Abstract
Bamboo is an economically important member of the grass family Poaceae with over 1500 documented uses. India has the 2nd largest reserve of bamboo in Asia after China. It is also called “Poor man’s timber”, “Green Gold” and “twenty-first century steel”. They are extensively used as raw material for various industries besides serving the rural population through several uses. Their immense versatility and utility have resulted in the genetic erosion and over-exploitation of bamboos leading to a constant decline in their natural populations. This has made it indispensable to conserve and propagate bamboo species. For utilization and conservation of genetic resources, germplasm characterization and screening are noteworthy. For the characterization of bamboo species, multiple attempts have been taken with the support of molecular markers and with the emergence of molecular biology, different molecular markers are now being used to study genetic variability, genetic diversity and genotype identification. These markers include RAPDs, ISSRs, SSRs, RFLPs and AFLPs. Other marker types which have been employed for molecular characterization in bamboo but not particularly in Indian bamboo can also be used as a potential marker choice such are SCoT, IRAP, SRAP, SNP. The present review majorly deals with the role of different molecular markers in the genetic diversity study of natural populations of Indian bamboo species. It will provide information on the availability of different molecular markers to identify diversity in Indian bamboo species. It will give an ease of access for different researchers working in the field of bamboo to design primer for different markers accordingly. Further, this fingerprinting-based diversity study will also help in proper breeding of bamboos for its improvement program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bamboo is a member of the Grass Family Poaceae and sub-family Bambusoideae which grows in many parts of the world. With more than 1575 species from 111 genus, it is quite distinct from regular grass because of its size, width, height, and branches at internodes (Singh et al. 2013; Xiu-hua et al. 2017). Bamboos does not seem to be the only non-timber forest trees having about 1500 documented uses but also one in every of the important cultivated crops (Azeez and Orege 2018). They are one of the foremost economically important plants with diverse applications in many industries and households (Nongdam and Tikendra 2014). With reference to the whole bamboo community, India with 160 species is second largest bamboo producing country after China with 800 species having the highest number (Bystriakova 2003).
Bamboo distribution: India
In India, bamboo forest area in 2019 increased by 3229 km2 compared to 2017 because of its environmental and commercial importance. Bamboo covers almost 22.5% (160,037 km2) of area of the entire Indian forest. The North-eastern states of India which comprises of seven sister states i.e., Assam, Arunachal Pradesh, Manipur, Mizoram, Meghalaya, Tripura and Nagaland contribute 25% to bamboo forest and have almost 100 plus species of bamboo which is equivalent to 50% of Indian bamboo species (Amom et al. 2020; Nongkynrih et al. 2019; Singh et al. 2017; Tamang et al. 2013). Other states of India cover approximately 40% of total bamboo area of India i.e., Madhya Pradesh with 14%, Maharashtra with 10%, Arunachal Pradesh with 10% and Odisha with 8%. Uttar Pradesh, West Bengal and Bihar have large area but in terms of bamboo area cover it is only 3226 km2 (Fig. 1).
Of the 160 species of bamboo in India, essential and commercial species of various genera are identified as Bambusa bambos (L.) Voss, B. balcooa Roxb., B. nutans Wall. Ex Munro, B. cacharensis R.B. Majumdar, B. tulda Roxb., B. polymorpha Munro, (Synonym of B. multilplex), B. multiplex (Lour.) Raeush. Ex. Schult., B. vulgaris Schrad., Bambusa pallida Munro, Bambusa polymorpha Munro, Dendrocalamus giganteus Munro, D. asper (Schult.) Backer, D. hamiltonii Nees & Arn. Ex Munro, Pseudoxytenanthera stocksii (Munro) T.Q. Nguyen, D. brandisii (Munro) Kurz, D. strictus (Roxb.) Nees, Pseudoxytenanthera ritcheyi (Munro) H.B. Naithani, Ochlandra scriptoria (Dennst.) C.E.C. Fisch, O. travancorica (Bedd.) Gamble, O. ebracteate Raizada & Chatterji, Thyrsostachys oliveri Gamble, Schizostachyum dullooa (Gamble) R.B. Majumdar, Drepanostachyum falcatum (Nees) keng f., Melocanna baccifera (Roxb.) Kurz, Thamnocalamus spathiflorus (Trin.) Munro, Gigantochloa rostrata K.M. Wong, G. atroviolacea Widjaja (Sharma and Nirmala 2015; Kaur et al. 2016; Singh and Kumari 2018). For commercial purpose, the ten prominent bamboo species used in India are Bambusa bambos, B. balcooa, B. nutans, B. tulda, Dendrocalamus hamiltoniis, D. strictus, Ochlandra scriptoria, O. ebracteata, O. travancorica, Melocanna baccifera (Seethalakshmi et al. 1998). Figure 2 represents some of the commercially important bamboo species grown in India.

(Source: www.chhajedgarden.com/blogs)
Commercial important bamboo species grown in India
Bamboo usage
Because of its excellent mechanical, chemical and physical properties along with enormous diversity of application in around 10,000 products, it is called “Poor man’s timber”, “Green Gold” and “twenty-first century steel” (Ahmad and Kamke 2003, 2005; Archila et al. 2018; Azeem et al. 2020). A major number of bamboo products is used not only by traditional artists but also by big industries such as fibre boards, charcoal, paper making, house scaffolding etc., (Awoyera and Ede 2017; Bajpai 2018; Nguyen et al. 2018; Tanpichai et al. 2019). Almost 35% of the bamboo produced is used in the paper industry (Tripathi et al. 2018). Bamboo is also an excellent and healthy source of food (Choudhury et al. 2012; Silva et al. 2020). Around 200 bamboo species are considered edible worldwide (Basumatary et al. 2017; Bhatt et al. 2003, 2004). It also has many nutrients majorly nitrogen (N), Phosphorous (P) and Potassium (K). Due to its mechanical property bamboo is considered suitable for the construction industry. Mainly Bambusa tulda, B. balcooa, B. nutans, B. polymorpha, B. pallida, D. hamiltonii and Melocanna baccifera which are available in India are used for construction purposes (Borah et al. 2008). Bamboo also have certain desirable characteristics which make them excellent candidates to be used as a source of fuel for example low ash and alkali index content.
Need for characterization of bamboo species
Due to genetic erosion and overexploitation of bamboo species, it becomes important to collect germplasm for the purpose of conservation (Goyal et al. 2012). In compliance with collection of germplasm, more awareness is needed in the identification and classification of bamboos species (Goyal et al. 2012). Characterization is an important link between utilization and conservation of germplasms (Nayak et al. 2003). Recently much significance has been placed on ex situ and in situ conservation of bamboo to set up bamboo collections with genotypes of interest. To avoid collection duplication, molecular markers can serve as an effective tool to avoid one from getting drowned in the genepool and to supplement the bamboo knowledge of local people. One of the research strategies needed for in situ conservation is to assess genetic variation present in specific areas to document its relationship to overall patterns of geographic differences among and within populations (Stapleton and Rao 1995). Bamboo species that have constricted distribution and are naturally rare may be extremely vulnerable to overharvesting or habitat changes. With an aim to conserve bamboo crop in the country Government of India initiated National Bamboo Mission (NBM) in 2006, under ministry of Agriculture, Government of India with an effort to increase the area under bamboo plantation in non-forest Government, private lands and further for the growth of bamboo industry many state governments (Kerala, Maharashtra, all North-East) have their states-run programs. Also, many state governments are providing subsidies for bamboo farming (Sharma and Nirmala 2015).
Identification and classification of bamboo through morphological characterization
At the earliest in the year 1896, J.S. Gamble based on various reproductive and vegetative characters identified old world bamboos. Later, Chatterjee and Raizada (1963) prepared different culm-sheath and other potential vegetative descriptors for the identification of 22 bamboo taxa. According to them, “size, texture, general appearance, blades, shape and texture of the sheath” proved to be efficient traits for distinguishing different species. Triplett and Clark (2003) assessed the diversity and relationship within the members of Chusquea culeou species complex due to geographical and ecological variations. They studied 14 floral or reproductive characters and 7 vegetative characters. The analysis based on reproductive and vegetative traits showed that the differences in the characters is continuous and cannot be considered or used to separate the species into a group that are morphologically distinct. Lately, Bhattacharya et al. (2006) characterized B. tulda from 17 eco-geographical locations in various districts of West Bengal. They reported 17 culm-sheath and 15 culm characters and the study was in accordance with the previous taxonomic classifications given by Gamble (1896). Using these 32 key characters (15 culm and 17 culm-sheath), phylogenetic relationship among 15 species of bamboo were studied by Das et al. (2008). The clustering pattern generated from the morphological descriptors were not in accordance with the taxonomic classification of Gamble (1896).
