Abstract
Coconut (Cocos nucifera L.) production sustains millions of smallholder farmers and their families around the globe, and also powers a thriving industry of climate-adaptive nutrition and biomaterials. Demand for high-quality planting materials has risen sharply in recent years, calling for greater understanding and potential utilization of the genetic diversity in coconut populations. Genetic diversity among several conserved coconut varieties in Vietnam was investigated to assess the suitability of 15 start codon targeted (SCoT) primers for future selective breeding programs. Amplification of 15 SCoT primers in 57 individuals from 19 coconut varieties revealed high intra-varietal diversity (Nei’s genetic diversity index = 0.237, Shannon’s Information index = 0.352), especially among the Vietnamese Dwarfs. Intriguingly, inter-varietal differentiation accounted for only 9.41% of total genetic variation. The designated SCoT primers were moderately informative. On average, each primer had 9.5 reproducible polymorphic bands per reaction. Polymorphism Information Content (PIC) per locus ranged between 0.217 and 0.390, with Resolving power (Rp) between 0.271 and 0.599. Principal Coordinate Analysis (PCoA) and Unweighted Pair Group Method with Arithmetic mean (UPGMA) analyses revealed distinct clusters for non-native Dwarf, native Dwarf, and Tall varieties. This paper represents the first-of-its-kind attempt to shed light on coconut genetic diversity in Vietnam using molecular markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coconut (Cocos nucifera L.) has long been appreciated worldwide as a multi-functional crop with economic, environmental and social significance (Niral and Jerard 2018). In Vietnam, commercialization of fresh coconuts and related products accounts for a significant percentage of household income in productive provinces, particularly in Ben Tre, Tien Giang, and Tra Vinh. Following molecular techniques developed for important crops such as rice (Oryza sativa L.) and corn (Zea mays L.), the coconut genetic diversity has increasingly been studied and characterized (Rajesh et al. 2018). In the past, diversity studies primarily related to the presence of polyphenols, isozymes, carotenols or protein markers. Amongst these early markers, analysis of polyphenols using high-performance liquid chromatography revealed the highest levels of polymorphism (Chempakam and Ratnambal 1993). Since the 1990s, DNA markers have been widely used to analyze coconut genetic diversity, with copious studies using Random Amplification of Polymorphic DNA (RAPD) and Simple Sequence Repeats (SSR) techniques (Ashburner et al. 1997; Everard et al. 1996; Hayati and Hartana 2000; Rivera et al. 1999; Perera et al. 2000; Meerow et al. 2003; Gunn et al. 2011; Loiola et al. 2016; Preethi et al. 2020). Earlier studies used other marker systems including Restriction Fragment Length Polymorphism (RFLP) (Lebrun et al. 1998), Inverse Sequence-Tagged Repeat (ISTR) (Rohde et al. 1996; Duran et al. 1997), Inter Simple Sequence Repeats (ISSR) (Manimekalai et al. 2006), WRKY Loci (Mauro-Herrera et al. 2007; Meerow et al. 2009) and Start Codon Targeted Polymorphism (SCoT) (Rajesh et al. 2015). Major achievements in coconut genetic study have successfully contributed to (i) differentiation based on the level of genetic variation in Tall and Dwarf varieties (Perera et al. 2000), (ii) construction of genetic maps (Rohde et al. 2002; Lebrun et al. 2001), (iii) construction of phylogenetic dendrograms providing evidence for origin and distribution (Upadhya et al. 2004; Dasanayaka et al. 2009), (iv) suggested qualitative trait loci (QTLs) for mite resistance and the pandan aroma (Shalini et al. 2007; Saensuk et al. 2016), and (v) identification of genes involved in somatic embryogenesis, lipid biosynthesis and salt tolerance (Rajesh et al. 2016a; Reynolds et al. 2019; Yang et al. 2021).
Start codon targeted (SCoT) polymorphism is a straightforward and powerful DNA marker system that was first presented by Collard and Mackill (2009). The single, random primers used in PCR reactions are based on the short conserved region surrounding the ATG translation start codon in plant genes on both DNA strands. Point mutations at the primer-binding site or indel mutations within the amplified regions contribute to varying banding patterns that have successfully been used to characterize polymorphism in rice (Collard and Mackill 2009), longan (Chen et al. 2010) and grapes (Zhang et al. 2011). A screening study by Rajesh et al. (2015), involving 25 markers as previously described by Collard and Mackill (2009), found that 15 showed consistent amplification patterns for a set of 23 coconut varieties, and were therefore recommended for further analysis. SCoT markers have also proved useful in identifying true hybrid seedlings in areca nut palms (Areca catechu L.), a member of the same Areca family as coconut. Based on a 1300 bp polymorphic band produced by primer SCoT11, a sequence-characterized amplified region (SCAR) marker, which can distinguish between true Areca nut Tall x Hirehalli Dwarf hybrid seedlings and false seedlings produced due to self-pollination of Hirehalli Dwarf mother palms was developed (Rajesh et al. 2016b). Here, the suitability of 15 SCoT primers was examined for future selective breeding programs in Vietnamese coconuts, while genetic diversity of a subset of varieties conserved at the Dong Go Coconut Research Center (Ben Tre, Vietnam) was also examined.
