Abstract
Monoclonal antibody YHD-06 generated by immunization with GM2 reacted with gangliosides with GM2-determinant, i.e., GM2, GalNAc-GM1b and GalNAc-GD1a, among which GalNAc-GD1a was characterized as an antigen of autoimmune peripheral neuropathies including Guillain-Barré syndrome. When glycolipids were examined by TLC-immunostaining with YHD-06 in seven human cervical carcinoma-derived cell lines, GM2 was found in all cell lines, amounting to 15.5 % to 57.5 % of total gangliosides. Whereas GalNAc-GD1a was present in three cell lines, amounting to 5.4–17.5 % of total gangliosides, and GalNAc-GM1b in four cell lines in amounts of less than 2 %. The elevated amounts of gangliosides with GM2 determinant were closely correlated with the relative intensities of gene expression of GalNAc transferase, this being characteristic of cervical carcinoma-derived cells. However, in tissues from patients with several histological types of cervical carcinomas, GM3 was ubiquitously expressed in amounts of more than 66 % of total gangliosides, GM2 was expressed in only five of 15 tissues, and both GalNAc-GM1b and GalNAc-GD1a were not even detected in trace amounts. Since GM1 was detected in all tissues in amounts of less than 0.06 μg/mg dried tissue, all cervical carcinoma tissues were revealed to exhibit GM2 synthesis, indicating that enhanced synthesis of gangliosides with GM2 determinant is a characteristic of cultivated cells in vitro. Similarly, although I3SO3-GalCer was not present in the squamous cell carcinoma (SCC) tissues, SCC-derived cells selectively expressed II3SO3-LacCer. Since enhanced synthesis of GM2 has been reported in SV-40 virus-transfected fibroblasts, papilloma virus might be involved in the expression of GM2 in cervical carcinoma-derived cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
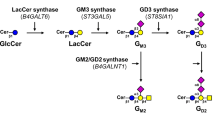
The carbohydrate moieties of glycosphingolipids are constructed by sequential addition of monosaccharides to the nonreducing terminals of precursor glycolipids with glycosyltransferases, and are classified into 13 core structures, whose tissue and cellular expression is strictly regulated in species-, tissue-, differentiation- and transformation-characteristic manners, and which are also involved in several cellular functions, including cell-to-cell recognition, adhesion and signal transduction [3, 4]. Structural modification of carbohydrate chains in baseline metabolic pathways for glycolipids in normal tissues frequently occurs during transformation to give cancer-related antigens, e.g. sialyl Lea antigen for diagnosis of pancreatic, gallbladder and colon carcinomas [5, 6], and Leb, LeY and H antigens for prediction of metastatic potential [7–9]. We have also observed that LeX and Leb are characteristically expressed in ovarian serous carcinoma KF28 cells exhibiting anti-cancer drug resistance, and enhanced expression of LeY in ovarian clear cell carcinoma RMG-1 cells on transfection of α1,2-fucosyltransferase results in high dissemination potential and anti-cancer drug resistance [10, 11]. On comparison of glycolipid compositions between KF28 cells and KF28-derived anticancer drug-resistant cells, and between RMG-1 cells and fucosyltransferase gene-transfected RMG-1 cells, globo-, lacto- and neolacto-series glycolipid profiles were found to be characteristically changed to cause alteration of the antigenicity of glycolipids, and their cellular functions including in anticancer drug-resistance and peritoneal dissemination [11, 12]. In addition, on comparison of glycolipids in tissues and cells of histologically defined ovarian carcinomas, i.e., serous, mucinous, endometrioid and clear cells, the amounts of sulfatides in the mucinous type were found to be strikingly higher than those in the other types, but the expression of globo- and ganglio-series glycolipids including Lewis antigens was not related with the above histological classifications [13–15]. Similar glycolipid profiles to those of ovarian carcinomas including Lewis antigens have been obtained for poor-, moderate- and well-differentiated types of endometrial carcinomas, but sulfatides have been found characteristically in well-differentiated endometrial carcinoma tissues, although their amounts were less than one-third of those in mucinous type ovarian carcinomas [15–17]. To further explore transformation-related changes in glycolipids of gynecological carcinomas, we subsequently determined them in tissues and cells of cervical carcinomas, whose onset was due to infection with papilloma virus, and found that all cells established from different types, i.e., squamous cell carcinomas, adenocarcinomas, adenosquamous carcinomas and small cell carcinomas, commonly expressed unique glycolipids with GM2-carbohydrate determinant belonging to ganglio-series, i.e., GM2, GalNAc-GM1b and GalNAc-GD1a. In particular, GalNAc-GD1a with GM2-determinant, which is a minor constituent in the human brain and is an antigen of autoimmune peripheral neuropathies [18, 19], was detected in relatively high amounts in three out of seven cell lines. Since elevated amounts of GM2 have been previously reported as an event after SV-40 virus-dependent transformation of human fibroblasts and neural cells, the expression of unique gangliosides with GM2 determinant in cervical carcinoma-derived cell lines is probably a papilloma virus-related phenomenon, which is not observed in endometrial and ovarian carcinomas-derived cell lines [20].