Need for molecular markers
Despite the fact that characterization of bamboo species has long been done based on morphological traits, yet the classification is not dependable since these characters are often influenced by environmental elements. Das et al. (2008) reported that only vegetative characters are inadequate to differentiate closely related species. The clustering pattern of the 15 species of bamboo studied were not in conformity with the taxonomic patterns of Gamble (1896). Bambusa atra (synonym of Neololeba atra (Lindl.) Widjaja), B. wamin E.G. Camus, B. striata (synonym of Bambusa vulgaris Schrad.) as these names were reported by Das et al. 2008. As per editor I have added the latin name of species. Similarly, D. strictus was clustered with B. atra, B. wamin and B. striata. The cycle of reproduction for bamboo is extensively long i.e. 3–120 years and hence the identification and classification with floral characteristics is difficult (Bhattacharya et al. 2009; Yesmin et al. 2015). The taxonomic classification in case of bamboo is mostly based on vegetative characteristics and are greatly influenced by environment (Ohrnberger 2002). Therefore, not considered reliable for taxonomic classification. Das et al. (2008) reported in his study that differentiation among and within bamboo species based on only morphological characterization are inefficient. B. wamin and B. striata of Bambusa genus were separated from the some of the other Bambusa species. Similarly, D. strictus was grouped with B. atra, B. wamin and B. striata (Yesmin 2015). Also, there are only limited numbers of genes which are involved in expression of morphological characteristics which does not give the exact scenario of the entire genome (Brown-Guedira et al. 2000). Ample and effectual molecular data are needed to separate and regroup many bamboo species for better evolutionary understanding, molecular phylogeny and determination of genetic diversity (Stapleton et al. 2009; Nilkanta et al. 2017).
Bamboo and DNA based molecular markers
DNA based molecular markers are way better than morphological markers as the former are cost-effective, highly informative, not tissue or age specific, neutral and are not influenced by environmental factors (Uchoi et al. 2017; Kumar et al. 2011). The use of DNA based molecular markers has given rise to significant improvement in understanding phylogenetic relationships within the bamboo species with the help of genetic diversity studies, which was previously constricted with limited phenotypic based characteristics (Das et al. 2008; Bhandari et al. 2021). The most practical and most important application of the use of molecular markers in bamboo is doubtlessly the precise identification of bamboo species and genetic variation assessment within species within a short period of time. Taxonomists and others working with bamboo are very much aware with the difficulties associated with identification and genetic variation assessment of bamboo genotypes, which itself is important for effectively selecting superior genotypes. But with the rise of molecular marker tools, which allows one to objectively assess genetic variation within species and to determine genotype irrespective of the environmental locations and other aspects contributing to morphological variability (Stapelton and Rao 1995).Various marker systems have been used in explaining the genetic diversity of bamboo using molecular markers such as Random Amplified Polymorphic DNA (RAPD) (Giels et al. 1997a,1997b; Hodkinson et al. 2000), restriction fragment length polymorphism (RFLP) (Friar and Kochert 1991, 1994), amplified fragment length polymorphism (AFLP) (Isagi et al. 2016), Sharma et al. (2009), simple sequence repeats (SSRs) (Tang et al. 2010), expressed sequence tags-SSR (EST-SSR) (Zhang et al. 2011), inter-simple sequence repeats (ISSRs) (Lin et al. 2010) and single nucleotide polymorphism (SNP) (Zhou et al. 2011). Suitably, this approach is extensively used for studying phylogenetic relationship (Amom et al. 2020; Liu et al. 2016), genetic diversity (He et al. 2019; Tanzeem et al. 2019), DNA fingerprinting (Afshari et al. 2016) and varietal identification (Odunayo et al. 2019).These above mentioned markers are frequently used for molecular characterization of Indian bamboos but there are some other DNA based molecular markers also which had been employed for the characterization of bamboos other than India like SCoT (Start codon Targeted) (Amom et al. 2020), IRAP (Inter- retrotransposon amplified polymorphism) (Li et al. 2020) and SRAP (Sequence related amplified polymorphism) (Feng et al. 2010; Zhu et al. 2013). Detail of the markers used to explore genetic diversity among different bamboo species of India by different groups of works was shown in Table 1. Many hybridization, PCR and sequence-based marker systems have acquired existence over a period of time.
Random amplified polymorphic DNA (RAPD)
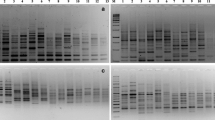
The most widely employed DNA marker for identification of rapid plant genotype is RAPD which is the cheapest and most simple molecular marker using arbitrary primers (Arumugam et al. 2019). The RAPD reactions are carried out on genomic DNA with the help of an arbitrary primer which results in the amplification of many discrete DNA products and these products are usually separated by gel electrophoresis. Genetic variation within individuals results from the sequence differences in the primer binding sites. The efficacy of RAPD markers in detection of DNA polymorphism and phylogenetic study in bamboos species has been previously studied by various researchers (Das et al. 2008; Nayak et al. 2003; Bhattacharya et al. 2006; Agnihotri et al. 2009; Desai et al. 2015; Waghmare et al. 2013). Nayak et al. 2003 in his work distinguished bamboo species of genera Dendrocalamus, Cephalostachyum, Dinochloa and Bambusa with the help of RAPD markers. They investigated genetic relationships in 12 species of bamboo using RAPD technique which started by using 30 ten-mer primers that differentiated 12 species and hence selectively reduced the set of primers. In total, 137 different polymorphic DNA bands were amplified by 10 selected primers. Similarly, Bhattacharya et al. (2006) characterized populations of B. tulda from west Bengal using RAPD marker for species identification and reported that no polymorphism detected between various populations of B. tulda and also no polymorphism between the phenotypic variants. Agnihotri et al. (2009) performed genetic fidelity test of tissue culture generated plant from D. hamiltonii using RAPD marker. Shalini et al. (2013) investigated genetic relationships in 10 bamboo species using 21 RAPD markers and reported 100% polymorphism between the studied species. Similarly, Desai et al. (2015) assessed the genetic diversity of 13 bamboo species using 120 RAPD markers in which 30 RAPD generated 645 fragments out of which 623 were polymorphic. The RAPD banding patterns and the dendrogram generated by RAPD analysis is represented in Figs. 2 and 3 respectively. The number of amplified fragments generated by primers varied from 6 (OPC-02, OPA-02, OPN-01, OPD-M4, OPO-15, OPD-18) to 29 (OPM-11), with size range 285–2168 bp (Fig. 3). The average polymorphic bands generated per primer is 20.76 (Desai et al. 2015). The UPGMA analysis based dendrogram grouped 13 bamboo genotypes in 3 main clusters (Fig. 4). Clusters 1 consisted of GAU-1 (Bambusa bambos from Anand, Gujarat),GFC-2 (Bambusa tulda from Anand, Gujarat) and GSF-5 (Bambusa multiplex from Anand, Gujarat), cluster II consisted GKU-3 (Bambusa balcooa from Kutch Gujarat), GVP-6 (Bambusa balcooa Anand, Gujarat), GKU-4 (Bambusa balcooa from Kutch, Gujarat) and OCT-9 (Bambusa multiplex from Anand, Gujarat) and cluster III consisted of GNU-7 (Bambusa vulgaris from Anand, Gujarat), OVP-8 (Bambusa vulgaris from Anand, Gujarat), YKE-11 (Phyllostachys vivax from Thiruvananthapuram, Kerala), YVI-2(Phyllostachys vivax from Anand, Gujarat), YNU-10 (Phyllostachys vivax from Anand, Gujarat) and YVP-13 (Phyllostachys vivax from Anand, Gujarat) (Desai et al. 2015).

Source: Desai et al. 2015)
RAPD amplification pattern of 13 Indian bamboo species generated by RAPD primer Legends: a 5′CCGCATCTAC 3′ b 5′ACAACGCCTC3′; lane M-1 kb ladder; lanes 1–13 thirteen bamboo species (

Source: Desai et al. 2015)
Dendrogram of 13 Indian bamboo species based on RAPD analysis (
Genetic relationship of four bamboo species (P. stocksii, P. ritchey, D. strictus and B. bambusa) collected from Maharashtra’s Raigad district was analysed by Waghmare et al. (2013) using PCR-RAPD technique. Makmur et al. (2020) evaluated 8 bamboo types using RAPD markers to investigate the differences in the generated DNA sequence. A total of 20 primers were selected and from that 12 primers with clear and prominent amplification were selected.
