Abstract
Ispaghul (Plantago ovata Forsk.) as an important medicinal plant has obtained a remarkable reputation due to therapeutic applications of seed mucilage. To determine the effect of in vitro-induced polyploidy on various characteristics of P. ovata, the terminal bud of two true leaves seedlings were separately treated with colchicine [0.1, 0.3 and 0.5% (w/v) for 6, 12 and 24 h] and trifluralin [7.5, 15 and 22.5% (w/v) for 24, 48 and 72 h] solutions. The ploidy level of induced tetraploids was determined via chromosome counting of root tip cells, and then confirmed through flow-cytometric analysis. Comparison the morphological, physiological, anatomical features of intact diploids and induced tetraploids revealed that tetraploids had considerable more height, thicker leaf, larger spike and seed, larger pollen grain and more seeds per spike. Moreover, the amount of chlorophyll (a, b, and total) and carotenoids, as well as chloroplast number in guard cells was further in tetraploids than diploids. Unlike density, stomata size in tetraploids was bigger than that one in diploids. It was also observed that seeds of tetraploids had more mucilage than diploids. In summary, we firstly developed the P. ovata tetraploids and suggested 0.3% colchicine for 24 h and 22.5% trifluralin for 72 h as the optimum treatments for inducing tetraploidy in P. ovata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changing drug trends has led to an increase in demand for biomass and bioactive compounds in medicinal plants (Sabzehzari and Naghavi 2018, 2019). Plantago genus was known as an important medicinal herb in the pharmacy industries (Shahriari et al. 2018). The genus contains several species-especially P. ovata-that are important due to agricultural and medicinal values. From an agricultural point of view, domestication and cultivation of P. ovata as alternatives of high-water consuming plants-such as corn and wheat-can be adopted for marginal agronomic areas (Koocheki et al. 2007; Bannayan et al. 2008). From a medicinal perspective, P. ovata seeds contain mucilage (Karimi et al. 2013), which is mainly utilized in the food and cosmetic industries (Dhar et al. 2005; Ebadi-Almas et al. 2012), and also medicine (Dhar et al. 2002, 2005; Saeedi et al. 2010). The oral use of mucilage helps decline the level of blood cholesterol (Dhar et al. 2002, 2005). Furthermore, in various countries especially China, India and Iran, P. ovata seeds are utilized to treat breathing difficulties, fever, cough, cold, urinary problems, gonorrhea, dysentery and gastrointestinal malfunction as an alternative of chemical medicines such as antibiotics (Bahmani et al. 2016). As a result, P. ovata can be considered as an economic crop with good export value and relatively high mucilage yield applicable in medicine industries (Koocheki et al. 2007; Bannayan et al. 2008; Ebadi-Almas et al. 2012). Economic analysis revealed that the USA is the chief importer of P. ovata seeds and consumes annually 8000 metric tons, which indicating the importance of P. ovata market in the world (Rehana et al. 2015).
Polyploidy is an important approach in improving medicinal plants, because polyploidies exhibit more bioactive compounds (Gao et al. 2002; Berkov and Philipov 2002; Majdi et al. 2010). In general, genome duplication results in larger fruits and flowers, further content of secondary metabolites and finally more yield (Predieri 2001; Roy et al. 2001; Gu et al. 2005; Urwin et al. 2007). Polyploidy also improves starch content as an important nutritional value of the plants, a phenomenon which favored the human selection of cereals during human evolution (Kumar et al. 2018; Denham et al. 2016). For example, the induced polyploidies of Avena sativa, Saccharum officinarum, Solanum tuberosum, Triticosecale, Triticum aestivum, Coffea Arabica, Fragaria ananassa, Nicotiana tabacum have demonstrated the remarkable pharmaceutical and agronomic benefits compared with intact diploids (Gao et al. 2002; Zhang et al. 2008). This approach can be induced by using different antimitotic chemicals. Among them, the most common used antimitotic agents are oryzalin, trifluralin, as well as colchicine (Salma et al. 2017). The approach of chromosome doubling by antimitotic agents consists of several steps, including an induction, re-growth, and confirmation phase, which used to identify polyploidies (Salma et al. 2017). Since, induction efficiency depends on various factors-such as antimitotic agents, its different concentrations and exposure durations, and explant types-a range of attempts was carried out in order to find the optimum conditions to polyploidy induction (Lavania 2005). To assess the result of polyploidization, morphological, anatomical and physiological characteristics can be assayed as a rapid technique. However, flow cytometry and chromosome count are used as dominant techniques for absolute confirmation (Doležel and Bartoš 2005; Dhooghe et al. 2011).