Materials and methods
Materials
Glycolipids were purified from various sources in our laboratory: GlcCer, LacCer, Gb3Cer, Gb4Cer, GM3 and IV3NeuAcα-nLc4Cer (sialyl paragloboside, SPG) from human erythrocytes, GalCer, I3SO3-GalCer (sulfatide), GM2, GM1, GD3, GD2, GD1a and GalNAc-GD1a from bovine brain, Forssman (Fs) glycolipid from equine kidney, fucosyl GM1 (FGM1) from bovine thyroid, GM1b and GalNAc-GM1b from murine spleen, GD1α from rat ascites hepatoma, and II3SO3-LacCer from rat kidney. GA1 (asialoGM1) and fucosylGA1 were prepared by hydrolysis of GM1 and FGM1 with neuraminidase (A. ureafacience) [21]. Cholera toxin B-subunit was purchased from Sigma (S. Louis, MO, USA), and an antibody against cholera toxin B-subunit was generated by immunization of a rabbit together with Freund’s complete adjuvant (Sigma). Murine monoclonal anti-sulfatide (TCS-1) and anti-GM2 (Y916) antibodies were also generated by a conventional procedure in our laboratory [22]. Murine monoclonal anti-Lea plus Leb (MSN-1), anti-sialyl Lea (CA19–9), and anti-GM3 (M2590) antibodies were kindly donated by Prof. Nozawa S., Keio University, Tokyo, Dr. Matsumoto K., Mikuri Immun. Research Inc. (Kyoto, Japan), and Seikagaku Co. (Tokyo, Japan), respectively.
Human cervical carcinoma-derived cell lines
The human cervical carcinoma-derived cell lines listed in Table 1 were purchased from the RIKEN Bio Resource Center (Wako, Saitama, Japan), and cells were cultured in RPM1 1640 medium (Nissui, Tokyo) supplemented with 10 % fetal calf serum (FCS). The type of papilloma virus in cell lines was determined by the National Institute of Infectious Diseases [23].
Cervical carcinoma tissues
Human cervical carcinoma tissues were obtained from university hospitals, Tokai and Keio Universities. Written informed consent to use carcinoma tissues for this study was obtained from all subjects, and the experimental protocol was approved by the Ethics Committees of both hospitals. Histological classification was performed according to the criteria of the International Federation of Gynecology and Obstetrics [24].
Analysis of glycolipids
Lipids were extracted from lyophilized tissues and cells with chloroform/methanol/water (20:10:1, 10:20:1 and 1:1:0, by vol.), and the combined extracts were used as the total lipid extracts. A part of each extract was applied to a DEAE-Sephadex column (A-25, acetate form; GE Healthcare Bioscience, Piscataway, NJ, USA) to isolate the neutral and acidic lipids. The total lipids, and neutral and acidic glycolipids thus obtained were applied to plastic-coated (Macherey-Nagel, Düren, Germany) and glass-coated (Merck, Darmstadt, Germany) TLC plates, which were then developed with chloroform/methanol/water (65:35:8, by vol.) and chloroform/methanol/0.5 % CaCl2 in water (55:45:10, by vol.) for glycosphingolipids, and chloroform/methanol/water (65:25:4, by vol.) for bacterial glycolipids, and the spots were visualized with orcinol-sulfuric acid reagent. Then the densities of orcinol-positive spots on TLC plates were determined by image analysis (NIH image). Standard lipids (0.1 to 1.5 μg), i.e., N-stearoyl derivatives of GlcCer, LacCer, Gb3Cer, Gb4Cer, Fs and GA1, FGM1 and FGA1, were developed on the same TLC plates for the preparation of standard curves [10–14]. For structural analysis, individual gangliosides were purified from the acidic lipids using a silica gel (Iatrobeads 6RS8060: Iatron Laboratory, Tokyo) column by gradient elution with chloroform/methanol/water (55:45: 2 and 10:90:4, by vol.). The purified glycolipids were analyzed by negative ion FAB-MS (JMS-700TKM; JEOL, Tokyo) as described previously [22].