This wide range use of RAPD technique is due to its low- cost, speed and efficiency which generate large marker numbers in a short period as compared to other techniques. RAPD technique has also attracted several criticisms pointing towards its reproducibility, despite numerous advantages. RAPD has many limitations which includes uncertain locus homology, dominance and sensitivity to reaction conditions. RAPD being neutrally selective has advantage over RFLP as the former does not use radioisotopes and is able to use low quality DNA and also the primers are more accessible. However, due to low annealing temperatures RAPDs are less reproducible. The disadvantages of RAPD also include polymorphism detection at a limited level, low resolution profile and it detects dominant allelomorphs. It is also unable to distinguish homozygotes and heterozygotes. Co-migration problems may raise questions like “Do same sized bands in the gel correspond to DNA fragments?” The presence of identical bands with the same molecular weight in different samples does not provide evidence that the samples share homologous fragments of DNA.
Inter simple sequence repeat (ISSR)
To overcome the limitations of RAPD markers, ISSR markers came into effect. ISSRs are regions that are present within the microsatellite repeats (Joshi et al. 2000; Poczai et al. 2000) and and provide considerable potential to determine inter- genomic and intra-genomic diversity in comparison to other arbitrary primers, since they bring out dissimilarity within unique regions of DNA at various locus simultaneously. Microsatellites have several properties such as high copy number, high variability among taxa and ubiquitous occurrence in the eukaryotic genome), make ISSRs immensely useful markers. For many scientists working on bamboo, ISSR has become a popular technique for the study of genetic relationships (Lin et al. 2010; Mukherjee et al. 2010; Sarwat 2012). Till date Genetic diversity study using ISSR based markers has been done by Mukherjee et al. 2010; Goyal and Sen 2014; Desai et al. 2015; Gami et al. 2015; Nilkanta et al. 2017). Mukherjee et al. (2010) reported genetic diversity and phylogenetic relationship in 22 bamboo taxa through EST and ISSR-based random markers. Similarly, Gami et al. (2015) assessed genetic variation within 20 bamboo accessions which were collected from different parts of India. The molecular marker approach used by them was ISSR which used 8 primers to distinguish twenty bamboo accessions. Genetic diversity of 13 bamboo genotypes were analysed by Desai et al. (2015) using RAPD and ISSR. Total 63 ISSR primers were used of which 12 ISSR primers gave a good amplification profile. 246 fragments were generated by the 12 ISSR primers in which 241 were polymorphic. Genetic diversity study of 29 accessions of bamboos encountered in North Bengal were done through ISSR technique (Goyal and Sen 2014) with the help of 9 ISSR primers which recorded 100% polymorphism between the studied species. Among the 9 ISSR primers used (Table 1), the highest number of bands (33) was produced by (TC)8G, while (TC)8A produced only 22 bands with each band averaging 27.11 distinct scorable bands. ISSR banding patterns of 29 accessions of bamboo generated by primer UBC824 and UBC815 are represented in Fig. 5.The range of Genetic similarity between the species was 0.613–0.960 with lowest value found between B. pallida (B12)and B. vulgaris ‘Vittata’ (B1), while the highest value was found between B. multiplex ‘Alphonse-Karr’ (B2) and B. multiplex ‘Riviereorum’ (B4) (Goyal and Sen 2014). The dendrogram assembled based on ISSR analysis showed that majority of the Bambusa species and its varieties were found to be clustered together, except for B. oliverianna (B9) and B. atra (B8) (Fig. 6). Nilkanta et al. (2017) investigated the population genetic structure and genetic diversity of M. baccifera using ISSR markers in 5 districts of Manipur.
Banding patterns of 29 accession of bamboo species generated by ISSR primers UBC-824 (a) and UBC-815 (b); M1-lamba DNA/EcoR1/Hind111 double digest ladder, M2-10 bp mol marker, Lanes B1 to B29 studied bamboo species (Source: Sen and Goyal 2014)
Dendrogram of 29 accession of bamboo generated by ISSR profiling (Source: Goyal and Sen 2014)
Since then, several authors have attempted RAPD technique for the study of genetic relationships among species of bamboo but there have been only a limited number of studies on bamboo using ISSR markers. Thus, keeping this in mind Goyal and Sen (2014) employed both RAPD and ISSR markers to study the phylogenetic relationship of 29 accessions found in North Bengal. The ISSR technique has been frequently applied for genetic diversity analysis in bamboo species as these are effective multi-locus markers and no prior knowledge of sequence is required. ISSR markers are used more than SSR and are also more reliable than RAPD markers mainly due to the method of detection and also longer primers are used, and hence more stringent PCR conditions.
It has several advantages as various loci can be assessed simultaneously which make ISSR a fast technique, also the obtained PCR products are specific to microsatellite sequence and are more reliable than the random primer sequence-based techniques. ISSR technique is economical and less time consuming than other microsatellite-based techniques as there is no requirement of characterization and cloning is required. However, there are certain disadvantages like sometimes the primers have less specificity to the scanned genome which could lead to questionable fingerprints and they are dominant hence unable to discriminate between heterozygotes and homozygotes.
Simple sequence repeat (SSR)
The accessibility of SSR markers in bamboo is very limited and only few simple sequence repeats (SSR) markers have been constructed for bamboo (Nayak and Rout 2005; Kaneko et al. 2008). However, the progress of SSR markers has traditionally been bounded by labor intensive and time-consuming methods of SSRs development. Hence, identification of simple sequence repeats (SSRs) from the expressed genome provides a substantial source of valuable markers. EST-SSRs linked with known function genes tend to be more extensively transferred at species and generic level (Bouck and Vision 2007; Bhandawat et al. 2019). In Bamboo, the first set of ten EST-SSRs were reported by Sharma et al. (2008) which would be valuable for phylogenetic studies and genetic diversity in bamboo and similarly Nayak and Rout (2005) isolated and characterized microsatellites in B. arundinacea and in other bamboos for cross species amplification. They characterized 6 microsatellites in a bamboo species, B. arundinacea, 3 polymorphic and 3 monomorphic. Another molecular characterization in bamboo species was done by Meena et al. (2019) of 19 populations of D. hamiltonii distributed over northeast Himalayas with the help of nuclear simple sequence repeats (nSSR). Nuclear microsatellite markers are distributed ubiquitously in the genome which are highly polymorphic, show co-dominant inheritance and are often transferable over related species (Fan et al. 2013). For this, total sixty-eight (68) nSSR primer pairs of B. arundinacea and D. latiflorus was tested in D. hamiltonii for their transferability, out of the 68 primers 17 showing polymorphic amplification was used for genotyping. The SSR gel pattern generated by 3 different primer sets is shown in Fig. 7 and the respective dendrogram analysis is shown in Fig. 8. For genetic mapping, genetic diversity and genotype fingerprinting studies, SSR markers have become the marker class of choice as SSR markers are highly reproducible, co-dominant and abundant in genome (Saha et al. 2004). Utilising SSR markers in polyploid species remained uncertain, because of their allelic and co-dominant nature. In such cases, SSR markers are analyzed by considering them as dominant markers. It has been examined that SSR markers are still an effective tool for genotyping polyploid species because reproducible alleles per locus are available in large numbers (Meena et al. 2020). The advantages of SSRs are that they are abundantly present and dispersed in all genomes with high levels of polymorphism in comparison to other markers. As a disadvantage, analysis of SSR is a time consuming and an expensive process especially when it comes to library creation.