Since, genome duplication affects the plant size and its secondary metabolites profile (Adaniya and Shirai 2001; Berkov and Philipov 2002; Jesus-Gonzalez and Weathers 2003; Majdi et al. 2010), polyploidy can be a promising approach to achieve the pharmaceutical and agronomic advantages in tetraploid of P. ovata (Dhooghe et al. 2011). Based on the mentioned advantages of polyploidy and on our previous study on P. Psyllium (Sabzehzari et al. 2019), present study was made to evaluate the effect of trifluralin and colchicine-induced polyploidy on morphological, physiological, anatomical, and cytological features of P. ovata medicinal plant and compare the induced tetraploids with their diploids.
Materials and methods
Plant material
Plantago ovata (2n = 2× = 8) seeds were provided from Pakanbazr Co. (http://www.pakanbazr.com/). In the following, chromosome doubling was made by colchicine and trifluralin on terminal buds, as discussed below.
Tetraploidy induction
For chromosome doubling, first a total of 100 seeds were planted in a culture tray with cocopeat content. Then, terminal buds of seedlings containing two true leaves, 30 days old, were treated by colchicine [0.1, 0.3 and 0.5% (w/v) for 6, 12 and 24 h] and trifluralin [7.5, 15 and 22.5% (w/v) for 24, 48 and 72 h] solutions. The concentrations of chemicals were selected based on LD50. In forth to six true leaves stage, seedlings were transferred to pots containing sand, clay and rotten manure (2:1:1) under normal greenhouse conditions (16 h of light period, 25–26 °C and 65% humidity). The experiment was carried out as a factorial in format of completely randomized design with two factors-including concentrations and durations of exposure-with three replications.
Identification and confirmation of the actual tetraploids
Three months after tetraploidy induction, the treated plants were compared with the controls in order to decline the workload and also rudimentary isolation of putative tetraploids. For achieving this goal, putative 4 × plants were marked according to the differences which observed in their leaves thickness, shape, color and size, in contrast with the 2 × plants. After marking deformed plants as putative tetraploids, two commonly techniques, flow-cytometry and chromosomal counting, were utilized to identify and validate actual tetraploids.
Chromosome counting
In order to counting the chromosome number, the root tips (0.5–1 cm long) were cut off at 11:00 a.m., and were pretreated with 4 °C for 12 h under refrigerator (physical pretreatment) and 0.002 g/mL C9H7NO solution for 3 h at 4 °C (chemical pretreatment), respectively. Carnoy’s I solution containing glacial-acetic acid and 95% ethanol (1:3) was used to fix the pretreated samples for 20 h and laid in 70% alcohol at 4 °C. Fixed samples were hydrolyzed in 1 N hydrochloric acid for 5 min at 60 °C, and then were washed with distilled water for 10–15 min and were stained with Aceto-orcein 2% in darkness at 25 °C for 60 min. In finally, stained samples with nearly 1–2 mm in length were squashed on a glass slide and their metaphase chromosomes were observed using Olympus microscope equipped with a camera.
Flow cytometry
In accordance with Doležel and Bartoš (2005), nearly 25 mg fresh leaf was chopped with a sharp blade in a petri-dish which consists of 1 mL modified WPB buffer (containing 10 mM Na2S2O5, 4 mM MgCl2·6H2O, 0.2 M Tris–HCl, 20 mM Na2EDTA, 86 mM NaCl, 0.1% (w/v) Triton X-100, 1% (w/v) Polyvinylpyrrolidone-10, pH = 7.5). Then, suspension was filtered via mesh screen with pore size of 30 and 50 μm and centrifuged at 2000 rpm for 5 min. Prepared samples were stained by 2 mL staining solution of Propidum Iodide (50 μg/mL) along RNase (1 μg/mL), in darkness for 30 min on ice. In final, stained samples were analyzed with a flow cytometer (BD FACSCanto™) and FloMax software. Leaf samples belong to 2 × plants were used as diploid reference. As a point, a total of 30 nuclei were studied in order to confirmation the ploidy level.