TLC-immunostaining
For TLC-immunostaining with anti-GM3, anti-GM2, anti-Lea plus Leb and anti-sialyl Lea antibodies, glycolipids were developed on plastic-coated TLC plates (Macherey-Nagel) with chloroform/methanol/0.5 % CaCl2 in water (55:45:10, by vol.), which were then blocked with blocking buffer (PBS containing 1 % polyvinylpyrrolidone and 1 % ovalbumin), and the spots were visualized by immunostaining with the above anti-glycolipid antibodies diluted 1:500 to 1:2000 with dilution buffer (PBS containing 3 % polyvinylpyrrolidone), followed by immunostaining with peroxidase-conjugated anti-murine IgG, A and M antibodies (1:1000; Jackson Immunoresearch Lab., PA, USA), and peroxidase substrates, 4-chloro-1-naphthol and H2O2, according to the procedure reported previously [22]. Standard glycolipids (10 to 500 ng), GM3, GM2, GalNAc-GM1b and GalNAc-GD1a, were developed on the same TLC plates for the preparation of standard curves. For detection of GM1, acidic lipids and standard GM1, 0.2–10 ng glycolipids were developed on plastic-coated TLC plates and after blocking of the plate, reaction with cholera toxin B-subunit (1 ng/10 mL) followed by anti-cholera toxin B-subunit antibodies (1:500) were performed, the following procedure being the same as that described above. The densities of spots on TLC plates were determined by image analysis (NIH image).
RT-PCR analysis
Total RNA was extracted from the cell lines with Isogen (Nippon Gene, Tokyo), reverse-transcribed to cDNA with reverse transcriptase (M-MuLV; Takara, Kyoto) and an oligo(dT) primer, and then subjected to PCR under the following conditions: SAT I (St 3 gal5, NM003896), sense primer, ttgagcacaggtatagcgtg, antisense primer, gggattttttctgccacctg; GalNAcT (B4galnt1, NM008080), sense primer, ggtcaggatcaaggagcaag, antisense primer, gatgctcctgaggggctgaa; GalT II (B3galt4, NM003782), sense primer, gactcctaccgcaacctcac, antisense primer, tcctgctcagcctctctctg; SAT IV (St 3 gal1, NM003033), sense primer, gaccctatgctggagaagag, antisense primer, aaatggtgcccgtggtgatg; SAT II (St8sia1, NM003034), sense primer, ttccagctgccattgaag, antisense primer, aagggccagaagccatag; 35 cycles of 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 40 s. The primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as controls. The resulting PCR products were electrophoresed on a 1.5 % agarose gel, stained with Gel Red Nucleic Stain (Biotium), and then examined under a UV transilluminator.
Results
Monoclonal anti-GM2 antibody
Monoclonal antibody YHD-06 established by immunization of mice with GM2 reacted with all gangliosides containing GM2-determinant, i.e., GM2, GalNAc-GM1b and GalNAc-GD1a, but not with the structurally related gangliosides, in particular with GD2, at all, indicating that GalNAcβ1-4Gal(3-2αNeuAc)β- of GM2-determinant is an epitope for antibody YHD-06 (Fig. 1). As determined by densitometry of the spots visualized on TLC densitometry after immunostaining with YHD-06 of the same amounts of glycolipids, the relative intensities of antibody binding as to GalNAc-GD1a and GalNAc-GM1b in comparison with that as to GM2 were 1.20 and 0.69, respectively (Fig. 1).
TLC and TLC-immunostaining of gangliosides. Several gangliosides (0.5 μg) were developed on TLC plates with chloroform/methanol/0.5 % CaCl2 in water (55:45:10, by vol.), and the spots were visualized with anti-GM2 antibody YHD-06 (a), and with orcinol-sulfuric acid reagent (b). GM1b, IV3NeuAcα-Gg4Cer; GalNAc-GM1b, IV3NeuAcα-, IV4GalNAcβ-Gg4Cer; GD1α, IV3NeuAcα-, III6NeuAcα-Gg4Cer; GalNAc-GD1a, II3NeuAcα-, IV3NeuAcα-, IV4GalNAcβ-Gg4Cer
Glycolipids in cervical carcinoma cell lines
Seven cell lines established from different histological types of cervical carcinomas, i.e. squamous cell carcinomas, adenocarcinomas, adenosquamous carcinomas and small cell carcinomas, were used in this experiment, and papilloma virus types 16 and 18 were detected in SKG-IIIb and Boku, and Hela and HCSC-1 cells, respectively (Table 1). When neutral glycolipids derived from 0.5 mg of dried cells were examined, those in SKG-IIIb cells were in less than chemically detectable amounts (0.05 μg), the other cells containing globo-series glycolipids, i.e., Gb3Cer and Gb4Cer, amounting to 25.2–78.6 % of the total neutral glycolipids (Fig. 2, Table 2). Also, the neutral glycolipid compositions were not correlated with the histologically defined origins of cells (Fig. 2). Whereas acidic glycolipids from all cell lines were found to be composed of gangliosides with long carbohydrate chains (Fig. 3). On TLC-immunostaining with anti-GM2 antibody YHD-06, all cell lines examined were revealed to contain GM2 amounting to 15.5–57.5 % of the total gangliosides, and squamous cell carcinoma-derived Boku cells, and adenocarcinoma-derived OMC-4 and HCA-1 cells contained both GalNAc-GM1b and GalNAc-GD1a, and small cell carcinoma-derived HCSC-1 cells contained only GalNAc-GM1b (Fig. 3c). After purification of GalNAc-GM1b from HCSC-1 cells and GalNAc-GD1a from Boku cells, their structures were further confirmed by negative ion FABMS, i.e., m/z 1775 and 1859 of molecular ion [M-H]− for GalNAc-GM1b with 18:0- and 24:0-containing ones, and m/z 2066 and 2150 of molecular ion [M-H]− for GalNAc-GD1a with 18:0- and 24:0-containing ones, as reported in the literature [25]. GalNAc-GD1a in Boku, OMC-4 and HCA-1 cells comprised 17.5 %, 5.4 % and 12.3 % of the total gangliosides, respectively, and their expression was not correlated with the histological types of cells (Table 2). GM1 and GD1a, a-series gangliosides, were abundant in SKG-IIIb, BOKU, OMC-4 and HCA-1 cells (Fig. 3a, b, and Fig. 4c), in which the GalNAc-transferase gene was intensely expressed, but were relatively low in HKUSP and Hela cells, as shown in Fig. 5, indicating that GalNAc- transferase is a key enzyme for the synthesis of gangliosides with long carbohydrate chains and with GM2-determinant. Although the galactosyl (GalT-II) and sialyl (SAT-IV) transferase genes were expressed intensely in all cell ines, the GD3 synthase (SAT-II) gene was only faintly expressed in Boku cells, in which GD3 and b-series gangliosides were in trace amounts. Whereas sulfoglycolipid, II3SO3-LacCer was characteristically detected in squamous cell carcinoma-derived SKG-IIIb, Boku and HKUSP cells, whose sulfotransferase (CST) gene expression was positively correlated with its amount (Figs. 3 and 5). However, although the sulfotransferase gene was expressed in OMC-4 and HCA-1 cells, sulfoglycolipids were not detected in these cells. As shown in Fig. 3 and Table 2, although there was only a trace amount of II3SO3-LacCer in SKG-IIIb cells, that in Boku and HKUSP cells comprised 4.0 % and 41.2 % of the total acidic glycolipids. As to Lewis glycolipids related with clinical diagnosis, i.e., Leb and sialyl Lea, they were only expressed in two out of seven cell lines, as shown in Fig. 4a and b. Thus, enhanced expression of gangliosides with GM2 determinant, particularly with unique structures, GalNAc-GM1b and GalNAc-GD1a, was a characteristic of cervical carcinoma-derived cell lines, which was not observed for endometrial and ovarian carcinoma-derived cell lines [11, 15].
TLC of neutral glycolipids from cervical carcinoma-derived cell lines. Neutral glycolipids, corresponding to 0.5 mg dried cells, were developed on TLC plates with chloroform/methanol/water (65:35:8, by vol.), and the spots were visualized with orcinol-sulfuric acid reagent. St, standard glycolipids, GalCer and Gb3Cer; Gb4, Gb4Cer; Gg4, Gg4Cer
TLC and TLC-immunostaining of acidic glycolipids from cervical carcinoma-derived cell lines. Acidic glycolipids, corresponding to 0.5 mg dried cells, were developed on TLC plates with chloroform/methanol/0.5 % CaCl2 in water (55:45:10, by vol.), and were visualized with orcinol-sulfuric acid reagent (a and b), and with anti-GM2 antibody YHD-06 (c). Sul, I3SO3-GalCer; SPG, IV3NeuAcα-nLc4Cer
TLC-immunostaining of neutral (a) and acidic (b and c) glycolipids from cervical carcinoma-derived cell lines. TLC was carried out as described in Fig. 3, and the spots were visualized with anti-Lea plus Leb antibodies (MSN-1) (a), anti-sialyl Lea antibodies (CA19–9) (b), and cholera toxin B-subunit (c). St, standard glycolipids; Leb glycolipid for a, sialyl Lea for b and GM1 for c
Glycolipids in cervical carcinoma tissues
To further characterize glycolipid expression in cervical carcinomas, tissues diagnosed as squamous cell carcinomas (SCC, 4 cases), adenocarcinomas (adeno, 6 cases), adenosquamous carcinomas (adeno SCC, 2 cases), and small cell carcinomas (small, 3 cases) were analyzed by TLC and TLC-immunostaining. The major neutral glycolipids were globo-series ones, i.e., GlcCer, LacCer, Gb3Cer and Gb4Cer, whose relative proportions were similar to those in ovarian and endometrial carcinoma tissues and were not correlated with the histological types (data not shown) [11, 13, 15]. Also, Leb in neutral glycolipids was detected in all tissues without any correlation with the histological types, but the rate of Leb expression in the tissues was exceedingly high in comparison to that in cell lines (Figs. 4a and 6f). As to acidic glycolipids, GM3 was ubiquitously expressed as the major acidic one in amounts of more than 66 % of the total gangliosides (Fig. 6b), and IV3NeuAcα-nLc4Cer, which migrated on a TLC plate to a position just below that of GM2, was also present in 10 out of 15 cancerous tissues (Figs. 6b and 7). In addition, GM2 was only detected in five out of 15 tissues and its concentration was less than 6 % of the total gangliosides (Table 3 and Fig. 6c). GalNAc-GM1b and GalNAc-GD1a were not detected even on application of acidic glycolipids corresponding to 5 mg dried tissue. However, GM1 was detected in all tissues, although its amounts were less than 0.06 μg/mg dried tissue, indicating that all cervical carcinoma tissues exhibit synthetic potential for a-series gangliosides including GM2 (Fig. 6d). While I3SO3-GalCer was present in all adenocarcinoma and small cell carcinoma tissues, but not in squamous cell carcinoma ones at all (Table 3, Figs. 6a and 7). Since sulfoglycolipid II3SO3-LacCer was selectively distributed in squamous cell carcinoma-derived cells, sulfotransferase, which was not originally expressed in tissues, was thought to be activated to sulfate LacCer in cells after cultivation.
TLC-immunostaining of acidic (a–e) and neutral (f) glycolipids from cervical carcinoma tissues. TLC was carried out as described in Fig. 3, and the spots were visualized with anti-sulfatide (TCS-1) (a), anti-GM3 (M2590) (b), anti-GM2 (YHD-06) (c), cholera toxin B-subunit (d), anti-sialyl Lea antibodies (CA19–9) (e), and anti-Lea plus Leb antibodies (MSN-1) (f). St, standard glycolipids; I3SO3-GalCer and II3SO3-LacCer for a, GM3 for b, GM2 for c, GM1 for d, sialyl Lea for E and Leb for F. SCC, squamous cell carcinoma; Adeno SCC, adenosquamous cell carcinoma; Small, small cell carcinoma; Adeno, adenocarcinoma. 1–6, patient numbers for respective cervical carcinomas
Discussion
GalNAc-GD1a was characterized as a component of the axolemma at nodes of Ranvier or paranodes of motor nerves. Since GalNAc-GD1a-containing cells have not been reported in the past, even neural tissue-derived cells, this is a first report of GalNAc-GD1a-containing cells. GalNAc-GD1a was characterized as a target antigen as to antibodies generated by infection with enterocolitis-associated bacteria such as Campylobacter jejuni, and with cytomegalovirus as a trigger on the onset of autoimmune neuropathies, such as the Guillain-Barré and Tolosa-hunt syndromes [18, 25–28]. Since GM2 determinant was also detected in the lipooligosaccharides from C. jejuni [29], it was revealed to exhibit a strong immunogenicity in the gut-associated immune system to yield anti-GM2 antibodies, and the resulting antibodies were thought to react with GalNAc-GD1a more strongly than with GM2 in neural tissues to disturb the neural functions through antibody-induced inflammation, leading to neuropathies. Detection of antibodies against GalNAc-GD1a was useful as a diagnostic marker for autoimmune neuropathies [18, 26–29], and Boku, OMC-4 and HCA-1 cells would be applicable as sources for preparation of GalNAc-GD1a and as target cells for the detection of anti-GalNAc-GD1a antibodies in sera of patients with autoimmune neuropathies. In fact, in our preliminary experiment, anti-GalNAc-GD1a IgG antibodies could be detected on immunohistochemical staining of Boku cells with sera of patients suffering from chronic motor axonal neuropathy [30].
On the contrary, since GM2, together with GM3, GD3 and GD2, is a melanoma-associated antigen, administration of monoclonal anti-GM2 antibodies, as well as vaccination of melanoma patients with GM2, have been applied as immunotherapy for melanomas [31]. The intravenous administration of monoclonal anti-GM2 and anti-GD2 antibodies revealed their anti-tumor effects, and no significant damage to normal tissues including neural ones was observed, suggesting that gangliosides in normal tissues are cryptic in nature to avoid binding with antibodies. Further characterization of the reactivities of ganglosides with GM2-determinant is necessary to understand the difference in antigenic properties between GM2 and GalNAc-GD1a, and to clarify the mechanism on the onset of neuropathies triggered by binding with antibodies. An approach for determining the reactivities of patient’s sera to several cervical carcinoma cell lines with different GM2, GalNAc-GM1b and GalNAc-GD1a compositions should help to verify the antigenic structures involved in autoimmunity.
As shown in this report, high amounts of gangliosides with GM2-determinant were observed in all cell lines originating from different types of cervical carcinomas, i.e., squamous cell carcinomas, adenocarcinomas, adenosquamous carcinomas and small cell carcinomas, and were a cervical carcinoma-characteristic phenotype, which was not observed for endometrial and ovarian carcinomas [13–15]. The enhanced synthesis of GM2, GalNAc-GM1b and GalNAc-GD1a was largely dependent on gene-expression of GalNAc-transferase, but the relative amounts of them differed among cell lines, probably due to the differences in their baseline metabolic pathways. One can suggest that enhanced synthesis of gangliosides with GM2-determinant in cervical carcinoma-derived cell lines is triggered by infection with papilloma virus, analogously to in the case of fibroblasts and neural cells after infection with SV-40 virus [20]. Papilloma viruses 16 and 18 were detected in SKG-IIIb and Boku cells, and Hela and HCSC-1 cells, respectively, but not in HKUSP, OMC-4 and HCA-1 cells. One can suggest that the virus might have disappeared from these cells, because patients with cervical carcinomas, whose onset was initiated by papilloma virus, and whose virus, after transformation, could not be detected in their cancerous tissues and sera have been reported [32]. To explore the involvement of papilloma virus in glycolipid metabolism, an experiment involving papilloma virus-infected keratinocytes is in progress in our laboratory [33].
Alteration of the glycolipid composition during the establishment of a cancer cell line has been frequently observed. In endometrial carcinoma tissues, I3SO3-GalCer was expressed in a well-differentiated type [16], but II3SO3-LacCer was newly synthesized after the transfer of cancer cells to cultivation, due to the disappearance of GalCer synthetase [17]. In this case, although the structures of sulfoglycolipids changed from I3SO3-GalCer to II3SO3-LacCer, a well-differentiated phenotype was maintained in cell lines, because the cancer cell nodules formed on intradermal transplantation of cells into nude mice demonstrated the gland-like structure as the well differentiated type, indicating that sulfoglycolipids are related with the well differentiated phenotype [17]. However, these phenotypes are frequently lost on continuous subculture for more than 50 passages, and recovered with suitable stimulation including contact with type 1 collagen [34]. Apparently, the circumstances surrounding cells in vitro and in vivo affect even the differentiation phenotypes including glycolipid compositions. The observations that I3SO3-GalCer was present in cervical adenocarcinomas and small cell carcinomas, but not in the respective carcinoma-derived cells, corresponded to the above findings for endometrial carcinoma-derived cell. Similarly, GM3 was present as the major ganglioside, but GM1 only in a trace amounts in all cervical carcinoma tissues, indicating that elongation of the carbohydrate chains of GM3 is suppressed in these tissues, although the metabolic pathway for a-series gangliosides is retained in all tissues. The mechanism of the activation of GalNAc-transferase for the synthesis of GM2 in cervical carcinoma-derived cells should be elucidated, and analyses of the activity and gene expression of all glycosyltransferases involved in the synthesis of a-series gangliosides should be carried out in comparison with those in the tissues.
References
IUPAC-IUB: Commission on biochemical nomenclature of lipids. Eur. J. Biochem. 179, 11–21 (1977)
Svennerholm L.: Chromatographic separation of human brain gangliosides. J. Neurochem. 10, 613–623 (1963)
Iwamori M.: A new turning point in glycosphingolipid research. Hum. Cell. 18, 117–133 (2005)
Yu R.K., Tsai Y.T., Ariga T., Yanagisawa M.: Structures, biosynthesis, and functions of gangliosides–an overview. J. Oleo. Sci. 60, 537–544 (2011)
Chhieng D.C., Rodriguez-Burford C., Talley L.I., Sviglin H., Stockard C.R., Kleinberg M.J., Barnes M.N., Partridge E.E., Khazaeli M.B., Grizzle W.E.: Expression of CEA, Tag-72, and Lewis-Y antigen in primary and metastatic lesions of ovarian carcinoma. Hum. Pathol. 34, 1016–1021 (2003)
Kim Y.S., Yuan M., Itzkowitz S.H., Sun Q.B., Kaizu T., Palekar A., Trump B.F., Hakomori S.: Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res. 46, 5985–5992 (1986)
Bevilacqua M.P., Stengelin S., Gimbrone Jr. M.A., Seed B.: Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 243, 1160–1165 (1989)
Goupille C., Marionneau S., Bureau V., Hallouin F., Meichenin M., Rocher J., Le Pendu J.: alpha1,2Fucosyltransferase increases resistance to apoptosis of rat colon carcinoma cells. Glycobiology. 10, 375–382 (2000)
Baldus S.E., Hanisch F.G., Pütz C., Flucke U., Mönig S.P., Schneider P.M., Thiele J., Hölscher A.H., Dienes H.P.: Immunoreactivity of Lewis blood group and mucin peptide core antigens: correlations with grade of dysplasia and malignant transformation in the colorectal adenoma-carcinoma sequence. Histol. Histopathol. 17, 191–198 (2002)
Iwamori M., Tanaka K., Kubushiro K., Lin B., Kiguchi K., Ishiwata I., Tsukazaki K., Nozawa S.: Alterations in the glycolipid composition and cellular properties of ovarian carcinoma-derived RMG-1 cells on transfection of the alpha1,2-fucosyltransferase gene. Cancer Sci. 96, 26–30 (2005)
Iwamori M., Iwamori Y., Kubushiro K., Ishiwata I., Kiguchi K.: Characteristic expression of Lewis-antigenic glycolipids in human ovarian carcinoma-derived cells with anticancer drug-resistance. J. Biochem. 141, 309–317 (2007)
Kiguchi K., Iwamori Y., Suzuki N., Kobayashi Y., Ishizuka B., Ishiwata I., Kita T., Kikuchi Y., Iwamori M.: Characteristic expression of globotriaosyl ceramide in human ovarian carcinoma-derived cells with anticancer drug resistance. Cancer Sci. 97, 321–326 (2006)
Tanaka K., Mikami M., Aoki D., Kiguchi K., Ishiwata I., Iwamori M.: Expression of sulfatide and sulfated lactosylceramide among histological types of human ovarian carcinomas. Hum. Cell. 28, 37–43 (2015)
Tanaka K., Takada H., Isonishi S., Aoki D., Mikami M., Kiguchi K., Iwamori M.: Possible involvement of glycolipids in anticancer drug resistance of human ovarian serous carcinoma-derived cells. J. Biochem. 52, 587–594 (2012)
Takehara K., Kubushiro K., Kiguchi K., Ishiwata I., