynthate in Satsuma mandarin. Plant Growth Regul 81, 277–282 (2017). https://doi.org/10.1007/s10725-016-0204-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0204-9