Differences between diploids and tetraploids
After identification of actual tetraploids through chromosome counting and flow cytometry techniques, the morphological characteristics including leaf thickness, spike and seed length, as well as seeds per spike were compared between tetraploid and diploid plants. To measure leaf thickness, a digital caliper was used, which own an acceptable accuracy. It is necessary to mention, the greenhouse conditions were considered similar for intact diploids and induced tetraploids.
In the physiological evaluation, each sample about 1.0 g of fresh leaves was homogenized in 10 mL of 80% acetone and kept in the dark for 8 h. The supernatant was made up to 25 mL by the addition of 80% acetone, and absorbance was determined at 470, 646 and 663 nm wavelengths for measuring the content of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid. These values were calculated through the equations of Lichtenthaler and Wellburn (1983).
In the anatomical evaluation, to avoid the outcomes related to the position of stomatal openness, all of leaves were accumulated between 12:00 and 1:00 p.m. Carnoy solution, as well as sterilized water were used to discoloring and washing the samples for 10 h and 15 min, respectively. Afterwards, the bottom epidermis was flake off and hereafter superposed on glass slide. It was employed a photomicroscope with 100 × objective lens to observe stomata, chloroplasts, and pollen grains. It is worth to mention, three vision fields were randomly sampled to determine chloroplast and stomata indicators.
Seed mucilage measuring
Mucilage of P. ovata seeds was ectracted by Sharma and Koul’s method (1986). In this method, three 25 g seed samples were used to measure the mucilage content in seeds.
Statistical analysis
The survival rate was measured via dividing the survived seedlings on treated seeds. The induction rate was also calculated through dividing the induced tetraploids on survived seedlings. Eventually, induction efficiency was estimated as survival rate × induction rate (Bouvier et al. 1994). ANOVA and Student’s t test at 5% probability level were conducted through SPSS V. 25 software. Duncan’s multiple-range test was also made to determine the significant differences among the means via the statistical program.
Results and discussion
In this study, we successfully induced tetraploidy in P. ovata by using colchicine and trifluralin and then revealed the differences between intact diploid and tetraploid plants (Fig. 1a–f; Tables 1–3).
Induction efficiency
Our results documented a more decline in survival rate by higher concentration and longer exposure time in all of treatments (Tables 1, 2). The similar findings were reported in Rosa (Khosravi et al. 2008); Tanacetum parthenium (Majdi et al. 2010); Lagerstroemia indica (Zhang et al. 2010); Paulownia tomentosa (Tang et al. 2010); Gerbera jamesonii (Gantait et al. 2011); Echinacea purpurea (Abdoli et al. 2013); Vitis vinifera (Acanda et al. 2015); Trachyspermum ammi (Sadat-Noori et al. 2017); Bletilla striata (Pan-pan et al. 2018). This toxicity of genome doubling agents could result from damaging to cells protoplast (Zeng et al. 2006).
In addition to survival rate, induction rate is another factor that determines induction efficiency (Sadat-Noori et al. 2017). Our results showed that induction rate was severely affected by exposure time, and also enough time for the effectiveness of trifluralin and colchicine on explants is an important criterion (Tables 1, 2). Given the survival and induction rate, the maximum of genome doubling efficiency was documented from 22.5% trifluralin for 72 h, and 0.3% colchicine for 24 h. Therefore, these two treatments are optimum for development of tetraploid plants in P. ovata.
Cytological differences
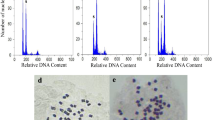
The findings of chromosome counting of root cells confirmed that chromosome number of diploid was 2n = 2× = 8, whereas that of the tetraploid was doubled (2n = 4× = 16) (Fig. 1d). The flow cytometry showed that diploids and tetraploids have single peak at ~ 75 and ~ 150 channels, respectively (Fig. 1c). Flow cytometry confirmed the tetraploid plants that already identified by chromosome counting. DNA content in tetraploids was recorded nearly twice than diploids, which suggested genome duplication was successfully achieved by using trifluralin and colchicine. The DNA ratio of tetraploids to diploids was not exactly two, probably due to the fact that tetraploids have the more content of DNA and thereby need the more staining time (Luo et al. 2018).
Morphological differences
A number of researches have reported that tetraploids have valuable farming characteristics such as the longer leaves, thicker stems and roots, as well as vigorous growth (Shao et al. 2003). Our results also demonstrated that leaf thickness, spike length, seed length and seeds per spike increased in tetraploids achieved from colchicine and trifluralin treatments (Table 3; Fig. 1a). These findings are in agreement with Abdoli et al. (2013) on E. purpurea, Tavan et al. (2015) on Thymus persicus, Pan-pan et al. (2018) on Bletilla striata, as well as Luo et al. (2018) on Taraxacum kok-saghyz.
Physiological differences
Our results uncovered the fact that amount of chlorophylls and carotenoid significantly increased in the leaves of tetraploids (Table 3). The effect of genome doubling on the chlorophyll extent has been documented as greener leaves in others plants such as Datura stramonium (Amiri et al. 2010), E. purpurea (Abdoli et al. 2013), H. reticulatus (Madani et al. 2015), Taraxacum koksaghyz (Luo et al. 2018), as well as Eclipta alba (Salma et al. 2018). However, there is a report that declared polyploidy induction can’t change the chlorophyll content in Cannabis sativa tetraploids (Bagheri and Mansouri 2015).
Anatomical differences
The results of anatomical evaluation revealed that chloroplast number in tetraploids was significantly more than that one in diploids (Table 3). Thus, it can be concluded the chloroplast number is associated with the level of ploidy, and can be used as a simple and effective parameter to distinguish the different ploidy levels. Similar findings have been reported on E. purpurea (Abdoli et al. 2013), and E. alba (Salma et al. 2018). In addition to chloroplast number in guard cells, it found that tetraploids stomata own more width and length than diploids (Table 3; Fig. 1e). However, the stomata density in diploids was more than that one in tetraploids. The similar documents have been also reported for Astragalus membranaceus (Chen and Gao 2007), L. indica (Zhang et al. 2010), T. parthenium (Majdi et al. 2010), Miscanthus (Głowacka et al. 2010), Centella asiatica (Kaensaksiri et al. 2011), E. purpurea (Abdoli et al. 2013), Crocosmia aurea (Hannweg et al. 2013), Mitracarpus hirtus (Pansuksan et al. 2014), Linum album (Javadian et al. 2017), as well as E. alba (Salma et al. 2018). The pollen grain size was also observed larger in tetraploids than diploids (Fig. 1f).
Seed mucilage content
Since mucilage is produced in the seeds (Gupta et al. 2018), it seems that larger seeds in tetraploids can be resulted in the more content of seed mucilage. To test this assumption, mucilage content of diploids and tetraploids seeds was measured.In the intact diploids, the mucilage yield and content were 0.6 g plant−1 and 14%, respectively. However, the mucilage yield and content in the induced tetraploids were 1.2 g plant−1 and 38%, respectively (Table 3).
Conclusion
Given to valuable pharmaceutical properties of P. ovata (Shahriari et al. 2018) and also the positive effect of polyploidy on its biomass and bioactive components, it needs to find the optimum conditions for producing tetraploids in P. ovata medicinal plant. Thus, we firstly in vitro induced P. ovata tetraploid plants via trifluralin and colchicine agents. Our findings indicated that 22.5% trifluralin for 72 h, as well as 0.3% colchicine for 24 h can be used as the optimum treatments for development of P. ovata tetraploids. We observed that tetraploids were larger than their intact diploids for height, leaf thickness, spike, seed, pollen grain, seeds per spike. Furthermore, chlorophyll (a, b, and total), carotenoid and chloroplast number in guard cells of tetraploids were more than diploids. Unlike density, stomata size in tetraploids was bigger than that one in diploids. In summary, for the first time we established an in vitro procedure for genome doubling in P. ovata medicinal plant. And also, we showed the procedure can increase seed mucilage content-as commercial product for food, cosmetic and pharmacy industries-in P. ovata tetraploids.
References
Abdoli M, Moieni A, Badi HN (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant 35:2075–2083
Acanda Y, Martinez O, Gonzalez MV, Prado MJ, Rey M (2015) Highly efficient in vitro tetraploid plant production via colchicine treatment using embryogenic suspension cultures in grapevine (Vitis vinifera cv. Menca). Plant Cell Tissue Organ Cult 123:547–555
Adaniya S, Shirai D (2001) In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. Sci Hortic 88:277–287
Amiri S, Kazemitabaar S, Ranjbar G, Azadbakht M (2010) The effect of trifluralin and colchicine treatments on morphological characteristics of jimsonweed (Datura Stramonium L.). Trakia J Sci 8:47–61
Bagheri M, Mansouri H (2015) Effect of induced polyploidy on some biochemical parameters in Cannabis sativa L. Appl Biochem Biotechnol 175:2366–2375
Bahmani M, Mirhosseini M, Fasihzadeh S, Karimian P, Rafieian-kopaei M (2016) Plantago: a plant for internists. Der Pharm Chem 8:84–91
Bannayan M, Nadjafi F, Azizi M, Tabrizi L, Rastgo M (2008) Yield and seed quality of Plantago ovata and Nigella sativa under different irrigation treatments. Ind Crops Prod 27:11–16
Berkov S, Philipov S (2002) Alkaloid production in diploid and autotetraploid plants of Datura stramonium. Pharma Biol. 40:617–621
Bouvier L, Fillon FR, Lespinasse Y (1994) Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitro. Plant Breed 113:343–346
Chen LL, Gao SL (2007) In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci Hortic 112:339–344
Denham TP, Iriarte J, Vrydaghs L (2016) Rethinking agriculture archaeological and ethnoarchaeological perspectives, 1st edn. Routledge, London, pp 44–57
Dhar MK, Kaul S, Friebe B, Gill BS (2002) Chromosome identification in Plantago ovata Forsk. through C-banding and FISH. Curr Sci 83(2):150–152
Dhar MK, Kaul S, Sareen S, Koul AK (2005) Plantago ovata: cultivation, genetic diversity, chemistry and utilization. Plant Genet Resour 3:252–263
Dhooghe E, Van-Laere K, Eeckhaut T, Leus L, Van-Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Doležel J, Bartoš J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110
Ebadi-Almas D, Karimzadeh G, Mirzaghaderi G (2012) Karyotypic variation and karyomorphology in Iranian endemic ecotypes of Plantago ovata Forsk. Cytologia 77(2):215–223
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011) Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tissue Organ Cult 106:485–493
Gao SL, Chen BJ, Zhu DN (2002) In vitro production and identification of autotetraploids of Scutellaria baicalensis. Plant Cell Tissue Organ Cult 70:289–293
Głowacka K, Jezowski S, Kaczmarek Z (2010) In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterization of induced polyploids in two Miscanthus species. Ind Crops Prod 32:88–96
Gu XF, Yang AF, Meng H, Zhang JR (2005) In vitro induction of tetraploid plants from diploid Zizyphus jujyba Mill. cv Zhanhua. Plant Cell Rep 24:671–676
Gupta M, Kaul S, Dhar MK (2018) Identification and characterization of some putative genes involved in arabinoxylan biosynthesis in Plantago ovate. 3 Biotech. https://doi.org/10.1007/s13205-018-1289-9
Hannweg K, Sippel A, Bertling I (2013) A simple and effective method for the micro-propagation and in vitro induction of polyploidy and the effect on floral characteristics of the South African iris, Crocosmia aurea. S Afr J Bot 88:367–372
Javadian N, Karimzadeh G, Sharifi M, Moieni A, Behmanesh M (2017) In vitro polyploidy induction: changes in morphology, podophyllotoxin biosynthesis, and expression of the related genes in Linum album (Linaceae). Planta 245:1165–1178
Jesus-Gonzalez L, Weathers PJ (2003) Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21:809–813
Kaensaksiri T, Soontornchainaksaeng P, Soonthornchareonnon N, Prathanturarug S (2011) In vitro induction of polyploidy in Centella asiatica (L.) urban. Plant Cell Tissue Organ Cult 107:187–194
Karimi D, Heidari B, Daneshnia SN, Dadkhodaie A (2013) Genetic variation and interrelationship of seed mucilage, swelling factor and agronomic traits in Plantago ovata wild accessions. Ann Biol Res 4:175–182
Khosravi P, Kermani MJ, Nematzadeh GA et al (2008) Role of mitotic inhibitors and genotype on chromosome doubling of Rosa. Euphytica 160:267–275
Koocheki A, Tabrizi L, NassiriMahallati M (2007) The effect of irrigation intervals and manure on quantitative and qualitative characteristics of Plantago ovata and Plantago psyllium. Asian J Plant Sci 6(8):1229–1234
Kumar A, Bharti B, Dubey RB (2018) Principles of crop improvement. Lap Lambert Academic Publishing, p 240
Lavania UC (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet Resour 3:170–177
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Luo Z, Iaffaldano BJ, Cornish K (2018) Colchicine-induced polyploidy has the potential to improve rubber yield in Taraxacum kok-saghyz. Ind Crops Prod 112:75–81
Madani H, Hosseini B, Dehghan E, Rezaei-chiyaneh E (2015) Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L. Acta Physiol Plant 37:55–60
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): morphological, physiological, cytological, and phytochemical changes. HortScience 45:16–21
Pan-pan H, Wei-Xu L, Hui-Hui L, Zeng-Xu X (2018) In vitro induction and identification of autotetraploid of Bletilla striata (Thunb.) Reichb.f. by colchicine treatment. Plant Cell Tissue Organ Cult 132:425–432
Pansuksan K, Sangthong R, Nakamura I, Mii M, Supaibulwatana K (2014) Tetraploid induction of Mitracarpus hirtus L. by colchicine and its characterization including antibacterial activity. Plant Cell Tissue Organ Cult 117:381–391
Predieri S (2001) Mutation induced and tissue culture in improving fruits. Plant Cell Tissue Organ Cult 64:185–210
Rehana K, Ovidiu T, Mihaela AM, Cameli S (2015) Industrial application of psyllium: an overview. Acta Univ Cibin Technol Ser 67:210–214
Roy AT, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20:489–495
Sabzehzari M, Naghavi MR (2018) Phyto-miRNA: a molecule with beneficial abilities for plant biotechnology. Gene 683:28–34
Sabzehzari M, Naghavi MR (2019) Phyto-miRNAs-based regulation of metabolites biosynthesis in medicinal plants. Gene 682:13–24
Sabzehzari M, Hoveidamanesh S, Modarresi M, Mohammadi V (2019) Morphological, anatomical, physiological, and cytological studies in diploid and tetraploid plants of Plantago Psyllium. Plant Cell Tissue Organ Cult 139:131–137
Sadat-Noori SA, Norouzi M, Karimzadeh G, Shirkool K, Niazian M (2017) Effect of colchicine induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult 130:543–551
Saeedi M, Morteza-Semnani K, Ansoroudi F, Fallahi S, Amin G (2010) Evaluation of binding properties of Plantago psyllium seed mucilage. Acta Pharm 60:339–348
Salma U, Kundu S, Mandal N (2017) Artificial polyploidy in medicinal plants: advancement in the last two decades and impending prospects. J Crop Sci Biotech 20:9–19
Salma U, Kundu S, Kumar-Hazra A, Ali N, Mandal N (2018) Augmentation of wedelolactone through in vitro tetraploid induction in Eclipta alba (L.) Hassk. Plant Cell Tissue Organ Cult 133:289–298
Shahriari Z, Heidari B, Dadkhodaie A, Richards CM (2018) Analysis of karyotype, chromosome characteristics, variation in mucilage content and grain yield traits in Plantago ovata and P. psyllium species. Ind Crops Prod 123:676–686
Shao J, Chen C, Deng X (2003) In vitro induction of tetraploid in pomegranate (Punica granatum). Plant Cell Tissue Organ Cult 75:241–246
Sharma PK, Koul A (1986) Mucilage in seeds of Plantago ovata and its wild allies. J Ethnopharmacol 17:289–295.
Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT (2010) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult 102:213–220
Tavan M, Mirjalili MH, Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult 122:573–583
Urwin NA, Horsnell J, Moon T (2007) Generation and characterization of colchicine-induced autotetraploid Lavandula angustifolia. Euphytica 156:257–266
Zeng HZ, Chen CW, Hong L, Liu JH, Deng XX (2006) In vitro induction, regeneration and analysis of autotetraploids derived from protoplasts and callus treated with colchicine in citrus. Plant Cell Tissue Organ Cult 87:85–93
Zhang Z, Dai H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159:59–65
Zhang QY, Luo FX, Liu L, Guo FC (2010) In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tissue Organ Cult 101:41–47
Acknowledgements
This study was carried out with the financial assistance of University of Tehran, as well as Persian Gulf University, Iran.
Author information
Authors and Affiliations
Contributions
All authors equally participated into this work.
Corresponding author
Ethics declarations
Conflict of interest
Authors have nothing to disclose with regard to commercial support.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sabzehzari, M., Hoveidamanesh, S., Modarresi, M. et al. Morphological, anatomical, physiological, and cytological studies in diploid and tetraploid plants of Ispaghul (Plantago ovata Forsk.). Genet Resour Crop Evol 67, 129–137 (2020). https://doi.org/10.1007/s10722-019-00846-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-019-00846-x