Materials and methods
Plant materials and DNA isolation
Genetic materials were sourced from 19 coconut varieties, comprising thirteen Dwarf and six Tall varieties (Table 1). Each variety was represented by three different individual palms. Fresh leaves were thoroughly collected from the Dong Go Coconut Research Centre (Giong Trom, Ben tre province, Vietnam). DNA samples were isolated from 57 samples using the extraction procedure of the GeneJet Plant Genome Extraction Kit (Thermo Scientific) as described by the manufacturer. The quality and quantity of DNA were verified using 1.0% agarose gel electrophoresis, a NanoDrop Spectrophotometer and PCR amplification of the plant’s housekeeping gene COX (mitochondrial cytochrome oxidase).
SCoT markers analysis
Three biological replicates were used for each variety to examine and account for the within-population genetic diversity of each variety. All samples were amplified by 15 SCoT primers, previously screened for clarity by Rajesh et al. (2015). Details of the primers, synthesized from Integrated DNA Technologies (Vietnam) are provided in Table 2. The reaction mixture had a total volume of 25 μL and contained 5 μL 5 × MyTaq Buffer, 0.25 μL MyTaq DNA Polymerase (Bioline), 2 μL primer (10 μM), 15.75 μL distilled water and 2 μL template DNA (3 ng/μL). Concentration of MyTaq DNA Polymerase in the reaction mixture was 0.05 units/μL. All PCR amplifications were carried out in a GeneQ™ Thermal Cycler (Bioer, China) with the following conditions: initial denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing for 1 min at variable melting temperature (Tm) for each primer as presented in Table 2 and then 72 °C for 1 min. The final extension at 72 °C lasted 7 min. Three microliters of PCR products from each reaction were mixed with 1.5 μL of 0.6 × GelRed™ (ABT, Vietnam), and separated on 1.5% agarose gel in 0.5 × TBE buffer by electrophoresis at 50 V for 45 min using HyperLadder™ 1 kb (Bioline) as a molecular weight standard. Gels were visualized under UV light in a UVP™ GelDoc-It® imaging system.
Data analysis
The gel images were analyzed by CLIQS 1D software, version 1.4 from TotalLab (Newcastle, UK). The bands were scored as either present (1) or absent (0), and their respective molecular weights were calculated in base pairs (bp). Band matching for each gel image was automatically carried out by the program. If the retardation factor (Rf) value of the bands was within 0.015 units of another, a match was declared.
The Polymorphic Information Content (PIC) of each locus was calculated using the formula for dominant markers as \(PIC = 1 - ~\mathop \sum \limits_{i}^{n} f_{i}^{2}\), where \(f_{i}\) is the frequency of the ith allele (De Riek et al. 2001). The Resolving power (Rp) of each locus was calculated according to a previous study as \(Rp = \sum Ib\), where \(Ib = 1 - {\text{(}}2~ \times ~{\text{|}}~0.5 - f_{i} |~)\) (Prevost and Wilkinson 1999). The heterozygosity (He) value of each variety was computed according to Nei’s formula for within population variation index as \(He = 1 - {\text{~}}\mathop \sum \limits_{{i = 1}}^{n} f_{i}^{2}\) (Nei 1978). The unbiased heterozygosity (uHe) value was calculated as \(uHe = \left[ {2N~ \div \left( {2N - 1} \right)} \right]*He\) where N is the number of samples. The Shannon’s Information index was determined using \(I = ~ - \sum f_{{i~}} \times ~\log _{2} (f_{i} )\) (Lewontin 1972). The Shannon’s Information index (uI) value was calculated as \(uI = \left[ {~2N~ \div \left( {2N - 1} \right)} \right]*I\).
Multi-dimensional scaling analysis was undertaken by applying the Principal Coordinate Analysis (PCoA) method to the dissimilarity matrix composed of populations’ pairwise Nei’s genetic distances. Analysis of MOlecular VAriance (AMOVA) was performed with 9999 permutations for all data. Hierarchical cluster analysis was achieved with the application of UPGMA to the matrix of Simple Matching similarity coefficients. Calculations and data visualizations were performed using Microsoft Excel (2010), Genalex 6.5 (Peakall and Smouse 2006, 2012), PopGene 1.32 (Yeh et al. 1997) and NTSYS-pc 2.1 (Rohlf et al. 2002).
Results
SCoT polymorphism
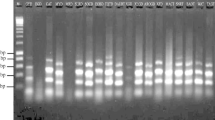
A total of 3774 amplicons were obtained, with ca. 251.6 bands per primer. The length of the bands ranged from 150 to 8500 bp. By matching molecular weights of the amplicons between corresponding replicates, we discovered 306 unique loci with an average of 20.4 unique resolvable bands amplified per primer. The number of loci with reproducible and polymorphic bands was 180, with an average of 9.5 bands per primer, ranging from 5 bands (SCOT19) to 17 bands (SCOT13). The Rp values ranged between 1.895 (SCOT04) and 9.263 (SCOT13), with an average of 5.733. Total Polymorphic Information Content (PIC) ranged from 1.518 (SCOT04) to 6.039 (SCOT13), with an average of 3.849. Average PIC over loci (PICave) ranged from 0.217 (SCOT04) to 0.390 (SCOT18) with an average of 0.316. Table 3 presents all data regarding number of amplicons and loci, Rp and PIC values for each SCoT primer. PCR amplification profiles, produced with two representative SCOT primers (SCOT04 and SCOT15), were shown in Fig. 5.
Genetic diversity analysis among coconut varieties
Based on allele distribution statistics presented in Table 4, the frequency of all reproducible polymorphic loci was greater than 5%, suggesting that no band was unique to a single variety. Numbers of bands were higher in XFD (158), AROD (151), XLGD (150) while MRD (29), EGD (37), and SLRD (45) were recorded the lowest number of bands. Furthermore, the number of bands presented at less than or equal to 25% of the population was observed in XFD (11) and in MYD, MRD, SLRD and TQYD (0). The number of less common bands presented at less than or equal to 50% of the populations was reported in XFD (50) and MRD (2). Likewise, the mean unbiased expected heterozygosity (uHe) ranged from 0.058 (MRD) to 0.410 (XFD), with an average of 0.237. The mean unbiased Shannon’s Information index ranged between 0.092 (MRD) and 0.592 (XFD), with an average of 0.352. Applying the two-tailed t-test and assuming unequal variances, the Nei’s genetic diversity index (uHe) of Vietnamese Dwarfs (M = 0.319, SD = 0.066) was higher than the non-native Dwarf varieties (M = 0.260, SD = 0.086), t(11) = 5.916, p < 0.001. AMOVA analysis presented in Table 5 indicated significant variation within varieties (90.57%, ΦPT = 0.094, p = 0.009).
Multi-dimensional scaling analysis
The first three axes of the PCoA plot derived from Nei’s genetic distances represented 34.96%, 10.45% and 7.81% of the cumulative variation among varieties. The 19 coconut varieties were separated into four distinct groups, as shown in Fig. 1. The clearest association was seen among six non-native Dwarf varieties—one Sri Lankan (SLRD), two African (GYD, EGD), two Malaysian Dwarfs (MYD, MRD) and one Filipino Dwarf (CAT). Four Vietnamese varieties, including three Dwarfs and one hybrid (XLGD, AROD, XGD and DAURT) were also clustered together. The third group was made up of four Tall varieties (WAT, BAOT, TAAT01 and MACT02) and one Dwarf (EOD). Two Dwarfs from Vietnam primarily harvested for drinking water (SOCD and TQYD) exhibited a close relationship, according to this analytical tool. As data showed, XFD and SNRT showed no meaningful association with any group.
Hierarchical clustering analysis
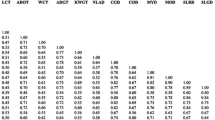
The average pairwise Simple Matching (SM) coefficient was 0.611. The pairwise Simple Matching (SM) coefficient was lowest in two Dwarf varieties from Sri Lanka (SLRD) and Vietnam (XFD) at 0.411, and highest in two Dwarf varieties from Sri Lanka (SLRD) and Malaysia (MRD) at 0.956. The Vietnamese XFD showed the lowest coefficients against all foreign Dwarf varieties. Bootstrap values for branches on the dendrogram constructed from UPGMA analysis ranged between 26 and 90%, as shown in Fig. 2. Two main clusters were identified, designated as ‘A’ and ‘B’ diverging at the similarity coefficient of 0.550, meaning that they shared 55% of the amplified bands. Each cluster contained two clades as A.1, A.2 and B.1, B.2. Cluster A was characterized by five varieties (DAURT, SOCD, XLGD, AROD and XFD) that all had small fruit sizes and high sugar levels of 7.5–8.5°Bx.. Within Cluster A, Clade A.1 contained one hybrid (DAURT) and two Dwarf (SOCD, XLGD) varieties from Vietnam, while Clade A.2 contained two Dwarf varieties from Vietnam (AROD and XFD). Cluster B contained both Tall and Dwarf varieties. Sub-cluster B.1 contained two clades with the highest similarity values (Clade B.1.1 and Clade B.1.2), while Clade B.1.1 contained six Dwarf varieties from Equatorial Guinea, Malaysia, Sri Lanka, the Philippines, and Vietnam (EGD, MYD, MRD, SLRD, CAT, and EOD). These varieties produced coconut water at medium quantity (133.3–220 g per nut), with sugar level of 6.75°Bx (data not shown). Clade B.1.2 contained three Tall varieties from Vietnam and West Africa (MACT02, WAT and TAAT01. These all had oil content in the upper-medium range (ca. 65% of dry weight) (data not shown). Sub-cluster B.2 contained a Vietnamese Dwarf variety (TQYD) and two Tall varieties from the Philippines (SNRT and BAOT). These varieties were less productive in fruit yield (80–90 fruits/tree/year).
Discussion
The SCoT system was designed with longer primers, modeled after short conserved regions flanking ATG translation start codon in plants. This characteristic confers higher reproducibility, with a greater possibility of amplifying meaningful regions of the genome compared to RAPD markers (Collard and Mackill 2009). This study suggested that SCoT primers can detect a high level of genetic diversity among coconut varieties, with an average PIC value of 0.316 ± 0.051 comparable to that obtained by SSR and RAPD primers (Rivera et al. 1999; Meerow et al. 2003; Rajesh et al. 2014). Thus, in Fig. 3, by plotting a scatter graph of markers based on their PIC values and number of reproducible unique loci, we identified a group of markers that performed well on both measures (SCOT18, SCOT13, SCOT15, SCOT21, SCOT22 and SCOT23) in contrast to another group with low values for both measures (SCOT04 and SCOT25). The primers of the first group should therefore be prioritized in future coconut studies.
The allele distribution revealed by SCoT primer amplification indicated a high degree of diversity in the seven Dwarf varieties originating from Vietnam (SOCD, EOD, TQYD, XGD, XFD, AROD and XLGD) compared to a relatively high degree of uniformity within each of the six non-native Dwarf varieties (GYD, EGD, CAT, MYD, MRD and SLRD), as shown in Fig. 4. The Nei’s genetic diversity index (uHe) of Vietnamese Dwarfs was higher than the non-native Dwarf varieties. Applying the two-tailed t-test and assuming unequal variances, the Nei’s genetic diversity index (uHe) of Vietnamese Dwarfs (M = 0.319, SD = 0.066) was higher than the non-native Dwarf varieties (M = 0.260, SD = 0.086), t(11) = 5.916, p < 0.001. The Nei’s genetic diversity index measures the probability that two individuals, chosen at random from the population, do not exhibit the same allele for the marker being studied. Therefore, our results suggested that within-population diversity for Vietnamese Dwarf varieties was high (Fig. 5).
Previous genetic marker studies on coconut revealed that Dwarf varieties tend to be more homozygous by virtue of their self-pollinating nature, resulting in less variability (Perera et al. 1998, 2000). The sampling procedure for this study was prudently carried out in the most established germplasm collection in Vietnam. Thus, the high within-population diversity for Vietnamese Dwarf varieties revealed above suggests that traditional farming practices in Vietnam have greatly encouraged cross-fertilization. Unlike other regions where monoculture coconut plantations are well-established, most coconut palms in Ben Tre, Vietnam are planted as mixtures of varieties as small enclosures with high density, often intercropped with several fruits and vegetables. The high density of palms coupled with the great diversity of pollinating insects might have contributed to cross-pollination even in traditionally self-pollinating Dwarfs. Dong Go Coconut Research Center selected native mother palms from surrounding small farms. Thus, results presented here most likely reflect the genetic diversity present in the Ben Tre region.
This high level of genetic diversity in Vietnamese Dwarf varieties has not been observed in previous studies. Compared to other regions such as China, America and Africa, Southeast Asia is regarded as the center of domestication of Dwarf coconuts. Hence, coconuts from this region tend to have a greater genetic diversity (Perera et al. 2000; Xiao et al. 2013). Nevertheless, in most studies that included Vietnamese Dwarfs, the genetic diversity of the Vietnamese group was not significantly different compared to populations from other Southeast Asian countries (Perera et al. 2003; Geethanjali et al. 2018). The allele distribution revealed by this study suggests that a closer examination of the genetic material of Vietnamese Dwarfs will be of interest, especially since greater diversity allows for better and more sustainable resistance to diseases such as lethal yellowing (Baudouin et al. 2009).
Shannon’s Information index was higher in Vietnamese Dwarf varieties (M = 0.471, SD = 0.066) than in non-native Dwarf varieties (M = 0.190, SD = 0.052), t(11) = 6.107, p < 0.001. Shannon’s index is a statistical measure of entropy, based on the weighted geometric mean of the frequency of each allele (Hennink and Zeven 1990; Spellerberg and Fedor 2003). If a marker has a greater number of alleles that are more evenly distributed across populations, this will result in a higher Shannon’s index value. The lower number of alleles in non-native varieties might be due to the small number of qualified foreign mother palms conserved at Dong Go Coconut Research Center, leading to higher uniformity in these sample populations.
Among the exotic varieties, the Nei’s genetic diversity index value for the Talls (M = 0.249, SD = 0.072) was not significantly higher than for the Dwarfs (M = 0.125, SD = 0.051), t(3) = 2.662, p = 0.076. Previous studies suggested that the greater diversity in Tall varieties was due to allogamous breeding behaviors but the limited number of varieties in this study precludes a statistically significant conclusion (Rivera et al. 1999; Teulat et al. 2000). There was also no significant difference in Nei’s index between the seven Vietnamese Dwarfs (M = 0.319, SD = 0.066) and the three Vietnamese Tall and hybrid varieties (M = 0.260, SD = 0.086), t(3) = 1.063, p = 0.366, or between all Dwarf varieties (M = 0.230, SD = 0.116) and all Tall varieties (M = 0.255, SD = 0.071), t(15) = 0.575, p = 0.574. This result further underscored the high degree of genetic diversity in Vietnamese Dwarf populations compared to previously studied populations.
Across all varieties, the moderate ΦPT value (9.41%) obtained from the AMOVA analysis indicated relatively little differentiation among coconut varieties. ΦPT is an analog of Wright’s fixation index (Fst). The value decreases with a greater degree of gene flow. A high value of ΦPT suggests significant barriers to inter-varietal breeding and, hence, high inter-varietal variance. By contrast, a lower value suggests the reverse, i.e. varieties are more similar to one another. Previous studies of coconut indicated that the degree of within-variety population was higher than among-variety, ranging from 53% in Sri Lankan varieties (Perera et al. 1998), 60% in South Pacific varieties (Ashburner et al. 1997), 67% in Mexican varieties (Zizumbo-Villarreal et al. 2006) and up to 98% in Kenyan varieties (Oyoo and Muhammed 2016). The within-population variation partition of coconut varieties conserved in Dong Go Coconut Research Center was toward the higher end of the spectrum at 90.59%. This is primarily due to the greatly shared diversity among aforementioned Vietnamese Dwarf varieties, which in turn suggests a degree of introgression, especially among the Xiem family (XFD, XLGD, XGD and AROD).
Both the PCoA plot and UPGMA dendrogram delineated six non-native Dwarf varieties as a separate group. Both methods also indicated that the Vietnamese Dwarf varieties were similar to one another, and that the Tall varieties could be separated into distinct clusters (WAT, SNRT, BAOT, TAAT01 and MACT02). The Vietnamese hybrid DAURT was suggested by both methods to share more alleles with the Dwarf variety of XLGD. This result indicated that SCoT primers can provide useful information about the type and origin of coconut varieties, as suggested by the pioneering study on SCoT markers in coconut (Rajesh et al. 2015).
In conclusion, this study is one of the first to characterize Vietnamese coconut varieties using genetic markers. The high level of within-population genetic diversity revealed in Vietnamese coconuts, especially among the Dwarf varieties, suggested a higher degree of inter-breeding in Vietnamese Dwarfs compared to foreign Dwarf populations. This underscored the influence of traditional farming practice at small householder farms, where mixtures of coconut varieties are planted at high density among intercrops that attract pollinating insects. Future breeding efforts to improve yield and disease-resistance traits can benefit from further investigation of such diversity. SCoT markers with higher differentiation power were also identified as relatively able to demonstrate relationship among coconut varieties with Vietnamese origin or foreign origin, and likewise demonstrate relationship among Dwarfs and among Talls. The SCoT-PCR system thus proved useful in the exploratory assessment of diversity in large coconut germplasm collections.
Data availability
Data supporting the findings of this study are available from the corresponding authors (Nguyen Bao Quoc and Nguyen Phuong Thao) upon reasonable request.
Abbreviations
- SCoT :
-
Start codon targeted
- QTL :
-
Quantitative trait locus
- PIC :
-
Polymorphism information content
- Rp :
-
Resolving power
- PCoA :
-
Principal coordinate analysis
- UPGMA :
-
Unweighted pair group method with arithmetic mean
References
Ashburner GR, Thompson WK, Halloran GM (1997) RAPD analysis of South Pacific coconut palm populations. Crop Sci 37(3):992–997
Baudouin L, Philippe R, Quaicoe R, Dery S, Dollet M (2009) General overview of genetic research and experimentation on coconut varieties tolerant/resistant to Lethal Yellowing. Oléagineux Corps Gras Lipides 16(2):127–131
Chempakam B, Ratnambal MJ (1993) Variation for leaf polyphenols in coconut cultivars. In Advances in Coconut Research and Development. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, India, pp. 51–53.
Chen H, He XH, Luo C, Zhu J, Li F (2010) Analysis on the genetic diversity of 24 longan (Dimocarpus longan) accessions by SCoT markers. Acta Horticulturae Sinica 37:1651–1654
Collard BC, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Report 27(1):86
Dasanayaka PN, Nandadasa HG, Everard JMDT, Karunanayaka EH (2009) Analysis of coconut (Cocos nucifera L.) diversity using microsatellite markers with emphasis on management and utilisation of genetic resources. J Nat Sci Found Sri Lanka 37(2):99–109
De Riek J, Calsyn E, Everaert I, Van Bockstaele E, De Loose M (2001) AFLP based alternatives for the assessment of distinctness, uniformity and stability of sugar beet varieties. Theor Appl Genet 103(8):1254–1265
Duran Y, Rohde W, Kullaya A, Goikoetxea P, Ritter E (1997) Molecular analysis of East African tall coconut genotypes by DNA marker technology. J Genet Breed 51:279–288
Everard JMDT, Katz M, Gregg K (1996) Inheritance of RAPD markers in the coconut palm Cocos nucifera L. Trop Agric Res 8:124–138
Geethanjali S, Rukmani JA, Rajakumar D, Kadirvel P, Viswanathan PL (2018) Genetic diversity, population structure and association analysis in coconut (Cocos nucifera L.) germplasm using SSR markers. Plant Gen Res 16(2):156
Gunn BF, Baudouin L, Olsen KM (2011) Independent origins of cultivated coconut (Cocos nucifera L.) in the Old World tropics. PLOS ONE 6(6):21143
Guo X, Elston R (1999) Linkage information content of polymorphic genetic markers. Hum Hered 49(2):112–118
Hayati PK, Hartana A (2000) Genetic diversity of Jombang coconut population based on RAPD markers. In International Conference of Science and Technology for Managing Plant Genetic Diversity in the 21st Century, pp. 12–16.
Hennink S, Zeven AC (1990) The interpretation of Nei and Shannon-Weaver within population variation indices. Euphytica 51(3):235–240
Lebrun P, N’cho YP, Seguin M, Grivet L, Baudouin L (1998) Genetic diversity in coconut (Cocos nucifera L.) revealed by restriction fragment length polymorphism (RFLP) markers. Euphytica 101(1):103–108
Lebrun P, Baudouin L, Bourdeix R, Konan JL, Barker JH, Aldam C, Ritter E (2001) Construction of a linkage map of the Rennell Island Tall coconut type (Cocos nucifera L.) and QTL analysis for yield characters. Genome 44(6):962–970
Lewontin RC (1972) The apportionment of human diversity. Evol Biol 6:381–398
Loiola CM, Azevedo AON, Diniz LE, Aragao WM, Azevedo CDDO, Santos PHA, Ramos HCC, Pereira MG, Ramos SRR (2016) Genetic relationships among tall coconut palm (Cocos nucifera L.) accessions of the International Coconut Genebank for Latin America and the Caribbean (ICG-LAC), evaluated using microsatellite markers (SSRs). PloS one 11(3):0151309
Manimekalai R, Nagarajan P, Kumaran PM (2006) Comparison of effectiveness of RAPD, ISSR and SSR markers for analysis of coconut (Cocos nucifera L.) germplasm accessions. Trop Agric Res 18:217–226
Mauro-Herrera M, Meerow AW, Borrone JW, Kuhn DN, Schnell RJ (2007) Usefulness of WRKY gene-derived markers for assessing genetic population structure: an example with Florida coconut cultivars. Sci Hortic 115(1):19–26
Meerow AW, Wisser RJ, Brown SJ, Kuhn DN, Schnell RJ, Broschat TK (2003) Analysis of genetic diversity and population structure within Florida coconut (Cocos nucifera L.) germplasm using microsatellite DNA, with special emphasis on the Fiji Dwarf cultivar. Theor Appl Gen 106(4):715–726
Meerow AW, Noblick L, Borrone JW, Couvreur TL, Mauro-Herrera M, Hahn WJ, Kun DN, Nakamura K, Oleas NH, Schnell RJ (2009) Phylogenetic analysis of seven WRKY genes across the palm subtribe Attaleinae (Arecaceae) identifies Syagrus as sister group of the coconut. PLoS One 4(10):7353
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89(3):583–590
Niral V, Jerard BA (2018) Botany, origin and genetic resources of coconut. In the coconut palm (Cocos nucifera L.)-research and development perspectives. Springer, Singapore
Oyoo ME, Muhammed N, Cyrus KN, Githiri SM (2016) Assessment of the genetic diversity of Kenyan coconut germplasm using simple sequence repeat (SSR) markers. Afr J Biotech 15(40):2215–2223
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peakall R, Smouse PE (2012) GENALEX 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Perera L, Russell JR, Provan J, McNicol JW, Powell W (1998) Evaluating genetic relationships between indigenous coconut (Cocos nucifera L.) accessions from Sri Lanka by means of AFLP profiling. Theor Appl Gen 96(3–4):545–550
Perera L, Russell JR, Provan J, Powell W (2000) Use of microsatellite DNA markers to investigate the level of genetic diversity and population genetic structure of coconut (Cocos nucifera L.). Genome 43(1):15–21
Perera L, Russell JR, Provan J, Powell W (2003) Studying genetic relationships among coconut varieties/populations using microsatellite markers. Euphytica 132(1):121–128
Preethi P, Rahman S, Naganeeswaran S, Sabana AA, Gangaraj KP, Jerard BA, Niral V, Rajesh MK (2020) Development of EST-SSR markers for genetic diversity analysis in coconut (Cocos nucifera L.). Mol Biol Rep 47(12):9385–9397
Prevost A, Wilkinson MJA (1999) New system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98(1):107–112
Rajesh MK, Jerard BA, Preethi P, Thomas RJ, Karun A (2014) Application of RAPD markers in hybrid verification in coconut. Crop Breed Appl Biotech 14(1):36–41
Rajesh MK, Sabana AA, Rachana KE, Rahman S, Jerard BA, Karun A (2015) Genetic relationship and diversity among coconut (Cocos nucifera L.) accessions revealed through SCoT analysis. Biotech 5(6):999–1006
Rajesh MK, Sabana AA, Rachana KE, Rahman S, Ananda KS, Karun A (2016) Development of a SCoT-derived SCAR marker associated with tall-type palm trait in arecanut and its utilization in hybrid (dwarf x tall) authentication. Ind J Gen Plant Breed 76:119–122
Rajesh MK, Fayas TP, Naganeeswaran S, Rachana KE, Bhavyashree U, Sajini KK, Karun A (2016) De novo assembly and characterization of global transcriptome of coconut palm (Cocos nucifera L.) embryogenic calli using Illumina paired-end sequencing. Protoplasma 253(3):913–928
Rajesh MK, Karun A, Parthasarathy VA (2018) Coconut biotechnology. In the coconut palm (Cocos nucifera L.)-research and development perspectives. Springer, Singapore
Reynolds KB, Cullerne DP, El Tahchy A, Rolland V, Blanchard CL, Wood CC, Singh SP, Petrie JR (2019) Identification of genes involved in lipid biosynthesis through de novo transcriptome assembly from Cocos nucifera developing endosperm. Plant Cell Physiol 60(5):945–960
Rivera R, Edwards KJ, Barker JHA, Arnold GM, Ayad G, Hodgkin T, Karp A (1999) Isolation and characterization of polymorphic microsatellites in Cocos nucifera L. Genome 42:668–675
Rohde W, Kullaya A, Rodriguez MJ, Ritter E (1996) Genome analysis of Cocos nucifera L. by PCR amplification of spacer sequences separating a subset of Copia-like Ecorirepelitive elements. Philippine J Crop Sci (philippines) 21:26–31
Rohde W, Sniady V, Herran A, Estioko L, Sinje S, Marseillac N, Angelique B, Patricia L, Dieter P, Alois K, Judith R, Norbert B, Rodriguez J (2002) Construction and exploitation of high density DNA marker and physical maps in the perennial tropical oil crops coconut and oil palm: from biotechnology towards marker-assisted breeding. Burotrop Bulletin 20:13–14
Rohlf FJ (2002) NTSYSpc: Numerical taxonomy system, ver. 2.1. Setauket, NY: Exeter Publishing Ltd.
Saensuk C, Wanchana S, Choowongkomon K, Wongpornchai S, Kraithong T, Imsabai W, Chaichoompu E, Ruanjaichon V, Toojinda T, Vanavichit A, Arikit S (2016) De novo transcriptome assembly and identification of the gene conferring a “pandan-like” aroma in coconut (Cocos nucifera L.). Plant Sci 252:324–334
Shalini KV, Manjunatha S, Lebrun P, Berger A, Baudouin L, Pirany N, Raganath RM, Prasad DT (2007) Identification of molecular markers associated with mite resistance in coconut (Cocos nucifera L.). Genome 50(1):35–42
Spellerberg IF, Fedor PJA (2003) A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Glob Ecol Biogeogr 12(3):177–179
Teulat B, Aldam C, Trehin R, Lebrun P, Barker JH, Arnold GM, Karp A, Baudouin L, Rognon F (2000) An analysis of genetic diversity in coconut (Cocos nucifera) populations from across the geographic range using sequence-tagged microsatellites (SSRs) and AFLPs. Theor Appl Genet 100(5):764–771
Upadhya A, Jayadev K, Manimekalai R, Parthasarathy VA (2004) Genetic relationship and diversity in Indian coconut accessions based on RAPD markers. Sci Hortic 99(3–4):353–362
Xiao Y, Luo Y, Yang Y, Fan H, Xia W, Annaliese S, Mason AS, Zhao S, Sager R, Qiao F (2013) Development of microsatellite markers in Cocos nucifera and their application in evaluating the level of genetic diversity of Cocos nucifera. Plant Omics 6(3):193–200
Yang Y, Bocs S, Fan H, Armero A, Baudouin L, Xu P, Xu J, This D, Hamelin C, Iqbal A, Qadri R, Zhou L, Li Z, Wu Y, Ma Z, Issali AE, Rivallan R, Liu N, Xia W, Peng M, Xiao Y (2021) Coconut genome assembly enables evolutionary analysis of palms and highlights signaling pathways involved in salt tolerance. Commun Biol 4(1):1–14
Yeh FC, Yang RC, Boyle TB, Ye ZH, Mao JX (1997) Popgene, version 1.32; the user-friendly shareware for population genetic analysis. Can Univ Alberta Mol Biol Biotechnol Centre 10:295–301
Zhang J, Guo D, Gong Y, Liu C, Li M, Zhang G (2011) Optimization of start codon targeted polymorphism PCR (SCoT-PCR) system in Vitis vinifera. J Fruit Sci 28(2):209–214
Zizumbo-Villarreal D, Ruiz-Rodriguez M, Harries H, Colunga-GarciaMarin P (2006) Population genetics, lethal yellowing disease, and relationships among mexican and imported coconut ecotypes. Crop Sci 46:2509–2516
Acknowledgements
We are grateful to the Dong Go Coconut Research Center for generous provision of leaf materials and information.
Funding
This research was funded by the Ministry of Science and Technology (MOST, Vietnam) under grant number ĐTĐL.CN-12/19.
Author information
Authors and Affiliations
Contributions
Conceptualization: NBQ, Data curation: NHMK, HNXM, NDNP, NBQ, Formal analysis: NHMK, Funding acquisition: NPT, Investigation: NHMK, NTQ, HNXM, NDNP, NPT and NBQ, Methodology: NBQ, NHMK, Validation: NBQ, NPT, Writing—original draft: NHMK, NBQ, Writing—review and editing: NBQ, NPT, NTQ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this article have no conflicts of interest.
Consent for publication
All authors have given their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khang, N.H.M., Quang, N.T., Mai, H.N.X. et al. Genetic characterization of coconut (Cocos nucifera L.) varieties conserved in Vietnam through SCoT marker-based polymorphisms. Genet Resour Crop Evol 69, 385–398 (2022). https://doi.org/10.1007/s10722-021-01237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-021-01237-x