Abstract
Colla corii asini (CCA) made from donkey-hide has been widely used as a traditional animal-based Chinese medicine. Chondroitin sulfate (CS), dermatan sulfate (DS) and hyaluronic acid (HA) are structurally complex classes of glycosaminoglycans (GAGs) that have been implicated in a wide range of biological activities. However, their possible structural characteristics in CCA are not clear. In this study, GAG fractions containing CS/DS and HA were isolated from CCA and their disaccharide compositions were analyzed by high sensitivity liquid chromatography-ion trap/time-of-flight mass spectrometry (LC-MS-ITTOF). The result showed that CS/DS/HA disaccharides were detected in the three lower salt fractions from anion-exchange chromatography. The sulfation patterns and densities of CS/DS chains in these fractions differed greatly, while HA chains varied in their chain lengths. The quantitative analysis first revealed that the amount of GAGs in CCA varied significantly in total and in each fraction. This novel structural information could help clarify the possible involvement of these polysaccharides in the biological activities of CCA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The major GAGs in animals are heparin sulfate (HS)/heparin (HP), CS/ DS and HA which have important biological activities in embryonic development, morphogenesis, immune response, inflammation mediation, pathogenic infection, tumor progression and invasion, angiogenesis and tissue regeneration [1,2,3,4]. These activities of GAGs are related to their structural diversity and ability to interact with a wide variety of proteins, i.e. enzymes, cytokines, growth factors, and extracellular matrix proteins [5,6,7,8,9].
CS/DS are complex linear polysaccharides which are initially constructed of repeating disaccharide units of glucuronate (GlcA) linked to N-acetylgalactosamine (GalNAc). These disaccharides are then subjected to several post-polymeric modification (i.e. epimerization and sulfation) to yield a variety of disaccharide structures. Epimerization occurs at some GlcA and then transforms into iduronate (IdoA), while sulfation occurs at C2 of IdoA and C4 or C6 of GalNAc [10]. HA is the simplest GAG, comprised of repeating disaccharide units of GlcA linked to N-acetylglucosmine (GlcNAc) without any sulfo groups in its backbone [11].
CCA, also called E Jiao, is a crude preparation of donkey-hide gelatin that has been widely used as a traditional animal-based Chinese medicine for about 2000 years. CCA has been predominantly used for the treatment of gynaecological diseases [12] and some chronic diseases [13]. Also, CCA has demonstrated various useful activities in the inhibition of hematological diseases [14, 15], anticoagulation [16], antitumor action [17, 18], immunomodulation [19], anti-inflammation [20], bone repair [21], and anti-aging [22]. However, the relationships between these biological activities and the chemical constituents of CCA are still not clear.
Several classes of compounds have been found in CCA gelatin, including amino acids, proteins, GAGs, volatile substances, inorganic substances, etc. [13]. Research on the active components within CCA have been mainly focused on proteins, peptides and other lower molecular weight ingredients. GAGs are important components in donkey skin. Our recent paper first reported that CCA contains a heterogeneous mixture of HS/HP with a variety of like domain structures in their constituent chains [23]. Many medicinal activities have been attributed to CCA, including ones in which GAGs are known to be potentially involved. However, the presence and structure of other GAGs, such as DS/CS, HA in CCA still remains unclear. Therefore, our current study further explored the structure of CS/DS and HA in CCA by high sensitivity LC-MS-ITTOF.
Materials and methods
Materials
CCA was obtained from Shan Dong Dong-E E-Jiao Co., Ltd. (Shandong, China). Eight CS/DS disaccharide standards (generated by the action of chondroitinases) and HA disaccharide standard were obtained from Iduron (Manchester, UK). Pronase (Streptomyces griseus) was purchased from Sigma Aldrich (St. Louis, MO., USA). Chondroitinase ABC (Proteus vulgaris; EC 4.2.2.4) was purchased from ADHOC (Beijing, China). Superdex™ 75 10/300 GL and DEAE-Sephacel were from GE Healthcare Life Sciences (Uppsala, Sweden).