Source: Meena et al. 2019). Lane M-100 bp DNA ladder; Lane 1 to 24 represents 24 random samples
SSR banding patterns generated by 3 different primer sets using 24 random Dendrocalamus hamiltonii samples (

Source: Meena et al. 2019)
Dendrogram showing genetic distance among nineteen populations of Dendrocalamus hamiltonii (
Restriction fragment length polymorphism (RFLP)
Konzen et al. (2017) recommended RAPD-RFLP for the analysis of divergence among bamboo species and their genetic diversity. They recommended RAPD-RFLP as an informative and reproducible technique for screening differences among species of bamboo. Providing an accurate and cost-effective method for identification of species and their characterization which would result in breeding, management and conservation of bamboo. PCR–RFLP is an alternative and simple technique for genotyping bamboos. In RFLP the PCR products are digested with different restriction enzyme combinations. The digested products are then separated with the help of gel electrophoresis. PCR–RFLP has been regularly conducted by digesting PCR products of Internal Transcribed Spacer (ITS) regions in plant (Biswas et al. 2016) and genomes and organelle gene. Based on the TrnL-trnF region, some studies have been performed on bamboo species schizostachyum and arundinaria to determine phylogeny respectively (Yang et al. 2007; Qiang et al. 2005). Multigene phylogenetic studies including TrnL-trnF have been performed to sort out significant phylogenetic groupings within Bambusoideae (Sungkaew et al., 2009). Similar study was conducted by Sen et al. (2014) to study the phylogeny of bamboos encountered in North Bengal (29 accessions) by amplification of TrnL-trnF region of chloroplast genome followed by restriction enzyme digestion usingTaq1,Alu1 and Hinf1 Restriction enzymes and showed considerable polymorphism among the bamboo species in non-coding regions of the genomic DNA(chloroplast).The different restriction digestion enzymes produced in total of Nineteen Scorable bands which ranged from 200 to 1986 bp. Out of the 19 bands,9 bands were polymorphic and the band number varied from 1 in HinfI and TaqI to 7 in AluI (Figs. 9, 10).Two clusters were made in the dendrogram formed. The first cluster consisted of 21 bamboo accessions and this cluster is further divided into 2 groups. The first group with 18 species, included 10 species of Bambusa from the 12 species of Bambusa studied. Two species Pseudosasa japonica (B25) and Himalayacalamus hookerianus (B21) were included in the second group of the first cluster. Phyllostachys nigra (B23) is completely isolated and in the similar way Shibataea kumasaca (B27) and Melocanna baccifera (B22) is separated from other bamboo species. The second cluster comprised of 5 species of which B. multiplex ‘Rivierorum’(B4), Bambusa multiplex ‘Alphanso Karr’ (B2) and Cephalostachyum latifolium (B13) clustered under the same sub-group (Fig. 11).
Gel showing amplified bamboo accessions with primer Tabc-f(TrnL-trnF) (Source: Sen et al. 2014)
Products of TrnL-trnF region of genome(chloroplast) digested with different restriction enzymes a Taq1; b Alu1; c Hinf1. Lane-B1 to B29 with different species studied; Lane M1-0.1-10 kb DNA ladder; Lane M2-lambda DNA/EcoRI/HindIII double digest DNA ladder (Source: Sen et al. 2014)
Dendogram based on digested products of TrnL-trnF genome with different restriction enzymes (Source: Sen et al. 2014)
Since RFLPs are co-dominant, they are able to distinguish between heterozygotes and homozygotes. They are highly reproducible, stable, locus specific and are transferable across the population hence prove to be a reliable method. The requirement of high quality and quantity of DNA put RFLPs on the back side and they are not neutrally selective as radiolabeled probes are required. These are time consuming, expensive and labor-intensive procedures which are many of its limitations.
Amplified fragment length polymorphism (AFLP)
AFLP is another class of markers which were screened for defining phylogenetic relationships and detecting genetic variability among bamboo species in India (Waikhom et al. 2012) and Singapore (Loh et al. 2000). The DNA fingerprinting technique Amplified fragment length polymorphism (AFLP) is a novel technique that allows precise characterization of DNA under tough experimental conditions. AFLP helps to explore the entire genome within a short period of time. Thus, this method is useful for identification of genotype within a short duration of time with precision. AFLP integrated with automated fluorescence dye-labelling is a recent technique and in comparison, with conventional AFLP technique has advantage of precision, enhanced resolution and analytical power for higher reproducibility and large-scale DNA fingerprinting (Ghosh et al. 2011). Mehta et al. (2011) for the first-time reported analysis of tissue culture raised plants and somatic embryogenesis using AFLP fingerprinting technique in B. nutans. Similarly, Ghosh et al. (2011) conducted AFLP analysis on 12 distinct edible species of bamboo from Manipur belonging to 5 genus of tribe Bambuseae (including Bambusa and Dendrocalamus) to study the genetic diversity in different bamboo species in this hotspot region. Pattanaik and Hall (2011) investigated relationships among ten Dendrocalamus and five outgroup species (Dinochloa maclellandii, Oxytenanthera abyssinica, Thyrsostachys siamensis, Melocalamus compactiflorus and B. balcooa) from subtribe Bambusinae. Waikhom et al. (2012) used AFLP technique with automated DNA sequencing to study the genetic relationship between D. hamiltonii and they also determined the specific association between biochemical traits (antioxidant and total cyanide content) and AFLP markers. Nag et al. (2013) studied 28 accessions of O. travancorica viz., OT1-OT28 collected randomly from dense forest of Kerala (southern region). Eight set of primers with good amplifications were utilized for the analysis of AFLP, which produced scorable band with an average of 75.3 bands per primer. The AFLP banding pattern of 28 accessions of O. travancorica generated by primer combination EACG-MCAC is depicted in Fig. 12. Dendrogram constructed based on AFLP analysis separated 28 accessions of O. travancorica into 3 major groups. Twenty accessions of OT collected from district Pathanamthitta were grouped into two different groups (I and II), while eight accessions of OT collected from Thrissur, Idukki and Ernakulam districts were grouped together in group II (Fig. 13). AFLP markers give fingerprints which are highly informative as a large number of bands are generated in this technique. Other advantages of AFLPs include quick scanning of the whole genome for polymorphism detection, high reproducibility and no need of prior information of sequence. On the other hand, there are some disadvantages of AFLP. Since there are huge quantities of information generated, the data obtained may need automated analysis which is a daunting task and also, they are dominant in nature. AFLPs have a time cluster at telomeres and centromeres in genetic mapping which may interfere with the data collection.
Gel showing amplified products generated by primer combination EACG-MCAC; lane M-20 bp DNA ladder, lane 1–28 amplified bands of different O. travancorica (Source: Nag et al. 2013)
Dendrogram showing relationship among O. travancorica based on 8 AFLP and 42 RAPD markers (Source: Nag et al. 2013)
Sequence characterized amplified regions (SCARs)
Sequence characterized amplified regions (SCARs) are PCR-based markers that are extension or modification of RAPD with better reproducibility because of higher annealing temperature (Rangsiruji et al. 2018). SCARs have been useful in genotypic identification (Rangsiruji 2018). Especially, at the seedling stage when the vital morphological characters are unable to differentiate. Das et al. (2008) designed 2 species-specific SCAR markers, ‘Tuldo609’ for B. tulda and ‘Balco836’ for B. balcooa from sequenced, putative species-specific, RAPD bands for accurate species identification. Figures 14 and 15 show the amplified band generated by designed SCAR primers ‘Balco836’ in B. balcooa and ‘Tuldo609’ in B. tulda respectively.
Gel showing presence of amplified band from the genomic DNA of Bambusa balcooa using ‘Balco836F’ and ‘Balco836R’ SCAR primer pairs and there are no amplified bands in the other 14 species of bamboo (Source: Das et al. 2008)
Gel showing presence of amplified band from the genomic DNA of Bambusa tulda using ‘Tuldo609F’ and ‘Tuldo609R’ SCAR primer pairs and also there is no amplified bands in the other 14 species of bamboo (Source: Das et al. 2008)
Other molecular markers employed to study bamboo species around the world
The availability of bamboo genome (http://www.bamboogdb.org, BambooGDB) have enabled different researchers to use other molecular marker techniques also and these markers have not been used frequently as compared in the molecular characterization of bamboo and that too specifically Indian bamboo (Poczai et al. 2013; Zhao et al. 2014). Some of these techniques which have been employed are SCoT (Start codon Targeted); IRAP (Inter-Retrotransposon amplified polymorphism); SRAP (Sequence related amplified polymorphism); SNP (Single nucleotide polymorphism); SRAP (Sequence related amplified polymorphism).
Start codon targeted (SCoT) polymorphism
Start Codon Targeted (SCoT) polymorphism is a novel molecular marker technique that targets ATG start codon regions in plant genes (Collar and Mackill 2009). It has various advantages over ISSR, AFLP and RAPD, as it produces more reliable and reproducible bands. This marker is also an effective technique for assessing genetic variation, population studies and DNA fingerprinting (Collar and Mackill 2009; Etminan et al. 2016; Hao et al. 2018. SCoT markers have been used to study the genetic fingerprinting and genetic diversity in rose (Agarwal et al. 2019), kalmegh (Tiwari et al. 2016), orchard grass (Zeng et al. 2014), Jatropha L. (Mulpuri et al. 2013) and mango (Luo et al. 2010). Amom et al. 2020 studied the efficiency of marker techniques such as RAPD, ISSR, iPBS and SCoT in determining the genetic relationship between four economically important and native bamboo species of North-east India (B. cacharensis, B. mizorameana, D. manipureanus, D. sikkimensis and D. hamiltonii). They reported that of all the above markers, SCoT markers produced the highest number of bands per primer depicting high efficiency compared to other markers.
Inter-retrotransposon amplified polymorphism (IRAP)
Another molecular marker group which is widely used in assessing genetic relationships and variability are Inter Retrotransposon Amplified Polymorphism (IRAP). These markers can be generated with the help of one or two primers and amplify the regions in between two long terminal repeats (LTRs). IRAP markers are widely employed in the study of cladistic relationship and genetic diversity analysis (Mansour 2008; Ramakrishnan et al. 2019; Gozukirmizi et al. 2015; Ramakrishnan 2019). LTR retrotransposons are highly abundant in bamboo (46%) which contribute to the diversity of the genome. These LTR retrotransposons are the main class (Class I) of mobile genetic elements in the genome of plants. The interspaces of retrotransposons can significantly vary among bamboo species because of their random behavior of insertion. Taking advantage of this feature, IRAP is an efficient marker system to evaluate the genetic diversity of plant species (Kalendar et al. 1999). To date, there is only one transposon-based marker reported by Li et al. (2020) from the genome of bamboo, particularly using IRAP markers to study the genetic diversity of 58 Asian bamboo species. The availability of bamboo genome (http://www.bamboogdb.org, BambooGDB) helped in a comprehensive investigation of LTR-retrotransposons and their copy numbers which enabled for development of 16 IRAP primers (CL3-F;CL4-R;CL15-R;CL22-F;CL22-R;CL34-F;CL34-R;CL37-F;CL37-R;CL42-R;CL54-R;CL58-F;CL59-F;CL61-F;CL62-F;CL63-R) with clear and distinguishable amplification which generated an average of 13.3 polymorphic alleles per primer. Based on IRAP analysis, 58 accessions of Phyllostachys were clustered into five groups. He concluded from the IRAP polymorphism pattern that LTR retrotransposons notably lack polymorphism among 58 bamboo accessions under study of their abundance in the genome of bamboo; only 29% IRAP markers showed polymorphism. This insufficiency of IRAP polymorphism suggested that the inter-LTR regions were conserved significantly in the studied bamboo species which implies that in the contribution of genome wide variation, LTRs are not much active and the genome of bamboo is still under evolution. Another type of retrotransposon-based marker technique is inter Primer Binding Site amplification (iPBS) which is not much used to study the genetic diversity in bamboo especially in Indian bamboo. Amom et al. (2020) has mentioned the use of iPBS and other markers like RAPD, RFLP and SCoT to check their efficiency in genetic relationship studies in five native bamboo species of North-east India.
Single nucleotide polymorphism (SNPs)
Because of their high abundance of source polymorphism, single nucleotide polymorphism (SNPs) has become a good choice of marker type but producing variable genetic markers with single-nucleotide polymorphisms (SNPs) is many a time difficult (Blair et al. 2013). However, next generation sequencing (NSGs) offers great possibility to produce large scale sequence data at reasonable cost from non-model organisms (Galindo and Ekblom 2011). For instance, the evolutionary relationship among 6 woody species of bamboo was resolved by the application of complete chloroplast genome (Zhang et al.2011). The relationship among closely related species is better resolved by the study of their nuclear data. However, temperate bamboo being tetraploid in nature introduces additional challenges for the development of SNP markers (Gielis et al. 1997a, b; Peng et al. 2013). One of the methods to reduced-representation of genomics is restriction site associated DNA (RAD) sequencing. RAD helps in the sequencing of short fragments of DNA flanking the cut sites of restriction enzymes, which allows identification of orthologous sequences across multiple samples to be targeted and score various genetic markers (Baird et al. 2008; Emerson et al. 2010). Till date, this RAD sequencing method is successfully applied to discover SNP in eggplants (Barchi et al. 2011), sunflowers (Andrew et al. 2013) and other organisms. Thus, RAD sequencing can identify a huge number of SNPs for analysis, even without a genome of reference. Wang et al. (2013) identified SNP markers to study the phylogenetic relationship among temperate bamboo species (Arundinaria faberi and Yushania brevipaniculata) using RAD sequencing. After filtering and grouping, Wang et al. (2013) recovered 29,443 presumed orthologs shared among the four populations of Arundinaria faberi and Yushania brevipaniculata, for a length of 2,129,079 bp which included 28,023 variable sites, out of which 13,650 were fixed between species; the rest were polymorphic. The rate of SNPs occurring was 0.95 SNP/RAD tag. To date, there are not any genetic diversity studies done in Indian bamboo using SNP markers.
Sequence related amplified polymorphism (SRAP)
Genetic diversity among bamboo species using SRAP (sequence-related amplified polymorphism). Due to overexploitation and genetic erosion, there was a requirement for bamboo germplasm establishment and in this context Zhu et al. (2013) collected 13 species of bamboo from different regions of China. Examination of genetic relationships among species was done using different morphological and molecular markers. For molecular relationship, SRAP analysis was done. The SRAP analysis consisted of amplification of ORFs (open reading frames) using PCR. For this, 2 types of primer combinations were used. The forward primer was 17 bp in length containing GC rich 14 nucleotides and the reverse primer was 18 bp in length containing AT rich 15 nucleotides which amplifies promoter and intronic regions preferentially, which are generally AT rich. Feng et al. 2010 using SRAP and AFLP molecular markers analyzed genetic relationships within 9 bamboo species. Total 359 bands were generated by 12 AFLP primer combinations and 258 bands by 24 SRAP primer combinations. But until now, there have been no such papers which have reported the use of SRAP technique to characterize Indian bamboo species.
Conclusion
Genetic erosion and over-exploitation of bamboo have made conservation and collection of bamboo germplasm indispensable. For this, accurate identification and evaluation of bamboo species is very important. Since, morphological markers are greatly influenced by environmental aspects and thus cannot be considered much reliable for identification and genetic relationship study. The knowledge of genetic diversity and relationship in bamboos is still constricted despite various studies due to irregular flowering pattern and improper phenotypic variations. With emerging molecular markers, the knowledge of diversity between different varieties is possible. For molecular characterization of bamboo, the different molecular markers used are RAPD, RFLP, AFLP, ISSR and SSR out of these the frequently used technique is RAPD because of its low-cost, speed and efficiency which generate large marker numbers in a short period as compared to other techniques. However due to several limitations associated with the use of RAPD, ISSRs are the marker of choice to study the genetic diversity in Indian bamboo species. RFLP, due to low polymorphism, is not used frequently. AFLP on the other hand, is technically tough to handle with multi step procedures, hence not chosen by many laboratories. Meanwhile, SCARs can be used efficiently for genotype identification with highest accuracy. While talking about other molecular marker types which are used around the world there are many which are employed to study plant diversity, out of which few are used in diversity studies of bamboo species. The availability of the bamboo genome database has given an insight into the development and use of SCoT, IRAP, SRAP and SNPs markers in bamboo species. This review will provide information on availability of different molecular markers to identify diversity in Indian bamboo species. It will give an ease of access to different researchers working in the field of bamboo to design primer for different markers accordingly. It will provide information on availability of markers to identify diversity in Indian bamboo species. It will give an ease of access for different researchers working in the field of bamboo to design primer for different markers accordingly. For ex-situ and in-situ conservation of available species of Bamboo, it is very important to fingerprint this for proper utilization. Apart from conservation, fingerprinting based diversity study will also help in proper breeding of Bamboos for its improvement program. This highlights the likelihood and future trends in molecular markers and its application in bamboo. Undoubtedly, the most important application is the precise identification of genotypes of bamboo and the genetic variation assessment within species.
Abbreviations
- RAPD:
-
Randomly amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
- AFLP:
-
Amplified fragment length polymorphism
- ISSR:
-
Inter simple sequence repeat
- SSR:
-
Simple sequence repeat
- SCAR:
-
Sequence characterized amplified region
- ScoT:
-
Start codon targeted
- IRAP:
-
Inter-retrotransposon amplified polymorphism
- SRAP:
-
Sequence related amplified polymorphism
- SNP:
-
Single nucleotide polymorphism
References
Agarwal A, Gupta V, Haq SUI, Jatav PK, Kothari SL, Kachhwaha S (2019) Assessment of genetic diversity in 29 rose germplasms using SCoT marker. J King Saud Univ-Sci 31(4):780–788
Afshari A, Jamshid A, Razmyar J, Rad M (2016) Genomic diversity of Clostridium perfringens strains isolated from food and human sources. Iran J Vet Res 17(3):160–164
Agnihotri RK, Mishra J, Nandi SN (2009) Improved in vitro shoot multiplication and rooting of Dendrocalamus hamiltonii Nees et Arn. Ex Munro: production of genetically uniform plants and field evaluation. Acta Physiol Plant 31:961–967
Ahmad M, Kamke FA (2003) Analysis of Calcutta bamboo for structural composite materials: surface characteristics. Wood Sci Technol 37:233–240
Ahmad M, Kamke FA (2005) Analysis of Calcutta bamboo for structural composite materials: physical and mechanical properties. Wood SciTechnol 39:448–459
Amom T, Tikendra L, Apana N, Goutam M, Sonia P, Koijam AS, Potshangbam AM, Rahaman H, Nongdam P (2020) Efficiency of RAPD, ISSR, iPBS, SCoT and phytochemical markers in the genetic relationship study of five native and economical important bamboos of North-East India. Phytochemistry 174:112–330
Andrew RL, Rieseberg LH (2013) Divergence is focused on few genomic regions early in speciation: incipient speciation of sunflower ecotypes. Evolution 67(9):2468–2482
Archila H, Kaminski S, Trujillo D, Escamilla E, Harries KA (2018) Bamboo reinforced concrete: a critical review. Mater Struct 51(4):1–18
Arumugam T, Jayapriya G, Sekar T (2019) Molecular fingerprinting of the Indian medicinal plant Strychnos minor Dennst. Biotechnol Rep 21:e00318
Awoyera PO, Ede AN (2017) Bamboo versus tubular steel scaffolding in construction: pros and cons. In: Hashmi S (ed) Reference module in materials science and materials engineering. Elsevier Publishers, Oxford Spires Business Park, Kidlington 1–12
Azeem MW, Hanif MA, Khan MM (2020) Medicinal plants of South Asia Chapter 3 Bamboo. In: Hanif MA, Nawaz H, Khan MM, Byrne HJ (ed) Elsevier, pp. 29–45
Azeez MA, Orege JI (2018) Bamboo, its chemical modification and products. Bamboo Curr Fut Prosp 25–48
Baird NA, Etter PD, Atwood TS et al (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 3:e3376
Bajpai P (2018) Brief Description of the Pulp and Papermaking Process. In: Biotechnology for Pulp and Paper Processing. Springer, Singapore pp. 9–26. https://doi.org/10.1007/978-981-10-7853-8_2
Barchi L, Lanteri S, Portis E, Acquadro A, Vale G, Toppino L, Rotino GL (2011) Identification of SNP and SSR markers in eggplant using RAD tag sequencing. BMC Genom 12(1):1–9
Basumatary A, Middha SK, Usha T, Basumatary AK, Brahma BK, Goyal AK (2017) Bamboo shoots as a nutritive boon for Northeast India: an overview. 3 Biotech 7:169. https://doi.org/10.1007/s13205-017-0796-4
Bhandari S, Tyagi K, Singh B, Goutam U (2021) Role of molecular markers to study genetic diversity in bamboo. Plant Cell Biotechnol Mol Biol 22(3 & 4):86–97
Bhandawat A, Sharma V, Singh P, Seth R, Nag A, Kaur J, Sharma RK (2019) Discovery and utilization of EST-SSR marker resource for genetic diversity and population structure analyses of a subtropical bamboo, Dendrocalamus hamiltonii. Biochem Genet 57:652–672
Bhatt B, Singha L, Singh K, Sachan MS (2003) Some commercial edible bamboo species of North East India: production, indigenous uses, cost-benefit and management strategies. Bamboo Science and Culture 17(1):4–20
Bhatt B, Singha L, Sachan MS, Singh K (2004) Commercial edible bamboo species of the North-Eastern Himalayan Region, India. J Bamboo Ratt 3:337–364
Bhattacharya S, Das M, Bar R, Pal A (2006) Morphological and molecular characterization of Bambusa tulda with a note on flowering. Ann Bot 98(3):529–535
Bhattacharya S, Ghosh JS, Das M, Pal A (2009) Morphological and molecular characterization of Thamnocalamus spathiflorus subsp. spathiflorus at population level. Plant Syst Evol 282:13–20
Biswas P, Chakraborty S, Dutta S, Pal A, Das M (2016) Bamboo flowering from the perspective of comparative genomics and transcriptomics. Front Plant Sci 7:1900
Blair MW, Cortes AJ, Penmetsa RV, Farmer A, Garcia NC, Cook DR (2013) A high-throughput SNP marker system for parental polymorphism screening, and diversity analysis in common bean (Phaseolus vulgaris L.). Theor Appl Genet 126(2):535–548
Borah ED, Pathak KC, Deka B, Neog D, Borah K (2008) Utilization aspects of Bamboo and its market value. Indian for 134(3):423–427
Bouck A, Vision T (2007) The molecular ecologist’s guide to expressed sequence tags. Mol Ecol 16(5):907–924
Brown-Guedira GL, Thompson JA, Nelson RL, Warburton ML (2000) Evaluation of genetic diversity of soybean introductions and North American ancestors using RAPD and SSR markers. Crop Sci 40:815–823
Bystriakova N, Kapos V, Lysenko I, Stapleton C (2003) Distribution and conservation status of forest bamboo biodiversity in the Asia-Pacific region. Biodivers Conserv 12:1833–1841
Cajee L (2018) Diversity of bamboo species and its utilization in the north-eastern region of India. Int J Res Appl Sci Eng Technol 6:3286–3299
Chatterjee RN, Raizada MB (1963) Culm sheaths as an aid to identification of bamboos. Ind For 89:744–756
Choudhury D, Sahu JK, Sharma GD (2012) Value addition to bamboo shoots: a review. J Food Sci Technol 49:407–414
Collard BC, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plan Mol Biol Rep 27:86–93
Das M, Bhattacharya S, Singh P, Filgueiras TS, Pal A (2008) Bamboo taxonomy and diversity in the era of molecular markers. Adv Bot Res 47:225–268
Desai P, Gajera B, Mankad M, Shah S, Patel A, Patil G, Narayanan S, Kumar N (2015) Comparative assessment of genetic diversity among Indian bamboo genotypes using RAPD and ISSR markers. Mol Bio Rep 42(8):1265–1273
Ekblom R, Galindo J (2011) Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 107(1):1–15
Emerson KJ, Merz CR, Catchen JM et al (2010) Resolving postglacial phylogeography using high-throughput sequencing. Proc Nat Acad Sci USA 107:16196–16200
Etminan A, Pour-Aboughadareh AR, Mohammadi R, AhmadiRad A, Noori A, Mahdavian Z, Moradi Z (2016) Applicability of start codon targeted (SCoT) and inter-simple sequence repeat (ISSR) markers for genetic diversity analysis in durum wheat genotypes. Biotechnol Biotechnol Equip 30(6):1075–1081
Fan L, Zhang MY, Liu QZ, Li LT, Song Y, Wang LF et al (2013) Transferability of newly developed pear SSR markers to other Rosaceae species. Plant Mol Biol Rep 31:1271–1282
Forest Survey of India, Ministry of Environment, Forest & Climate Change Government of India (2019) Bamboo resources of the Country. Indian State of Forest Report ed. 16 vol 2, Dehradun, Uttarakhand, India
Friar E, Kochert G (1991) Bamboo germplasm screening with nuclear restriction fragment length polymorphisms. Theor Appl Genet 82(6):697–703
Friar E, Kochert G (1994) A study of genetic variation and evolution of Phyllostachys (Bambusoideae: Poaceae) using nuclear restriction fragment length polymorphisms. Theor Appl Genet 89(2):265–270
Gamble JS (1896) Bambuseae of british India. Ann Royal Bot Gard 7(1):133
Gami B, Syed BA, Patel B (2015) Assessment of genetic diversity in bamboo accessions of India using molecular markers. Int J Appl Sci Biotechnol 3(2):330–336
Ghosh S, Devi SW, Mandi S, Talukdar NC (2011) Amplified fragment length polymorphism-based study of phylogenetic relationship and genetic variability among some edible bamboo species of North East India. J Plant Mol Biol Biotechnol 2(8):15
Gielis J, Everaert I, De Loose M (1997a) Genetic variability and relationships in Phyllostachys using random amplified polymorphic DNA. Linn Soc Symp Acad Lond 19:107–124
Gielis J, Everaert I, Goetghebeur P, Deloose M (1997b) Bamboo and molecular markers. In bamboo, people and the environment, Vol. 2 Biodiversity and genetic conservation. INBAR Tech Rep 8:45–67
Goyal AK, Sen A (2014) Molecular phylogeny of North Bengal bamboos inferred through PCR-RFLP based on the trnL-trnF region. Exp 19(1):1304–1315
Goyal AK, Ghosh PK, Dubey PK, Sen A (2012) Inventorying bamboo biodiversity of North Bengal: a case study. Int J Fundam Appl Sci 1:5–8
Gozukirmizi N, Yilmaz S, Marakli S, Temel A (2015) Retrotransposon based molecular markers; tools for variation analysis in plants. In: Taški-Ajdukovic K (ed) Applications of molecular markers in plant genome analysis and breeding. Research Signpost, Kerala
Hao J, Jiao K, Yu C, Guo H, Zhu Y, Yang X, Zhang S, Zhang L, Feng S, Song Y, Dong M, Wang H, Shen C (2018) Development of SCoT-based SCAR marker for rapid authentication of Taxus media. Biochem Genet 56:1–12
He TY, Qu YQ, Chen LY, Xu W, Rong JD, Chen LG, Fan LL, Tarin MWK, Zheng YS (2019) Genetic diversity analysis of Dendrocalamopsis beecheyana var. Pubescens based on ISSR markers. Appl Ecol Environ Res 17:12507–12518
Hodkinson TR, Renvoize SA, Chonghaile GN, Stapleton CMA, Chase MW (2000) A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (Bambusoideae, Poaceae). J Plant Res 113:259–269
Isagi Y, Oda T, Fukushima K, Lian C, Yokogawa M, Kaneko S (2016) Predominance of a single clone of the most widely distributed bamboo species Phyllostachys edulis in East Asia. J Plant Res 129:21–27
Joshi SP, Gupta VS, Aggarwal RK, Ranjekar PK, Brar DS (2000) Genetic diversity and phylogenetic relationship as revealed by inter-simple repeat (ISSR) polymorphism in the genus Oryza. Theor appl Genet 100:1311–1320
Kalendar R, Grob T, Regina M, Suoniemi A, Schulman AH (1999) IRAP and REMAP: two new retrotransposon-based DNA fingerprinting techniques. Theor Appl Genet 98:704–711
Kaneko S, Franklin DC, Yamasaki N, Isagi Y (2008) Development of microsatellite markers for Bambusa arnhemica (Poaceae: Bambuseae), a bamboo endemic to northern Australia. Conserv Genet 9(5):1311–1313
Kaur JP, Kardam V, Pant KK, Naik SN, Satya S (2016) Characterization of commercially important Asian bamboo species. Eur J Wood Wood Prod 74(1):137–139
Konzen RE, Peron R, Ito MA, Brondani GE, TSA SM (2017) Molecular identificationof bamboo genera and species based on RAPD-RFLP markers. Embrapa Agropecuaria Oeste-Artigo em periodic indexado
Kumar R, Chandrashekhar N (2014) Fuel properties and combustion characteristics of some promising bamboo species in India. J Forest Res 25(2):471–476
Kumar PP, Turner IM, Rao AN, Arumuganathan K (2011) Estimation of nuclear DNA content of various bamboo and rattan species. Plant Biotechnol Rep 5:317–322
Li S, Ramakrishnan M, Vinod KK, Kalendar R, Yrjala K, Zhou M (2020) Development and deployment of high-throughput retrotransposon-based markers reveal genetic diversity and population structure of Asian bamboo. Forests 11(1):31
Lin XC, Lou YF, Liu J, Peng JS, Liao GL, Fang W (2010) Cross breeding of Phyllostachys species (Poaceae) and identification of their hybrids using ISSR markers. Genet Mol Res 9:1398–1404
Loh JP, Kiew R, Set O, Gan LH, Gan YY (2000) A study of genetic variation and relationship within the bamboo subtribe Bambusinae using amplified fragment length polymorphism. Ann Bot 85:607–612
Lou YF, Lin XC, He QJ, Guo XQ, Huang LC, Fang W (2010) Analysis on genetic relationship of Puji-bamboo species by AFLP and SRAP. Mol Plant Breed 8(1):83–88
Luo C, He X, Chen H, Ou S, Gao M (2010) Analysis of diversity and relationships among mango cultivars using Start Codon Targeted (SCoT) markers. Biochem Syst Ecol 38(6):1176–1184
Makmur MF, Larekeng SH, Restu M (2020) Genetic diversity of eight types of bamboo based on Random Amplified Polymorphic DNA markers. Plant Arch 20(2):2333–2337
Mansour A (2008) Utilization of genomics retrotransposon as cladistic makers. J Cell Mol Biol 7(1):17–28
Meena RK, Bhandhari MS, Barhwal S, Ginwal HS (2019) Genetic diversity and structure of Dendrocalamus hamiltonii natural metapopulation: a commercially important bamboo species of northeast Himalayas. 3 Biotech 9(2):60
Meena RK, Bhandari MS, Ginwal HS (2020) Usage of microsatellite markers for characterization of polyploids: a case study in reference to hexaploid bamboo species. Silvae Genet 69(1):94–97
Mehta R, Sharma V, Sood A, Sharma M, Sharma RK (2011) Induction of somatic embryogenesis and analysis of genetic fidelity of in vitro-derived plantlets of Bambusa nutans Wall., using AFLP markers. Eur J for Res 130:729–736
Mukherjee AK, Ratha S, Dhar S, Debata AK, Acharya PK, Mandal S, Panda PC, Mahapatra AK (2010) Genetic relationships among 22 taxa of Bamboo revealed by ISSR and EST-based random primers. Biochem Genet 48:1015–1025
Mulpuri S, Muddanuru T, Francis G (2013) Start codon targeted (SCoT) polymorphism in toxic and non-toxic accessions of Jatropa curcas L. and development of a codominant SCAR marker. Plant Sci 207:117–127
Nag A, Gupta P, Sharma V, Sood A, Ahuja PS, Sharma RK (2013) AFLP and RAPD based genetic diversity assessment of industrially important reed bamboo (Ochlandra travancorica Benth). J Plant Biochem Biotechnol 22(1):144–149
Nayak S, Rout GR (2005) Isolation and characterization of microsatellites in Bambusa arundinacea and cross species amplification in other bamboos. African J Biotechnol 4(2):151–156
Nayak S, Rout GR, Das P (2003) Evaluation of the genetic variability in bamboo using RAPD markers. Plant Soil Environ 49(1):24–28
Nguyen DM, Grillet AC, Diep TMH, Bui QB, Woloszyn M (2018) Influence of thermo-pressing conditions on insulation materials from bamboo fibers and proteins-based bone glue. Ind Crops Prod 111:834–845
Nilkanta H, Amom T, Tikendra L, Rahaman H, Nongdam P 1389 (2017) ISSR marker based population genetic study of Melocanna baccifera (Roxb.) Kurz: a commercially important bamboo of Manipur, North-East India. Hindawi Scientifica 1-9
Nongdam P, Tikendra L (2014) The nutritional facts of bamboo shoots and their usage as important traditional foods of northeast India. Int Sch Res Notices 679073:1–17
Nongkynrih C, Kumar Y, Mipun P (2019) Bamboos: diversity and its utilization in Meghalaya, northeast India. Plant Arch 19(2):3106–3311
Odunayo J, Abeeb A (2019) Molecular evaluation of Garcinia kola Heckel accessions using RAPD markers. Am J Mol Biol 9:41–51
Ohrnberger D (2002) The bamboos of the World. Second impression. Elsevier, Amsterdam
Pattanaik S, Hall JB (2011) Molecular evidence for polyphyly in the woody bamboo genus Dendrocalamus (subtribe Bambusinae). Plant Syst Evol 291:59–67
Peng Z, Lu Y, Li L, Zhao Q, Feng Q, Gao Z, Lu H et al (2013) The draft genome of the fast growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat Genet 45(4):456–461
Poczai P, Varga I, Laos M, Cseh A, Bell N, Valkonen HP, Hyvonen J (2013) Advances in plant gene- targeted and functional markers: a review. Plant Methods 9(1):6
Qiang Z, Yu-long D, Chen XU, Huiyu Z, Min-ren H, Ming-xiu W (2005) A preliminary analysis of phylogenetic relationships of Arundinaria and related genera based on nucleotide sequences of nrDNA (ITS region) and cpDNA (trn LF intergenic spacer). J Forest Res 16(1):5–8
Ramakrishnan M, Zhou M, Pan C, Hanninen H, Yrjala K, Vinod KK, Tang D (2019) Affinities of terminal inverted repeats to DNA binding domain of transposase affect the transposition activity of bamboo Ppmar2 mariner-like element. Int J Mol Sci 20(15):3692
Rangsiruji A, Binchai S, Pringsulaka O (2018) Species identification of economic bamboos in the genus Dendrocalamus using SCAR and multiplex PCR. J Sci Technol 40(3):640–647
Saha MC, Mian MAR, Eujayl I, Zwonitzer JC, Wang L, May GD (2004) Tall fescue EST-SSR markers with transferability across several grass species. Theor Appl Genet 109:783–791
Sarwat M (2012) ISSR: a reliable and cost-effective technique for detection of DNA polymorphism Methods Mol Biol 862:103–21
Shalini A, Meena RK, Tarafdar S, Thakur S (2013) Evaluation of genetic diversity in bamboo through DNA marker and study of association with morphological traits. Bull Environ Pharmacol Life Sci 2(8):78–83
Sharma RK, Gupta P, Sharma V, Sood A, Mohapatra T, Ahuja PS (2008) Evaluation of rice and sugarcane SSR markers for phylogenetic and genetic diversity analyses in bamboo. Genome 51:91–103
Sharma V, Bhardwaj P, Kumar R, Sharma RK, Sood A, Ahuja PS (2009) Identification and cross-species amplification of EST derived SSR markers in different bamboo species. Conserv Genet 10:721–724
Sharma M and Nirmala C, (2015) Bamboo Diversity of India: an update. In: 10th World bamboo congress, (17–22 September 2015), Damyang, Korea. World Bamboo Organization, Plymouth, MA, USA
Silva MF, Menis-Henrique MEC, Felisberto MHF, Goldbeck R, Clerici MTPS (2020) Bamboo as an eco-friendly material for food and biotechnology industries. Curr Opin Food Sci 33:124–130
Singh SR, Singh R, Kalia S, Dalal S, Dhawan AK, Kalia RK (2013) Limitations, progress and prospects of application of biotechnological tools in improvement of bamboo—a plant with extraordinary qualities. Physiol Mol Biol Plants 19:21–41
Singh P, Kumari P (2018) Endemic bamboos of India—conservation status
Singh L, Jaiswal A, Thul ST, Purohit HJ (2017) Ecological and economic importance of bamboos. In: Dutt S, Tyagi A, Bhati HP, Singh H (ed.), Professor M. U. Charaya Felicitation, Advances in life sciences, S. R. Scientific Publications 10:132–140
Stapleton CMA, Rao VR (1995) Genetic diversity studies and conservation: progress and prospectus. IV th International Bamboo Congress, Bali
Stapleton C, Chonghaile GN, Hodkinson TR (2009) Molecular phylogeny of Asian woody bamboos: Review for the Flora of China. Bamboo Sci Cult 22(1)
Sungkaew S, Stapleton CM, Salamin N, Hodkinson TR (2009) Non Monophyly of the woody bamboos (Bambuseae: Poaceae): a multi-gene region phylogenetic analysis of Bambusoideaes. J Plant Res 122:95–108
Tamang DK, Dhakal D, Gurung S, Sharma N, Shrestha D (2013) Bamboo diversity, distribution pattern and its uses in Sikkim (India) Himalaya. Int J Sci Res Publ 3(2):1–6
Tang DQ, Lu JJ, Fang W, Zhang S,Zhou MB (2010) Development, characterization and utilization of genebank microsatellite markers in Phyllostachys pubescens and related species. Mol Breed 25:299–311
Tanpichai S, Witayakran S, Srimarut Y, Woraprayote W, Malila Y (2019) Porosity, density and mechanical properties of the paper of steam exploded bamboo microfibers controlled by nano fibrillated cellulose. J Mater Res Technol 8:3612–3622
Tanzeem F, Ashutosh S, Vageeshbabu S, Somashekar P, Srinivasa Rao M (2019) Genetic diversity estimates of Santalum album L. through microsatellite markers: implications on conservation. Am J Plant Sci 10:462–485
Tiwari G, Singh R, Sing N, Choudhury DR, Paliwal R, Kumar A, Gupta V (2016) Study of arbitrarily amplified (RAPD and ISSR) and gene targeted (SCoT and CBDP) markers for genetic diversity and population structure in Kalmegh. Ind Crops Prod 86:1–11
Tripathi SK, Mishra OP, Bhardwaj NK, Varadhan R (2018) Pulp and papermaking properties of bamboo species Melocanna baccifera. Cellul Chem Technol 52(1–2):81–88
Triplett J, Clark LG (2003) Ambiguity and an American bamboo: the Chusquea culeou species complex. Bamboo Sci Cult 17:21–27
Uchoi A, Malik SK, Choudhary R, Kumar S, Pal D, Rohini MR, Chaudhury R (2017) Efficiency of RAPD and ISSR markers system in accessing genetic variation of Indian citron (Citrus medica L.) cultivars collected from different parts of India. Indian J Biotechnol 16:346–356
Waghmare VN, Bagde US (2013) Random amplified polymorphic DNA based genetic characterization of four important species of Bamboo, found in Raigad district, Maharashtra State, India. Afr J Biotechnol 12(28):4446–4452
Waikhom SD, Ghosh S, Talukdar NC, Mand SS (2012) Assessment of genetic diversity of landraces of Dendrocalamus hamiltonii using AFLP markers and association with biochemical traits. Genet Mol Res 11(3):2107–2121
Wang XQ, Zhao L, Eaton DAR, Guo ZH (2013) Identification of SNP markers for inferring phylogeny in temperate bamboos (Poaceae: Bambusoideae) using RAD sequencing. Mol Ecol Resour 13(5):938–945
Xiu Z, Ping Z, Zhen Z, Li Z, Feng N, Guang N, Yan H, Lei O (2017) Sap flow-based transpiration in Phyllostachys pubescens: applicability of the TDP methodology, age effect and rhizome role. Trees 31:765–779
Yang HQ, Peng S, Li DZ (2007) Generic delimitations of Schizostachyum and its allies (Gramineae: Bambusoideae) inferred from GBSSI and trnL-F sequence phylogeneis. Taxon 56(1):45–54
Yeasmin L, Ali MN, Gantait S, Chakraborty S (2015) Bamboo: an overview on its genetic diversity and characterization. 3 Biotech 5(1):1–11
Zeng B, Zhang Y, Huang L, Jiang X, Luo D, Yin G (2014) Genetic diversity of orchard grass (Dactylis glomerata L.) germplasms with resistance to rust diseases revealed by Start Codon Targeted (SCoT) markers. Biochem Syst Ecol 54:96–102
Zhang YJ, Ma PF, Li DZ (2011) High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLoS ONE 6(5):e20596
Zhao H, Peng Z, Fei B, Li L, Hu T, Gao Z, Jiang Z (2014) BambooGDB: a bamboo genome database with functional annotation and an analysis platform. Database (Oxford), Article ID bau006
Zhou G, Meng C, Jiang P, Xu Q (2011) Review of carbon fixation in bamboo forests in China. Bot Rev 77:262
Zhu S, Liu T, Tang Q, Fu L, Tang SH (2014) Evaluation of bamboo genetic diversity using morphological and SRAP analyses. Rus J Genet 50(3):267–273
Acknowledgements
I would like to thank Dr. Veenu Joshi (Asst. Professor, Center for Basic Sciences, Pt. RSU, Raipur, India) for giving me this opportunity to associate with Indira Gandhi Krishi Vishwavidyalaya, Raipur, CG. I am also grateful to Dr. Penna Suprasanna (Head of Plant Stress Physiology and Biotechnology, Nuclear Agriculture & Biotechnology Division, Bhabha Atomic Research Center, Trombay, Mumbai, India) for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boban, S., Maurya, S. & Jha, Z. DNA fingerprinting: an overview on genetic diversity studies in the botanical taxa of Indian Bamboo. Genet Resour Crop Evol 69, 469–498 (2022). https://doi.org/10.1007/s10722-021-01280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-021-01280-8