Abstract
Carbohydrates are an important regulator of canola seed oil content. However, understanding the physiological regulation by carbohydrates governing seed oil accumulation is fragmented. In the present study, activities of sucrose and starch catalytic enzymes, including neutral and alkaline invertase, sucrose synthase (SUS), and starch phosphorylase, and biosynthetic enzymes, including sucrose phosphate synthase and ADP-glucose pyrophosphorylase, were compared in developing silique and seed of high oil content and low oil content lines (HOCL and LOCL, respectively). The results showed that the HOCL had significantly higher total soluble sugar concentration in the developing silique wall and seed during the seed lipid accumulation stage. Strikingly, all the enzymes showed very strong activities at 20 days after anthesis in the silique wall of the HOCL, which was in agreement with the higher amounts of corresponding gene expression. The result indicated that the homeostasis of the high efficiency of cleavage and biosynthetic of carbohydrates in the HOCL was beneficial for the rapid volume expansion of silique and transportation of carbohydrates, i.e., sucrose and starch, to the seed for utilization. At seed deposition stage, all the enzymes exhibited significantly higher activities in the HOCL than in the LOCL, which was helpful for increased production of carbohydrates. The results of gene expression at seed oil deposition stages revealed that one of the SUS genes, SUS3, showed significantly higher transcript amount in the HOCL than in the LOCL at all stages while most of other genes showed no significant differences between two lines at developing stages. Therefore, SUS3 might be played vital roles in the sucrose cleavage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canola seed accumulates considerable amounts of lipids during seed filling and uses photosynthetic assimilates such as sucrose and starch as biosynthetic substrate (Agrawal et al. 2008; Houston et al. 2009). There are three ways to provided photosynthetic assimilates in canola. The developing silique is presumably the main source of carbohydrates. Other contributors consist of green tissues, such as flower pedicel and newly expanded leaves enclosing branches. Further, carbohydrates transported from other senescing leaves are the third pathway (Gammelvind et al. 1996; Hua et al. 2012). However, approximately 70 % of the carbohydrate comes from developing siliques (King et al. 1997). Therefore, the developing silique is a key photoassimilate-providing organ for seed development in canola.
Although canola seed lipid biosynthesis begins with carbohydrate metabolism, the destinations of the intermediates from carbohydrate metabolism vary (i.e., pyruvate in the tricarboxylic acid cycle and fatty acid synthesis) (Hajduch et al. 2006; Agrawal et al. 2008; Houston et al. 2009). Thus, substrate competition between these pathways exists, and such competition may affect the allocation efficiency of carbohydrates into fatty acid biosynthesis. Consequently, the presence of a sufficient amount of carbohydrates is the first step to guarantee a substrate for lipid biosynthesis.
When the photosynthetic products are synthesized, assimilation of the carbohydrates becomes the prerequisite for fatty acid biosynthesis and other biosynthetic pathways (Ruuska et al. 2002). Generally, carbon metabolism starts from the breakdown of sucrose during canola seed filling (White et al. 2000). Invertase and sucrose synthase (SUS) are two enzymatic systems that catalyze sucrose in consequential metabolism (Schmalstig and Hitz 1987; Gutiérrez-Miceli et al. 2002; Fallahi et al. 2008). In canola, considerable amounts of hexose accumulate in the early developing silique and seed (mainly in the form of liquid endosperm) (King et al. 1997; Hill et al. 2003). The higher hexose content in early developing silique might be correlated with the higher expression of acid invertase activity than in other stages (King et al. 1997). However, SUS plays a key role in sucrose breakdown in the developing seed (King et al. 1997). Therefore, different enzymatic systems for carbohydrate metabolism in silique and seed tissues may exist.
Besides sucrose, starch is also an important carbohydrate that is involved in seed development. However, unlike sucrose, starch in the seed is purportedly partially synthesized from sucrose and is not a photosynthetic product (da Silva et al. 1997; Andriotis et al. 2010). Multiple functions of starch in oilseed and Arabidopsis are suggested. First, it is a carbon skeleton for lipid biosynthesis (Angeles-Núñez and Tiessen 2011; Sanjaya et al. 2011). Second, starch is a source of carbon for embryo establishment (da Silva et al. 1997). Third, seed starch is involved in cell division and differentiation during seed development (Andriotis et al. 2010).
In addition to the physiological changes, a considerable number of proteins related to carbohydrate metabolism have been identified via proteomic profiling during seed-filling stage, and such proteins were developmentally regulated. For example, three proteins encoding SUS were isolated and showed maximum expression fold during the mid-seed filling stage in rapeseed (Hajduch et al. 2006; Agrawal et al. 2008). Furthermore, different oil crops show distinct patterns of expression of proteins related to carbohydrate metabolism, including differences in transcript abundance at different developmental stages and different numbers of enzymatic species at the same developmental stages (seed filling) (Agrawal et al. 2008). For example, there were seven isolated proteins encoding SUS in soybean during sucrose cleavage into UDP-glucose, whereas three were found in oilseed rape (Agrawal et al. 2008; Houston et al. 2009). However, characterization of biochemical evidences from carbohydrate metabolism aspects such as enzymatic activities and relative gene expression in seeds with different oil content during seed filling stage are lacking.
In our previous study, we compared the carbohydrate profiles of high oil content line (HOCL) and low oil content line (LOCL) canola, as well as silique chlorophyll and seed oil contents. The results revealed that the HOCL had significant advantages in the production of more carbohydrates over the LOCL. The correlation between carbohydrate and seed oil content uncovered the importance of soluble hexose and seed starch in oil content (Hua et al. 2014). However, the differences of activities of enzymes, such as invertase and SUS, during seed development in different canola seed oil content lines remain unknown. Furthermore, the expression pattern of carbohydrate metabolism-related genes is not understood as well. Hence, the aim of the study was to (1) compare the cleavage activity of sucrose and starch in the HOCL and LOCL, and (2) investigate the expression pattern of carbohydrate-related genes.
Materials and methods
Plant materials and crop husbandry
The field experiment was performed over two growth seasons in 2010–2011 and 2011–2012 at the experimental station of the Zhejiang Academy of Agricultural Sciences, Hangzhou, China. Two recombinant inbred lines (RILs) with high and low oil content were bred by our group through crossing of Huyou 15 and Zheshuang 6 in 2000. Seeds of the two lines were sown in a seedling bed both on 5 October 2010–2011 and 2011–2012. The seedlings in uniform were transplanted after 1 month. Field practices, including disease and pest control, were carried out to maximize the canola seed yield and quality. The soil type in the experimental station was loamy clay (loamy, mixed, and thermic Aeric Endoaquepts). Urea was used as fertilizer and was manually applied into soil at 150 kg N ha−1 before transplanting. An additional 75 kg N ha−1 nitrogen fertilizer (urea) was topdressed around the end of January for each season. No irrigation was applied during the growing seasons because of the sufficient rainfall (1048.3 and 1217.5 mm) in the Hangzhou region.
Experiment design and sampling
The experiment used a randomized complete block design with two canola lines differing seed oil content as experimental treatments. Three replications were performed. The seedlings were planted in 20 m × 3.2 m plots. Each plot was composed of eight rows with 0.2 m spacing between plants and 0.35 m spacing between rows. Flowers in the main inflorescence were tagged from anthesis. Siliques were sampled at 5 days intervals starting from 10 to 65 days after anthsis (DAA). Siliques were collected from similar positions in the main inflorescence to minimize experimental error. Furthermore, margin plants were not chosen to avoid border effect, and five plants were selected for sampling at each developmental stage. The developing siliques and seeds were immediately immersed in liquid nitrogen upon detachment from the main inflorescence of plants and kept at −80 °C until use.
Total soluble sugar concentration measurement
Samples were dried at 80 °C to constant dry weight. The dry sample was ground to a powder using a pulverizer (Wenlin Dalin Machine Com., Ltd., China). The powder was extracted with 30 mL of 80 % (V:V) ethanol. Total soluble sugar was estimated using the method described by Dubios et al. (1956).
Enzyme determination
Invertase, SUS, and sucrose phosphate synthase (SPS) extraction and determination were performed using the method described by King et al. (1997). Starch phosphorylase (SS) and ADP-glucose pyrophosphorylase (AGPase) activities were measured according to the method of Smith (1990), Smith et al. (1989), and da Silva et al. (1997). Enzymatic activity was expressed as activity per milligram of enzyme.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis
Developing seeds (30, 40, and 50 DAA) were detached from silique walls and immediately frozen in liquid nitrogen and kept under low temperature (−80 °C) until use. The sampling stages were based on the previous study, which were key stages for seed lipid deposition (Hua et al. 2014). The samples were ground into powder with liquid nitrogen. Total RNA was extracted using the RNeasy Plant Mini Kit (QIAGEN, Cat. No. 74904) including a treatment with DNase I (Takara, Cat. No. D2215). The RNA was used for cDNA synthesis using a two-step RT-PCR kit (TAKARA, Cat. No. DRR019A) according to the manufacturer’s instructions. The resultant cDNA was subjected to qRT-PCR analysis (LightCycler® 480 system, Roche). The enzymatic genes used for detection and their corresponding primers were listed in Supplementary Table 1. The transcript amount was calculated according to Livak and Schmittgen’s (2001) method. The genes were searched on the Brassica rapa database (www.brassicadb.org/brad/) along with their homologs on Arabidopsis (www.arabidopsis.org). BnACTIN was used as the housekeeping gene. Selection of the reference gene was based on the screening of several housekeeping genes. Because BnACTIN was the most stable among these genes in silique and seed tissues at different developmental stages (Supplementary Table 2), it is reliable for the selection of the gene.
Statistical analysis
Statistical analysis was performed using SPSS (version 17.0). ANOVA was performed on the total soluble sugar concentration and enzymatic activities in tissues. Before analysis, the data sets were assessed using Bartlett’s test, and were found to be homoscedastic. Two-way ANOVA was then performed using growth season and plant line as main factors to determine whether there were any significant differences between growth seasons. The analyses showed there was no significant effect of growth season and the data from different seasons were then combined. Transcription levels were the mean of three biological replicates. The mean values were compared using Duncan’s test at a probability of 0.05.
Results
Dynamics of total soluble sugar and seed oil content in developing silique wall and seed of the HOCL and LOCL
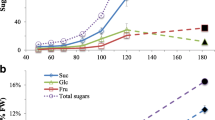
In general, both silique wall and seed showed a similar trend in total soluble sugar concentration during seed development (Fig. 1a, b). Total soluble sugar concentration increased from 10 DAA and peaked at 25 DAA in both tissues. However, total soluble sugar concentration in the silique wall and seed decreased to the initial level at 50 and 55 DAA, respectively (Fig. 1a, b). Total soluble sugar concentration in the HOCL was significantly higher than in the LOCL before 50 DAA in both tissues. At 25 DAA, total soluble sugar concentration in the silique wall and seed of the HOCL was 1.19- and 1.29-fold higher than that of the LOCL, respectively (Fig. 1a, b).
Dynamic of total sugar concentration in developing silique (a) and seed (b) from 10 to 65 days after anthesis (DAA) and seed oil content at 30, 40, and 50 DAA (c) in the combination of two growth seasons (2010–2011 and 2011–2012) of canola high oil content line (HOCL) and low oil content line (LOCL). Seed oil content was cited from Hua et al. (2014). Error bars represent SEs. Asterisk at each developmental stage indicates a significant difference at 5 % probability between HOCL and LOCL in averaged year
From 30 to 50 DAA, an increasing trend of seed oil content was observed (Fig. 1c). The seed oil content in the HOCL was significantly higher than in the LOCL during lipid deposition stages (Fig. 1c). On average, the seed oil content was 7.4 % higher in the HOCL than in the LOCL (Fig. 1c).
Dynamics of sucrose and starch cleavage enzyme activities in the developing silique wall and seed of the HOCL and LOCL
Nuetral invertase activity
Neutral invertase (NI) activity in the silique wall of the HOCL showed a peak at 20 DAA, and the activity was averagely fourfold higher than in other developing stages (Fig. 2a). NI activity from 25 to 40 DAA (early stage of seed lipid deposition) was weaker than from 45 to 55 DAA (middle to late stage of seed lipid deposition) in the silique wall of the HOCL (Fig. 2a). For the LOCL, the silique wall exhibited higher NI activity at an early developmental stage (10 DAA) and then decreased (Fig. 2a). NI activity in the silique wall of LOCL from 15 to 45 DAA was relatively stable. However, from 45 DAA onward, the NI activity in the silique wall drastically increased, and the maximum activity was obtained at 65 DAA in the LOCL (Fig. 2a). When the two lines were compared for NI activity in the silique wall, the HOCL showed higher activity than the LOCL at 20 and 25 DAA (6.1- and 2.2-fold increase, respectively; Fig. 2a). A reverse trend was detected at 60 and 65 DAA, i.e., NI activity in the HOCL was only 47.9 and 16.9 % of that in the LOCL, respectively (Fig. 2a).
Dynamic of sucrose and starch cleavage enzymatic activities in the combination of two growth seasons (2010–2011 and 2011–2012) of canola high oil content line (HOCL) and low oil content line (LOCL) from 10 to 65 days after anthesis. a, b neutral invertase activities in developing silique and seed, c, d alkaline inverase activity in developing silique and seed, e, f sucrose synthase activities in developing silqie and seed, g, h starch phophorylase activity in developing silique and seed. Error bars represent SEs. Asterisk at each developmental stage indicates a significant difference at 5 % probability between HOCL and LOCL in averaged year
Seed NI activity in the HOCL exhibited very few fluctuations from 0 to 35 DAA and then sharply peaked at 40 DAA (Fig. 2b). NI activity from 40 to 50 DAA in the HOCL seed decreased to a level similar to that from 10 to 35 DAA (Fig. 2b). Seed NI activity in the LOCL was high at 10 DAA and then decreased to the lowest point across all developmental stages (Fig. 2b). A slight increasing in NI activity was maintained from 15 to 40 DAA and then rapidly increased until 60 DAA in the LOCL. Seed NI activity in the HOCL from 15 to 45 DAA was significantly higher than in the LOCL (Fig. 2b). The opposite trend was observed from 50 to 60 DAA in both lines. The highest seed NI activity at 40 DAA in the HOCL was almost equal to that in the LOCL at 60 DAA (Fig. 2b).
Alkaline invertase activity
Silique wall alkaline invertase (AI) activity was divided into four sequential stages: 10–20, 25–35, 40–60, and 65 DAA in the LOCL (Fig. 2c). Weak AI activity was found from 10 to 20 DAA and from 40 to 60 DAA. Higher activity occurred between 25 and 35 DAA, with the highest values appearing at 65 DAA. Significantly higher activity was found from 10 to 25 DAA in the HOCL than in the LOCL. Activity in the HOCL was especially high at 20 DAA and was 58-fold higher than in the LOCL at this stage (Fig. 2c). However, at the silique maturation stage, a significantly high AI activity was observed in the LOCL, which was 24-fold higher than that in the HOCL (Fig. 2c).
AI activity in seeds of both lines was initially the same at about 7 nmol h−1 mg−1. In the HOCL, except for a peak at 30 DAA, seed AI activity was constant at about 7 nmol h−1 mg−1 from 10 DAA until 45 DAA after which activity dropped to a low level at 50 and 55 DAA before rising again. In the LOCL, seed AI activity dropped from its initial level to approximately 2 nmol h−1 mg−1 and remained at this level apart from a small peak at 30 DAA and a larger peak at 60 DAA (Fig. 2d).
Sucrose synthase activity
The trend of SUS activity was similar to the two invertases in the developing silique wall of the HOCL (Fig. 2e). Low and stable SUS activity was observed during the silique developing stage except at 20 DAA in the HOCL. The SUS activity in developing silique wall of the LOCL decreased from 10 to 25 DAA and increased at 30 DAA. However the activity decreased again from 30 to 45 DAA in the LOCL. The highest SUS activity in the LOCL was observed at 65 DAA (Fig. 2e). The peak activity of SUS in the HOCL at 20 DAA was 12.0-fold higher than that in the LOCL. However, the SUS activity in the LOCL at 65 DAA was 9.5-fold higher than that in the HOCL. Furthermore, the SUS activity at 20 DAA in HOCL was 1.9-fold higher than that in LOCL at 65 DAA (Fig. 2e).
Seed SUS activity in the HOCL increased from 10 to 20 DAA and then declined drastically until 55 DAA (Fig. 2f). The recovery of the seed SUS activity was observed from 60 DAA onward, which reached the similar level of 10 DAA in the HOCL. The LOCL seed SUS activity rapidly decreased from 10 to 20 DAA and maintained at a similar level from 20 to 35 DAA. However, the seed SUS activity in the LOCL increased from 35 DAA onward, and the maximum value was obtained at 60 DAA. The seed SUS activity in the HOCL was significantly higher than that in the LOCL from 15 to 40 DAA, whereas the opposite trend was found from 40 to 60 DAA. In particular, the seed SUS activity at 10 and 60 DAA in the LOCL increased by 14 and 41 %, respectively, compared with that in the HOCL at 15 DAA (Fig. 2f).
Sarch phosphorylase activity
Starch phosphorylase (SS) activity in the silique wall was very high in both HOCL and LOCL compared with other enzymes, such as invertase and SUS (Fig. 2g). The kinetics of SS activity in the HOCL peaked at 20 DAA. The SS activity in the silique wall of the LOCL was highest at 35 DAA. However, the SS activity of the LOCL at 35 DAA was much less than that of the HOCL at 20 DAA, a reduction of 73 % was observed (Fig. 2g).
Seed SS rapidly increased from 10 to 45 DAA and decreased drastically thereafter in the HOCL (Fig. 2h). The seed SS in the LOCL dropped to the lowest point from 10 to 15 DAA. Although SS activity in the LOCL exhibited an increasing trend from 15 DAA onward, the increment was very limited until 40 DAA. However, the SS activity rapidly increased from 40 to 45 DAA in the LOCL and reached the very close point with the HOCL. The superiority of SS activity in the HOCL over the LOCL was maintained at early to middle seed developmental stages (from 15 to 40 DAA), whereas the trend was reversed at late seed developmental stages (after 45 DAA). Although no significant difference was found in the seed SS activity between HOCL and LOCL at 45 DAA (both attained the maximum value), very significant differences between the two lines were observed from 15 to 40 DAA. On average, the seed SS activity in the HOCL was 3.5-fold higher than in the LOCL from 15 to 40 DAA (Fig. 2h).
Dynamics of sucrose and starch biosynthetic enzyme activities in the developing silique wall and seed of the HOCL and LOCL
Sucrose phosphate synthase activity
Sucrose phosphate synthase (SPS) activity in the silique wall exhibited dynamics similar to the invertase and SUS activity during the developmental stages in the HOCL (Fig. 3a). Silique wall SPS exhibited higher activity at early and late developmental stages than during middle developmental stages (from 15 to 45 DAA) in the LOCL. In general, a significantly higher SPS activity was observed from 20 to 30 DAA in the HOCL compared with the LOCL. Other developmental stages (10, 35, and 50–65 DAA) exhibited the opposite results: significantly higher enzymatic activity was found in the LOCL than in the HOCL. At 20 DAA, the SPS activity in the HOCL was 6.5-fold higher than in the LOCL (Fig. 3a).
Dynamic of sucrose and starch synthesis enzymatic activities in the combination of two growth seasons (2010–2011 and 2011–2012) of canola high oil content line (HOCL) and low oil content line (LOCL) from 10 to 65 days after anthesis. a, b sucrose phosphate synthase activity in developing silique and seed, c, d ADP-glucose pyrophosphorylase activity in developing silique and seed. Error bars represent SEs. Asterisk at each developmental stage indicates a significant difference at 5 % probability between HOCL and LOCL in averaged year
Seed SPS activity fluctuated across all developmental stages in the HOCL, and activity varied from 18 to 153 nmol h−1 mg protein−1 (Fig. 3b). The SPS activity in the LOCL was almost constant from 15 to 40 DAA and was significantly lower than that in the HOCL. However, the SPS activity rapidly increased from 40 DAA onward, and the highest value was obtained at 60 DAA in the LOCL. The SPS activity from 45 to 60 DAA in the LOCL was significantly higher than in the HOCL. At 60 DAA, the SPS activity in the HOCL decreased by 54 % compared with the LOCL (Fig. 3b).
AGPase activity
AGPase activity (Fig. 3c) in the silique wall of the LOCL varied between 10 and 56 nmol h−1 mg−1 and showed peaks of activity at 35, 50, and 65 DAA. In the HOCL, activity was initially similar to that of the LOCL; however, there was a sharp increase in activity at 20 DAA to 160 nmol−1 h−1 mg after which the activity dropped to a minimum at 40 DAA before rising again until 50 DAA and then declining.
Seed AGPase activity (Fig. 3d) in the HOCL increased from 10 to 20 DAA and remained high until 30 DAA after which it declined. In contrast, AGPase activity in the LOCL decreased from 10 to 15 DAA and then remained low until 40 DAA after which peaks of activity were found at 45 and 60 DAA.
Quantification of carbohydrate metabolic related genes in the developing silique wall and seed of the HOCL and LOCL
Since a significant peak of each carbohydrate enzymatic activity appeared in the silique of the HOCL at 20 DAA, we correspondingly measured the carbohydrate metabolic gene expression amount at this stage both in the HOCL and LOCL silique. Results showed that all the genes had higher expression amounts in the silique of the HOCL at 20 DAA. High expression amount of SPS genes in both lines could result in the strong activity of SPS, and hence the higher synthesized silique total soluble sugar concentration at 20 DAA. A starch degradable gene, ATSS2, also showed very high expression amount in the silique of the HOCL at 20 DAA (Fig. 4). Taken together, the HOCL took a priority to drive the higher activity of carbohydrate metabolic enzymes via strong expression of relative genes.
Transcript amount of carbohydrate related transcript in developing silique at 20 days after anthesis (DAA) of canola high oil content line (HOCL) and low oil content line (LOCL). Transcripts of the BnACTIN were monitored and used as a reference. Error bars represent SEs. Asterisk at each developmental stage indicates a significant difference at 5 % probability between HOCL and LOCL
Transcription was measured during seed lipid deposition stages (30, 40, and 50 DAA) as well. Seven genes encoding invertase were selected for analysis. Four of the seven genes encoded cell wall-binding forms, two had products localized in the cytoplasm, and one in the vacuole (Supplementary Table 1). With the exception of the vacuole invertase gene (VAC-INV) at 30 DAA, the transcription of all invertase genes was not significantly different between two lines at the time points measured. The transcript amounts of the three cell wall-binding invertase genes, ATCWINV2, ATCWINV4, and ATCWINV6, increased in both lines from 30 to 50 DAA (Fig. 5a). For ATCWINV1, the transcription increased from 30 to 40 DAA both in the HOCL and LOCL and then declined at 50 DAA. The two cytoplasmic invertase genes showed different expression modes in two lines at the different development stages. Transcription of CINV1 increased in both lines from 30 to 50 DAA. For CINV2, there was little difference in transcription at 30 to 40 DAA, but transcription had increased by more than twofold at 50 DAA. For the vacuole invertase gene (VAC-INV) at 30 DAA, the amount of transcript in the HOCL was 25 % of that in the LOCL. However, the amount of transcript dropped by 88 % in HOCL and by 98 % in the LOCL at 40 and 50 DAA (Fig. 5a).
Dynamics of carbohydrate related transcript in developing seed at 30, 40, and 50 days after anthesis (DAA) of canola high oil content line (HOCL) and low oil content line (LOCL). a Invertase; b sucrose synthase; c sucrose phosphate synthase; d starch phosphorylase; e ADP-glucose pyrophosphorylase; f sugar transporter. Transcripts of the BnACTIN were monitored and used as a referece. Error bars represent SEs. Asterisk at each developmental stage indicates a significant difference at 5 % probability between HOCL and LOCL. ND in (b) was an abbreviation for non-detected
There were several isoforms of SUS. Therefore, we chose five homolog genes with Arabidopsis in the B. rapa database (www.arabidopsis.org and www.brassicadb.org/brad/). The expression patterns of these genes were different (Fig. 5b). First, transcript amounts of the five SUS genes were differentially detected. In general, the order of expression intensity was SUS3 > SUS2 > SUS5 > SUS1 > SUS6 in both lines. Second, transcript amounts of the SUS genes changed differentially as seed development (Fig. 5b). For example, SUS2 and SUS5 increased from 30 to 50 DAA in both lines. However, expression amount of SUS3 increased from 30 to 40 DAA in both lines and then decreased to 22 and 21 % in the HOCL and LOCL from 40 to 50 DAA, respectively. The SUS6 transcript was detected in the HOCL at one stage (30 DAA), whereas this transcript was detected at three stages in the LOCL. A gene, SUS3, was highly expressed throughout the seed developmental stages in the HOCL. Transcript amount of SUS1 in the LOCL was significant higher than that in the HOCL at 30 DAA while there were no significant difference of SUS2, SUS5, and SUS6 between two lines. At 40 DAA, with the exception of SUS3, all the SUS genes showed no markedly differences between two lines. At 50 DAA, two genes, SUS2 and SUS3 exhibited significant difference between two lines. The transcript amount of SUS2 in the LOCL was 1.8-fold higher than in the HOCL while that of SUS3 in the HOCL was 5.4-fold higher than in the LOCL (Fig. 5b).
Transcript amounts of SPS genes increased during seed development in both HOCL and LOCL (Fig. 5c). Furthermore, all the SPS genes showed more increments from 30 to 40 DAA than that from 40 to 50 DAA in both lines. At 30 DAA, transcript of SPS1F in the HOCL was significantly higher than that in the LOCL while SPS1 and SPS3F showed the same trend but the differences were not significant between both lines. At 40 and 50 DAA, three SPS genes exhibited little difference between two lines (Fig. 5c).
Although three starch degradation genes showed an increasing trend in expression as the seed developed, a higher increment was usually found at 30 to 40 DAA (Fig. 5d). The increased fold of transcript amount of three starch degradation genes in the HOCL from 30 to 40 DAA was close. As for the LOCL, ATSS2 increased by 11.2-fold, whereas ATSS3 increased by 3.7-fold from 30 to 40 DAA. As for the two lines, ATSS2, ATSS3, and SSIV, no significant difference of the transcripts were detected at the measured stages (Fig. 5d).
The transcript amount of ADG-glucose pyrophosphorylase genes increased as seed development progressed (Fig. 5e). However, the increment of the transcript of APS1 was small from 40 to 50 DAA in both lines. Unlike APS1, the transcript amount of APS2 from 40 to 50 DAA showed fast increasing, which more than three times in two lines. As for the two lines, a significant difference of transcript amount of APS2 was found only at 30 DAA (Fig. 5e). The expression amount of APS2 at 30 DAA in the HOCL was markedly higher than in the LOCL, which was increased by 82.4 % (Fig. 5e).
Three sugar transporter (SUT) genes were measured in the HOCL and LOCL during seed development (Fig. 5f). SUT4 was the most strongly expressed in both lines as compare with other two SUT genes. For the SUT1, the transcript amount in the HOCL showed similar amount at 30 and 50 DAA while that was higher than that at 40 DAA (Fig. 5f). However, the transcript amount of SUT1 in the LOCL showed decreasing state from 30 to 50 DAA (Fig. 5f). For the SUT2, the transcript amount kept increasing in the HOCL while that increased firstly and then decreased slightly from 30 to 50 DAA in the LOCL (Fig. 5f). For the SUT4, both lines showed increased from 30 to 40 DAA and then decreased from 40 to 50 DAA. However, the transcript amount of SUT4 in the HOCL dropped drastically while that slightly in the LOCL from 40 to 50 DAA, which declined by 66 and 17 %, respectively (Fig. 5f). As for the two lines, significant higher transcript amount of SUT1 was observed at 50 DAA in the HOCL, which was 6.5-fold higher than that in the LOCL (Fig. 5f). However, a reverse trend was found in the SUT4 that the transcript amount in the LOCL was significantly higher than that in the HOCL, which increased by 2.4 times (Fig. 5f).
Discussion
Previous investigations documented that carbohydrate profile, which includes fructose, glucose, sucrose, starch, and cellulose contents, differed significantly between HOCL and LOCL both in developing silique wall and seed in canola (Hua et al. 2014). To further elucidate the physiological mechanism underlying the effect of carbohydrate metabolism on the seed oil content in the HOCL and LOCL, a systematic comparison of carbohydrate metabolism-related enzymes, including invertase, SUS, SPS, SS, and AGPase activities and their corresponding genes, was performed using the two lines during seed development. Significant difference of total soluble sugar concentration was found between HOCL and LOCL during seed lipid accumulation. However, both lines showed the maximum concentration at early seed developmental stage (25 DAA). The result agreed with other reports (King et al. 1997; Hill et al. 2003). Considerable total soluble sugar deposition was observed in the early developing silique wall and seed (mainly liquid endosperm) supported the viewpoint these compounds played key roles in maintaining cellular turgidity for their rapid volume expansion (da Silva et al. 1997). Another piece of evidence for this inference was the small quantity of lipids accumulated (<350 mg g−1) in canola seed though the seed oil content was markedly different at the early stage (Hua et al. 2014).
A unique phenomenon was observed in all enzymes in the silique wall. Activities were extraordinarily higher in the HOCL than in the LOCL at 20 DAA. However, the enzyme activities exhibited low levels in other stages during silique development in the HOCL. The higher enzymatic activity in silique of the HOCL might reflect its advantage over the LOCL during silique development. Because silique is the essential photosynthetic organ (Gammelvind et al. 1996), rapid catalysis of the photo-assimilates is required because release of the small molecular sugar is very important for its volume expansion and transportation into the developing seed. Previous studies reported that rapid growth of silique occurred from initial flowering to 40 DAA (Hua et al. 2014). Thus, more carbohydrates accumulation is an essential issue for silique growth, and coordination of the breakdown of sucrose and starch by related enzymes is required to supply enough substrate. Unlike the seed, the silique is a simple organ from physiological and anatomic viewpoints. The ultra-structure of the silique illustrates that chloroplast and vacuole are the two major cell organelles, especially after 40 DAA (Hua et al. 2014). Hence, silique cell wall thickening is another important event in plant development. The process of cell wall thickening required the considerable deposition of cellulose (Taylor et al. 1992; Taylor 2008). The present study showed that higher carbohydrate metabolic enzyme activities were observed at late silique developing stage in the LOCL. Therefore, it was inferred that strong activities of carbohydrate degradation enzymes at late developing stage in the LOCL was mainly responsible for cell wall thickening. The higher carbohydrate metabolic enzymatic activities at the early stage in the HOCL and at the late stage in the LOCL both existed in carbohydrate synthesis and breakdown. The phenomenon underlies the homeostasis for carbohydrate synthesis and cleavage in silique. The viewpoint was also supported by the evidence from gene expression analysis. Strong expression of SPS genes in the silique of the HOCL and LOCL at 20 DAA reveals the high demand of carbohydrate synthesis for silique development. However, significantly higher SPS expression amount in the silique of the HOCL enables it to obtain higher enzymatic activity and hence the higher carbohydrate concentration. Besides the high expression amount of carbohydrate synthesis, all the carbohydrate catalytic genes, in particular, AtSS2, showed much higher in the silique of the HOCL, which made the feasibility of higher catalytic enzyme activity including SUS, invertase, and starch synthase. Therefore, enhancing the carbohydrate metabolic enzymatic activity at early developmental stage and reducing the activity during silique wall secondary thickening might be an important way to provide carbohydrates that would support the utilization of seed oil biosynthesis.
A common characteristic was observed, i.e., all measured carbohydrate metabolic enzymes in the seed showed higher activities in the HOCL than in the LOCL from 15 to 40 DAA. This is a key stage for seed lipid deposition. The result revealed the high efficiency of carbohydrate catalysis for its utilization. Although both invertase, including neutral and alkaline ones and sucrose synthase can cleave sucrose (Miron et al. 2002; Chandra et al. 2015), the seed SUS activity was evidently higher than invertase activity. The result indicated that SUS was a kind of enzymes that predominantly cleaved sucrose, but not invertase, during seed oil accumulation stages. This finding was in accordance with previous reports (King et al. 1997).
Besides sucrose, many reports suggested that starch played important roles for substrate supply and seed mass enrichment as starch was degraded by associated enzymes during seed development (Déjardin et al. 1997; Cuellar-Ortiz et al. 2008; Howard et al. 2012). The present study showed the higher activity of starch phosphorylase than other enzymes, which was in accordance with previous report (da Silva et al. 1997). However, da Silva et al. (1997) revealed that the activity of starch phosphorylase was mainly detected at extraplastidial. Consequently, they concluded that it was impossible for this enzyme to participate in starch degradation. However, they also demonstrated that isoforms of starch phosphorylase were found using activity gel analysis in plastid (da Silva et al. 1997). Thus, this enzyme might be involved in the function of starch degradation in the plastid of rapeseed embryo (da Silva et al. 1997). In the present study starch phosphorylase was believed to have played an important role in starch degradation because of the large and significant higher starch phosphorylase activity in the seed of the HOCL was noted during seed lipid deposition The higher catalytic activity of starch phosphorylase in the HOCL should be an important attribute to seed oil content when the cleaved starch into small molecular sugars can be used as substrate during lipid biosynthesis. The main questions of this hypothesis were which isoform of starch phosphorylase and how the activities of those isoforms were allocated. At late seed developmental stages, the enzymatic activity in the LOCL was higher than that in the HOCL. One of the explanations was the occurrence of other secondary metabolic events, i.e., seed cellulose accumulation. A previous study showed that seed cellulose content in the HOCL was significantly higher than in the LOCL (Jiang and Deyholos 2010; Hua et al. 2014). Hence, the increased activity of carbohydrate metabolic enzymes is necessary to produce a greater number of small molecular sugars, such as UDP-glucose and fructose for cellulose biosynthesis.
To further analyze the difference of carbohydrate metabolism-related enzymatic activities, gene expression analysis via real-time PCR was conducted. Interestingly, genes encoding invertase, which was localized in different organelles, had different expression modes. Cell wall binding invertase usually obtained high transcript amounts except for ATCWINV1 at later seed lipid deposition stage. This result was reasonable because most of the seed’s secondary metabolic events, in particular cellulose biosynthesis, occurred during seed dehydration and maturation (Kimura and Kondo 2002; Liepman et al. 2010; Wang et al. 2011). In the current study, there was no significant difference of transcript amount of cell wall- and cytoplasmic-binding INVERTASE gene between both lines in the developing seeds. The result support the previous study that invertase was not a dominant kind of sucrose cleavage enzyme (King et al. 1997). Amazingly, very high transcript amount of vacuole-binding invertase was observed in both lines at 30 DAA. This result revealed the importance of this kind of invertase at the early stage of seed lipid deposition. The ultra-structure of seed cells at 30 DAA illustrated that many vacuoles existed in the cell of the HOCL and LOCL, and there were very fewer oil bodies (Hua et al. 2014). Thus, the higher transcript amount of VAC-INV was reasonable. Although the LOCL showed fourfold higher expression of VAC-INV than the HOCL, this result uncovered that cleaved sucrose might be used for seed expansion but had little association with seed lipid deposition because of the increasing seed volume and small percentage of oil content (King et al. 1997, Nägele et al. 2010; Hua et al. 2014).
SUS is a small family gene and has been reported to occur in other plant species (Barratt et al. 2001; Bieniawska et al. 2007; Horst et al. 2007). In Arabidopsis, two isoforms (SUS2 and SUS3) were much more tissue-specific than the other isoforms, and both genes increased the expression level as the seed matured (Fallahi et al. 2008). The trend of SUS gene expression during seed development in the present study was in agreement with that in Arabidopsis, i.e., SUS1 showed decreasing tendency, whereas SUS2 increased during the seed lipid deposition stage (Bieniawska et al. 2007; Fallahi et al. 2008). Higher level of SUS3 might play an important role among isoforms even if no direct association was found between gene expression and enzyme activity. Hirose et al. (2008) suggested that SUS3 might function in carbohydrate allocation. The suggestion was possible regardless of the utilization of allocated carbohydrates for fatty acid biosynthesis or other metabolic event. Although SUS6 was not detected at 40 and 50 DAA in the HOCL, the transcripts were very weak in both lines at 30 DAA that relative to BnACTIN. In rice, the expression amount of SUS6 was very low (Hirose et al. 2008). Thus, it is possible that no actual effect on the final SUS activity.
All SS genes showed an increasing trend during seed development. However, the SS genes in HOCL usually showed lower expression levels than those in LOCL. In general, the expression levels of the three SS genes were weak compared with invertase and SUS genes even though it showed very high activity. These results indicated that high expression of the corresponding gene did not necessary result in high enzymatic activity.
Although sucrose transporter existed in plant cells, functions of this kind of genes, i.e., in carbon transportation and partitioning, are poorly understood (Sauer and Tanner 1993; Doidy et al. 2012). Because sucrose metabolism is distributed universally, wide association of sucrose transporter with other traits is predicted (Li et al. 2011; Gong et al. 2015). For example, Li et al. (2011) identified sucrose transporter alleles in Brassica napus that significantly correlated with seed yield traits, including number of effective branches, siliques per plant, and seed weight. In the present study, SUT4 had higher transcript amount than SUT1 and SUT2. However, no superiority was found in the seed of the HOCL. Until now, the molecular regulation of carbohydrate delivery between tissues, such as silique and seed, has not been reported.
Sucrose phosphate synthase and AGPase are the two enzymes involved in sucrose and starch biosynthesis (Geigenberger et al. 1999; Halford et al. 2011; Hädrich et al. 2012; Tuncel et al. 2014). However, in the present study, similar transcripts were obtained between two lines. This phenomenon may be due to the sourcing of the two carbohydrates (mainly from siliques) but not synthesized in the seed, even if some researchers insisted that developing embryos possess the potential to synthesize carbohydrates (da Silva et al. 1997).
Conclusively, the higher seed oil deposition in the HOCL was attributed importantly to its higher silique and seed sugar concentration. Furthermore, the higher sugar concentration could be a result of carbohydrate synthetic and catalytic enzymes in the coverage of sucrose and starch metabolism at 20 DAA in silique and key stage of seed lipid accumulation in the seed of the HOCL. Moreover, the extremely higher carbohydrate enzymatic activity in the silique of the HOCL was in accordance with the higher corresponding gene expression amounts at 20 DAA. Very high amount of SUS3 in the HOCL from 30 to 50 DAA revealed the importance of sucrose cleavage involving in the rapid seed lipid biosynthesis. The higher carbohydrate enzymatic activity and most gene expression amounts at 50 DAA in the LOCL was uncovered the destination of cleaved sugars to the cellulose biosynthesis. Based on the results in the current study, further identification of the key genes such as SUS3 and their molecular regulative network linking to canola seed oil content should be addressed.
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- DAA:
-
Days after anthesis
- HOCL:
-
High oil content line
- LOCL:
-
Low oil content line
- SPS:
-
Sucrose phosphate synthase
- SS:
-
Starch phosphorylase
- SUS:
-
Sucrose synthase
References
Agrawal GK, Hajduch M, Graham K, Thelen JJ (2008) In-depth investigation of soybean seed-filling proteome and comparison with a parallel study of rapeseed. Plant Physiol 148:504–518
Andriotis VME, Pike MJ, Kular B, Rawsthorne S, Smith AM (2010) Starch turnover in developing oilseed embryos. New Phytol 187:791–804
Angeles-Núñez JG, Tiessen A (2011) Mutation of the transcription factor LEAF COTYLEDON 2 alters to the chemical composition of Arabidopsis seeds, decreasing oil and protein content, while maintaining high levels of starch and sucrose in mature seeds. J Plant Physiol 168:1891–1900
Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C (2001) Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol 127:655–664
Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49:810–828
Chandra A, Verma PK, Islam MN, Grisham MP, Jain R, Sharma A, Roopendra K, Singh K, Singh P, Verma I, Solomon S (2015) Expression analysis of genes associated with sucrose accumulation in sugarcane (Saccharum spp. hybrids) varieties differing in content and time of peak sucrose storage. Plant Biol. doi:10.1111/plb.12276
Cuellar-Ortiz S, De La Arrieta-Montiel M, Acosta-Gallegos J, Covarrubias AA (2008) Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ 31:1399–1409
da Silva PMFR, Eastmond PJ, Hill LM, Smith AM, Rawsthorne S (1997) Starch metabolism in developing embryos of oilseed rape. Planta 203:480–487
Déjardin A, Rochat C, Wuillème S, Boutin JP (1997) Contribution of sucrose synthase, ADP-glucose pyrophosphorylase and starch synthase to starch synthesis in developing pea seeds. Plant Cell Environ 20:1421–1430
Doidy J, Grace E, Kühn C, Simon-Plas F, Casieri L, Wipf D (2012) Sugar transporters in plants and their interactions with fungi. Trends Plant Sci 17:413–422
Dubios M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination sugars and related substances. Anal Chem 28:350–356
Fallahi H, Scofield GN, Badger MR, Chow WS, Furbank RT, Ruan Y (2008) Localization of sucrose synthase in developing seed and siliques of Arabidopsis thaliana reveals diverse roles for SUS during development. J Exp Bot 59:3282–3295
Gammelvind LH, Schjoerring JK, Mogensen VO, Jensen CR, Bock JGH (1996) Photosynthesis in leaves and siliques of winter oilseed rape (Brassica napus L.). Plant Soil 186:227–236
Geigenberger P, Reimholz R, Deiting U, Sonnewald U, Stitt M (1999) Decreased expression of sucrose phosphate synthase strongly inhibits the water stress induced synthesis of sucrose in growing potato tubers. Plant J 19:119–129
Gong X, Liu M, Zhang L, Ruan Y, Ding R, Ji Y, Zhang N, Zhang S, Farmer J, Wang C (2015) Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol Plant 153:119–136
Gutiérrez-Miceli FA, Rodríguez-Mendiola MA, Ochoa-Alejo N, Méndez-Salas R, Dendooven L, Arias-Castro C (2002) Relationship between sucrose accumulation and activities of sucrose phosphatase, sucrose synthase, neutral inverase and soluble acid invertase in micropropagated sugar plants. Acta Physiol Plant 24:441–446
Hädrich N, Hendriks JHM, Kötting O, Arrivault S, Feil R, Zeeman SC, Gibon Y, Schulze W, Stitt M, Lunn JE (2012) Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal turnover in Arabidopsis thaliana leaves. Plant J 70:231–242
Hajduch M, Casteel JE, Hurremeyer KE, Song Z, Agrawal GK, Thelen JJ (2006) Proteomic analysis of seed filling in Brassica napus. Developmental characterization of metabolic isoenzymes using high-resolution two-dimensional gel electrophoresis. Plant Physiol 141:32–46
Halford NG, Curtis TY, Muttucumaru N, Postles J, Mottram DS (2011) Sugar in crop plants. Ann Appl Biol 158:1–25
Hill LM, Morley-Smith ER, Rawsthorne S (2003) Metabolism of sugars in the endosperm of developing seeds of oilseed rape. Plant Physiol 131:228–236
Hirose T, Scofield GN, Terao T (2008) An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci 174:534–543
Horst I, Welham T, Kelly S, Kaneko T, Sato S, Tabata S, Parniske M, Wang TL (2007) TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol 144:806–820
Houston NL, Hajduch M, Thelen JJ (2009) Quantitative proteomics of seed filling in castor: comparison with soybean and rapeseed reveals differences between photosynthetic and nonphotosynthetic seed metabolism. Plant Physiol 151:857–868
Howard TP, Fahy B, Craggs A, Mumford R, Leigh F, Howell P, Greenland A, Smith AM (2012) Barley mutants with low rates of endosperm starch synthesis have low grain dormancy and high susceptibility to preharvest sprouting. New Phytol 194:158–167
Hua W, Li RJ, Zhan GM, Liu J, Li J, Wang XF, Liu GH, Wang HZ (2012) Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. Plant J 69:432–444
Hua S, Chen Z, Zhang Y, Yu H, Lin B, Zhang D (2014) Chlorophyll and carbohydrate metabolism in developing silique and seed are prerequisite to seed oil content of Brassica napus L. Bot Stud 55:34
Jiang Q, Deyholos MK (2010) Transcriptome analysis of secondary-wall-enriched seed coat tissues of canola (Brassica napus L.). Plant Cell Rep 29:327–342
Kimura S, Kondo T (2002) Recent progress in cellulose biosynthesis. J Plant Res 115:297–302
King SP, Lunn JE, Furbank RT (1997) Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol 114:153–160
Li F, Ma C, Wang X, Gao C, Zhang J, Wang Y, Cong N, Li X, Wen J, Yi B, Shen J, Tu J, Fu T (2011) Characterization of Sucrose transporter alleles and their association with seed yield-related traits in Brassica napus L. BMC Plant Biol 11:168
Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV (2010) Arabidopsis—a powerful model system for plant cell wall research. Plant J 61:1107–1121
Livak JK, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Miron D, Petreikov M, Carmi N, Shen S, Levin I, Granot D, Zamski E, Schaffer AA (2002) Sucrose uptake, invertase localization and gene expression in developing fruit of Lycopersicon esculentum and the sucrose-accumulating Lycopersicon hirsutum. Physiol Plant 115:35–47
Nägele T, Henkel S, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG (2010) Mathematical modeling of the central carbohydrate metabolism in Arabidopsis reveals a substantial regulatory influence of vacuolar invertase on whole plant carbon metabolism. Plant Physiol 153:260–272
Ruuska SA, Thomas G, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14:1191–1206
Sanjaya Durrett TP, Weise SE, Benning C (2011) Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotech J 9:874–883
Sauer N, Tanner W (1993) Molecular biology of sugar transporters in plants. Bot Acta 106:277–286
Schmalstig JG, Hitz WD (1987) Contributions of sucrose synthase and invertase to the metabolism of sucrose in developing leaves: estimation by alternate substrate utilization. Plant Physiol 85:407–412
Smith AM (1990) Enzymes of starch synthesis. In: Lea PJ (ed) Methods in plant biochemistry, vol 3. Academic Press, London 99, pp 93–102
Smith AM, Bettey M, Bedford ID (1989) Evidence that the rb locus alters the starch content of developing pea embryos through an effect on ADP glucose pyrophosphorylase. Plant Physiol 89:1279–1284
Taylor NG (2008) Cellulose biosynthesis and deposition in higher plants. New Phytol 178:239–252
Taylor JG Jr, Owen TP, Koonce LT, Haigler CH (1992) Dispersed lignin in tracheary elements treated with cellulose synthesis inhibitors provides evidence that molecules of the secondary wall patterning. Plant J 2:959–970
Tuncel A, Cakir B, Hwang S, Okita TW (2014) The role of the large subunit in redox regulation of the rice endosperm ADP-glucose pyrophosphorylase. FEBS J 281:4951–4963
Wang Q, Zhang X, Li F, Hou Y, Liu X, Zhang X (2011) Identification of a UDP-glucose pyrophosphorylase from cotton (Gossypium hirsutum L.) involved in cellulose biosynthesis in Arabidopsis thaliana. Plant Cell Rep 30:1303–1312
White JA, Todd J, Newman T, Focks N, Girke T, de Iláduya OM, Jaworski JG, Ohlrogge JB, Benning C (2000) A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol 124:1582–1594
Acknowledgments
Great appreciations were expressed to the anonymous reviewers for their critical review. This work was supported by the Major State Basic Research Development Program of China (973 program) (2015CB150205); Natural Science Foundation of Zhejiang Province (LY12C13006); Program for Zhejiang Leading Team of S & T Innovation (2011R50026-04 and 2012C12902-1); the earmarked fund for China Agriculture Research System (CARS-13); the key and integrated technology for canola high yield improvement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Hua, S., Zhang, D. et al. Comparison on the carbohydrate metabolic enzyme activities and their gene expression patterns in canola differing seed oil content. Plant Growth Regul 78, 357–369 (2016). https://doi.org/10.1007/s10725-015-0098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0098-y