Methonal (HPLC grade) was provided by Merck KGaA (Darmstadt, Germany). 2-aminoacridone (AMAC, ≥98%), sodium cyanoborohydride (99%), dimethylsulfoxide (≥99.5%) and AR-grade reagents (ammonium hydrogen carbonate, sodium chloride (NaCl), acetic acid) were all purchased from Sigma Aldrich (St. Louis, MO., USA). Sodium hydroxide (≥98%) and AR-grade reagents (sodium acetate, sodium dihydrogen phosphate, disodium hydrogen phosphate) were all obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All HPLC solutions were prepared using MilliQ (Millipore, Watford, Herts., UK) ultra-pure water.
Extraction and partial purification of GAGs
GAGs in CCA was prepared by method described in our previous paper [23]. Briefly, CCA solution (1.25 g CCA in 10 mL of water) was digested by 0.5 mL of pronase (2.5 mg/mL) in 0.24 M sodium acetate and 1.9 M NaCl, pH 6.5 overnight at 37 °C. The GAG-containing fraction was precipitated by saturated sodium acetate/ethanol solution, then was collected by centrifugation and dried at 55 °C. The re-dissolved precipitate was applied to a 5 mL DEAE-Sephacel column pre-equilibrated with 0.1 M NaCl, 15 mM sodium acetate, pH 6.5 containing 0.01% v/v Triton X-100. After washing with the same solution, the CS/DS and HA containing GAG fractions were stepwise eluted with 60 mL of different concentrations of NaCl (0.25, 0.5, 1.0, 1.5 and 2.0 M NaCl) in 15 mM sodium acetate, pH 6.5 containing 0.01% (v/v) Triton X-100. The collected fractions were then dialyzed against Milli-Q water for 3 days at 4 °C followed by freeze-drying.
Preparation and recovery of GAG disaccharides
As CS/DS and HA chains can both be completely digested by chondroitinase ABC at pH 8.0 [24], GAG fractions from DEAE-Sephacel chromatography were exhaustively digested to CS/DS/HA disaccharides with 10 mIU of chondroitinase ABC in 50 μl of 20 mM, sodium phosphate buffer pH 8.0 containing 0.3% NaCl at 37 °C for 24 h as described by the instruction of enzyme. All digested samples were then separated by size-exclusion HPLC (SE-HPLC) on a Superdex™ 75 10/300 GL column eluted with 0.1 M ammonium hydrogen carbonate at a flow rate of 0.4 mL/min [23]. The disaccharide fractions were collected, incubated at 55 °C for 48 h to remove ammonium hydrogen carbonate, and then concentrated on a rotary vacuum concentrator.
AMAC labelling of CS/DS disaccharides
Dried CS/DS and HA disaccharide standards or purified GAG disaccharides from SE-HPLC (as above) were redissolved in 10 μL of 0.1 M AMAC in 85% dimethylsulfoxide / 15% acetic acid and incubated at room temperature for 20 min. At this point, 10 μL of 1 M sodium cyanoborohydride was added and the mixture was left for 4 h at 45 °C [25]. Finally, the reaction mixtures were diluted with 50% (v/v) aqueous dimethylsulfoxide for the following LC-MS analysis.
LC/MS-ITTOF analysis
The structural and compositional analysis of CS/DS and HA disaccharides were carried out on a LC/MS-ITTOF system (Shimadzu Corp., Kyoto, Japan) which was equipped with a binary gradient pump (LC-20 AD), autosampler (SIL-20 AC), degasser (DGU-20A3), photodiode array detector (SPD-M20A), communication base module (CBM-20A) and a column oven (CTO-20 AC).
Separations were performed on an ODS-2 HYPERSIL column (4.6 × 250 mm, 5 μm; Waters, Milford, MA, USA) at a flow rate of 0.3 ml/min at 45 °C. Eluent A was 40 mM ammonium acetate (pH 5.6) in water, whereas eluent B was methanol. A linear elution gradient was applied for isocratic 15% B in 5 min, then from 15% to 41% B in 45 min. The photodiode array detection was performed from 190 to 800 nm.
The mass spectrometer was equipped with an electrospray ionization source and was operated in the negative mode. Mass spectroscopic analyses were carried out on a full-scan mass spectrometer with a mass range of 200–1500 m/z. Liquid nitrogen was used as the nebulizing gas at a flow rate of 1.5 L/min. The curved desolvation line and heat block temperatures were both 200 °C. The interface voltages were set at −3.5 kV, and the detector voltage was 1.6 kV. The IT and TOF area vacuums were maintained at 1.8e-002 Pa and 1.6e-004 Pa, respectively.
Calculation
Quantitative analyses of CS/DS and HA disaccharides by fluorescence were performed using calibration curves as described by Volpi [26]. Briefly, increasing amounts of AMAC labelled CS/DS disaccharide standards (20, 30, 40, 70 and 120 ng) and HA disaccharide standard (240, 260, 280, 300 and 320 ng) were all analyzed by LC-MS, then the linearity of each disaccharide was assessed based on the amount of disaccharide and its peak intensity measured in the extract ion chromatogram (EIC).
Results and discussion
Partial purification of GAGs
Our most recent paper has developed an effective method for the isolation of the GAG fraction from the complex CCA mixture [23]. In order to investigate the structure and heterogeneity of the CS/DS and HA chains in CCA, the crude GAG fraction containing free GAG chains or/and GAG-peptide complexes was isolated by protease digestion and ethanol precipitation, then was further separated by stepwise salt-elution, using 0.2, 0.5, 1.0, 1.5 and 2.0 M NaCl steps, giving D1 to D5 fractions respectively. Large amounts of material were eluted in the low salt fractions (D1 and D2), while relatively small amounts of material were eluted in the 1.0 (D3), 1.5 (D4) and 2.0 M (D5) NaCl steps (See Fig. S1). These suggested that D1 and D2 might probably correspond to non-sulfated or low-sulfated GAGs, while D3 to D5 might be the relatively highly sulfated GAGs.
As CS/DS species can be sulfated to varying degrees, all the above five elution fractions (D1 to D5) were dialyzed and then freeze dried for subsequent enzymatic digestion, followed by disaccharide purification and analysis.
Purification of GAG disaccharides
SE-HPLC is a useful tool for purifying the GAG (likes HS/HP) disaccharides of CCA from other anionic contaminants for subsequent LC-MS-ITTOF structural analysis [23]. Therefore, the obtained D1 to D5 fractions were completely digested by chondroitinase ABC to disaccharides, which were then further purified by SE-HPLC on Superdex™ 75 column in 0.2 M ammonium hydrogen carbonate. As shown in Fig. 1D, the eight standard CS/DS disaccharides (all originally derived from chondroitinase-digested CS/DS and therefore contained the same characteristic uronate C4–5 unsaturation as our disaccharide samples) were eluted from the Superdex™ 75 column between 38 and 44 min. All the profiles for the five fractions (D1-D5, corresponding to Fig. 1A-1E, respectively) from DEAE-Sephacel chromatography contain a wide range of anionic contaminants, from low molecular weight ones to high molecular weight ones (mainly other GAGs, i.e. HS/HP [23], which are not degradable by chondroitinase ABC). The isolated disaccharide samples derived from D1 to D5 fractions were heated to remove ammonium hydrogen carbonate and then were AMAC-labelled by reductive amination for the following disaccharide analysis.
Purification of CS/DS and HA disaccharides by SE-HPLC on a Superdex™ 75 column. Five fractions, D1 (A), D2 (B), D3 (C), D4 (D) and D5 (E) from DEAE-Sephacel chromatography (Fig. S1) were digested with chondrotinase ABC and the released disaccharides were then purified by SE-HPLC. Elution was monitored by UV absorbance at 232 nm. The eight standard CS/DS disaccharides were eluted between 38 and 44 min on this column (F), so this fraction range (indicated by horizontal bars), was collected from each of the D1-D5 digested samples (profiles A-E, respectively)
Disaccharide analysis of GAG fractions
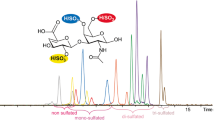
By combination with fluorescent labelling, LC-MS has recently become widely used to analyze CS/DS/HA disaccharides derived from biological samples [26, 27]. In this study, disaccharide compositional analysis of the AMAC-labelled disaccharide samples were performed by reverse-phase LC-MS using EIC detection (shown in Fig. 2A-2E). Because chondroitinase ABC has activity to both CS/DS and HA chains [24], the eight standard CS/DS and HA disaccharides were AMAC labeled and then quantitatively analyzed by LC-MS (Fig.2D&E; chemical structures shown in Table 1). The calibration curves for all disaccharide standards showed a linear response with high correlation coefficients (provided in Table 2). The preparation of GAG and its disaccharides were performed in duplicate. The resulting average disaccharide compositions are shown in Table 3.
Extracted ion chromatograms of AMAC labeled CS/DS and HA disaccharides analyzed by LC/MS-ITTOF. Profiles A-C correspond to the EIC profiles of disaccharides in the fractions D1-D3 (Fig. S1). Profile D is the EIC of the eight AMAC labeled standard CS/DS disaccharides, which are (TriS) ΔUA(2S)-GalNAc(4S,6S), (SB) ΔUA(2S)-GalNAc(4S), (SD) ΔUA(2S)-GalNAc(6S), (SE) ΔUA-GalNAc(4S,6S), (2S) ΔUA(2S)-GalNAc, (4S) ΔUA-GalNAc(4S), (6S) ΔUA-GalNAc(6S) and (0S) ΔUA-GalNAc. Profile E is the EIC of standard HA disaccharide
By comparison with the EIC profiles of CS/DS and HA standard disaccharides, we detected CS/DS and HA disaccharides in D1-D3 fractions (Fig.2A-2C) which were eluted from DEAE column at 0.25, 0.5 and 1.0 M NaCl, suggesting that CS/DS and HA were present in CCA. However, no CS/DS/HA disaccharides were found in D4 and D5 fractions which had been eluted from DEAE column at higher salt concentrations (i.e. 1.0 and 1.5 M NaCl), suggesting that the constituents in D4 and D5 fractions are mainly highly sulfated HS/HP [23].
CS/DS disaccharides were detected in the EIC profiles of D2 and D3 fractions. D2 fraction contained three CS/DS disaccharides, ΔUA-GalNAc(4S), ΔUA-GalNAc(6S) and ΔUA-GalNAc at the level of 94.3%, 4.1% and 1.6% (See Table 3). In D3 fraction, three disaccharides, ΔUA-GalNAc(4S), ΔUA-GalNAc(6S) and ΔUA-GalNAc(4S,6S) were detected at the level of 65.9%, 2.6% and 31.5%. Table 3 also shows comparisons of the overall levels of 2-O-, 4-O-, 6-O-sulfation and total sulfation, as calculated from the disaccharide compositions. The level of 4-O-sulfation of CS/DS chains in both fractions were significantly higher than 6-O-sulfation. 2-O-sulfation of CS/DS does not exist in D2 fraction, however it occurred in D3 fraction at level of 31.5%. Consequently, the overall sulfation density of CS/DS chains in D2 fraction is 33.1% lower than the ones in D3 fraction. These results showed that the sulfation pattern and density of CS/DS chains in both fractions are clearly significantly different, suggesting that CS/DS in CCA is structurally heterogeneous.
In contrast, HA disaccharide was found in D1 to D3 fractions, indicating that the length of HA in three fractions may be different, probably having higher molecular weight of HA in D3 fraction, which could be due to the natural polydispersity of HA or/and the degradation of HA during the production of CCA. CCA was prepared from Equues asinus L. donkey-hide by decoction under pressure and concentrating [28]. These production procedures might cause the degradation of GAGs, such as CS/DS [29] and HA.
Calculation by quantitative disaccharide analysis (Table 4) showed that the amounts of HA from 1 g of CCA were 1508, 1745 and 405 ng in D1 to D3 fractions respectively. In contrast, the amounts of CS/DS disaccharides were 55 and 283 ng in D2 and D3 fractions. The total amount of CS/DS (338 ng) was 10 times lower than that of HA (3658 ng), and 15 times lower than HS/HP (5150 ng) [23].
Our current study demonstrated the distribution and the possible structure of HA and CS/DS in CCA. CCA has shown clinical functions of anti-aging and bone repair, but their possible mechanisms are still unknown. The presence and polydispersity of HA might be one of the important factors contributing to these activities, as HA has a role in tissue regeneration and cosmetic, nutricosmetic effects [7, 30, 31]. Also, CS/DS has been related to blood coagulant and tumor invasion and metastasis [6, 32, 33], therefore the structural characterization of CS/DS from CCA may provide possibilities for further elucidating the medicinal properties of CCA.
In conclusion, this paper shows that CCA contains a heterogeneous mixture of CS/DS and HA, in which CS/DS possesses a heterogeneous structure with different sulfation patterns and densities in its constituent chain, while HA structure might vary in its chain length. The presence of these complex GAGs raises the question as to whether they may contribute in any way to the biological/medicinal properties attributed to CCA.
Heading
Structural analysis of GAGs from CCA
Abbreviations
- AMAC:
-
2-aminoacridone
- CCA :
-
Colla corii asini
- CS:
-
chondroitin sulfate
- DS:
-
dermatan sulfate
- EIC:
-
extracted ion chromatogram
- GAG:
-
glycosaminoglycan
- GalNAc:
-
N-acetylgalatosamine
- GlcA:
-
glucuronate
- GlcNAc:
-
N-acetylglucosmine
- HA:
-
hyaluronic acid
- HP:
-
heparin
- HS:
-
heparan sulfate
- IdoA:
-
iduronate
- LC-MS-ITTOF:
-
liquid chromatography-ion trap/time-of-flight mass spectrometry
- NaCl:
-
sodium chloride
- ΔUA:
-
4,5-unsaturated uronate (generated by chondroitinase excision)
- SE-HPLC:
-
size-exclusion HPLC
References
Iozzo, R.V.: Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 6(8), 646–656 (2005). https://doi.org/10.1038/nrm1702
Bulow, H.E., Hobert, O.: The molecular diversity of glycosaminoglycans shapes animal de-velopment. Annu. Rev. Cell Dev. Biol. 22, 375–407 (2006). https://doi.org/10.1146/annurev.cellbio.22.010605.093433
Couchman, J.R.: Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 26, 89–114 (2010). https://doi.org/10.1146/annurev-cellbio-100109-104126
Iozzo, R.V., Sanderson, R.D.: Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 15(5), 1013–1031 (2011). https://doi.org/10.1111/j.1582-4934.2010.01236.x
Yamada, S., Sugahara, K.: Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr Drug Discov Technol. 5(4), 289–301 (2008). https://doi.org/10.2174/157016308786733564
Mizumoto, S., Yamada, S., Sugahara, K.: Molecular interactions between chondroitin-der- matan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 34, 35–42 (2015). https://doi.org/10.1016/j.sbi.2015.06.004
Bukhari, S.N.A., Roswandi, N.L., Waqas, M., Habib, H., Hussain, F., Khan, S., Sohail, M., Ramli, N.A., Thu, H.E., Hussain, Z.: Hyaluronic acid, a promising skin rejuvenating bio- medicine: a review of recent updates and pre-clinical and clinical investigations on cos- meticand nutricosmetic effects. Int. J. Biol. Macromol. 120, 1682–1695 (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.188
Liu, D.F., Shriver, Z., Venkataraman, G., El Shabrawi, Y., Sasisekharan, R.: Tumor cell surface heparan sulfate as cryptic promoters or inhibitors of tumor growth and metastasis. P Natl Acad Sci USA. 99(2), 568–573 (2002). https://doi.org/10.1073/pnas.012578299
Perrimon, N., Bernfield, M.: Specificities of heparan sulphate proteoglycans in develop- mental processes. Nature. 404(6779), 725–728 (2000). https://doi.org/10.1038/35008000
Bulow, H.E., Hobert, O.: The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Bi. 22, 375–407 (2006). https://doi.org/10.1146/annurevcellbio.22.010605.093433
Whistler, R.L., Olson, E.J.: The Biosynthesis of Hyaluronic Acid. 12(4), 299–319 (1957). https://doi.org/10.1016/S0096-5332(08)60211-8
Su, N.J., Li, B., Wang, F., Quan, S., Yang, C.L., Shan, D.H., Xing, F.Q.: Clinical effective-ness of colla corii asini on improving uterine receptivity in controlled ovarian stimulation. J. Trop. Med. 9, 155–157 (2009). https://doi.org/10.16437/j.cnki.1007-5038.2011.10.007
Wang, D.L., Ru, W.W., Xu, Y.P., Zhang, J.L., He, X.X., Fan, G.H., Mao, B.B., Zhou, X.S., Qin, Y.F.: Chemical constituents and bioactivities of colla corii asini. Drug. Discov. Ther. 8, 201–207 (2014). https://doi.org/10.5582/ddt.2014.01038
Song, Y.M., Mao, G.N., Huang, X.S., Du, Y.M.: Study on hemopoiesis and anti-fatigue eff-ects of colla corri asini effervescent granules in mice. Prog. Vet. Med. 32, 83–86 (2011). https://doi.org/10.16437/j.cnki.1007-5038.2011.10.007
Peng, L., Yao, S.Y., Wei-Zhong, F.: Evaluation on angelica and donkey-hide gelatin oral liquid in improving iron deficiency anemia in rats. Pract. Prev. Med. 19, 265–267 (2012)
Fan, H.Z., Liu, Y.X., Xie, K.Q., Zhang, A.: Characterization and quantification of derma- tan sulfate from donkey skin. China J. Chin. Mater. Med. 19(8), 477–480 (1994)
Liu, P.M., You, J.H., Tian, S.S., Xie, X.J., Xie, F.S.: Donkey hide gelatin drug-containing serum induced lung cancer PG cells apoptosis in animal experiments. Pract. J. Med. Pharm. 22, 426–427 (2005). https://doi.org/10.14172/j.cnki.issn1671-4008.2005.05.031
Liu, P.M., Cai, B.C., Xie, X.J., Tian, S.S., You, J.H., Xie, F.S.: Effect of donkey hide gelatin drug-containing serum on expressing of gene P53 in leukemia K562 cells. Pharm. Clin.Chin. Mat. Med. 21, 33–35 (2005). https://doi.org/10.13412/j.cnki.zyyl.2005.06.016
Song, Y.M., Mao, G.N., Kang, R.R., Mao, R.J.: Effect of colla corri asini effervescent granules on immune function in mice. Progr. Vet. Med. 32, 73–75 (2011). https://doi.org/10.16437/j.cnki.1007-5038.2011.09.011
Chen, B.F.: Clinical observation on triple therapy of Ejiao and Weifuchun for chronic atr-ophic gastritis combined with peptic ulcer. J. Clin. Exp. Med. 10, 1622–1623 (2011)
Chang, D.Y., Yang, J., Dong, F.H.: The effect of E-jiao on the multiplicative and differen-tiative function of osteoblast of wistar rats cultured in vitro. Chin. J. Gerontol. 29, 3230–3232 (2009)
Gao, Y., Dong, F.H., Zheng, J.: Influence of E Jiao on related genes expression during bone repair. Chin. J. Orthop. Trauma. 17, 520–523 (2004)
Du, J.Y., Liu, S., Liang, Q.T., Lin, J.H., Jiang, L.L., Chen, F.E., Wei, Z.: Analysis of Heparan sulfate/heparin from Colla corii asini by liquid chromatography-electrospray ion trap mass spectrometry. Glycoconj. J. 36(3), 211–218 (2019). https://doi.org/10.1007/s10719-019-09868-0
Grondahl, F., Tveit, H., Akslen-Hoel, L.K., Prydz, K.: Easy HPLC-based separation and quantitation of chondroitin sulphate and hyaluronan disaccharides after chondroitinase ABC treatment. Carbohydr. Res. 346(1), 50–57 (2011). https://doi.org/10.1016/j.carres.2010.10.025
Yang, B., Chang, Y., Weyers, A.M., Sterner, E., Linhardt, R.J.: Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography–mass spectro-metry. J. Chromatogr. A. 1225, 91–98 (2012). https://doi.org/10.1016/j.chroma.2011.12.063
Volpi, N.: High-performance liquid chromatography and on-line mass spectrometry detecti- on for the analysis of chondroitin sulfates/hyaluronan disaccharides derivatized with 2-ami-noacridone. Anal. Biochem. 397(1), 12–23 (2010). https://doi.org/10.1016/j.ab.2009.09.030
Volpi, N., Galeotti, F., Yang, B., Linhardt, R.J.: Analysis of glycosaminoglycan-derived, precolumn, 2-aminoacridone-labeled disaccharides with LC-fluorescence and LC-MS detect- ion. Nature Protocol. 9(3), 541–558 (2014). https://doi.org/10.1038/nprot.2014.026
China Pharmacopoeia Committee: Drug Standard of Department of Health in the People’s Republic of China, vol. 1–20, p. 450. People’s Health Press, Beijing (1995)
Fan, H.Z., Xu, T.Z.: Degradation of donkey skin components in sol process. China J. Chin. Mater. Med. 19(9), 543–545 (1994)
Neuman, M.G., Nanau, R.M., Oruna-Sanchez, L., Coto, G.: Hyaluronic acid and wound h-ealing. J Pharm Pharm Sci. 18(1), 53–60 (2015). https://doi.org/10.18433/j3k89d
Gupta, R.C., Lall, R., Srivastava, A., Sinha, A.: Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front Vet Sci. 6, (2019). https://doi.org/10.3389/fvets.2019.00192
Trowbridge, J.M., Gallo, R.L.: Dermatan sulfate: new functions from an old glycosamino- glycan. Glycobiology. 12(9), 117R–125R (2002). https://doi.org/10.1093/glycob/cwf066
Thelin, M.A., Bartolini, B., Axelsson, J., Gustafsson, R., Tykesson, E., Pera, E., Oldberg, A., Maccarana, M., Malmstrom, A.: Biological functions of iduronic acid in chondroitin/ dermatan sulfate. FEBS J. 280(10), 2431–2446 (2013). https://doi.org/10.1111/febs.12214
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC 21343013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 99 kb)
Rights and permissions
About this article
Cite this article
Huang, H., Liu, S., Du, J. et al. Structural analysis of glycosaminoglycans from Colla corii asini by liquid chromatography-electrospray ion trap mass spectrometry. Glycoconj J 37, 201–207 (2020). https://doi.org/10.1007/s10719-019-09904-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-019-09904-z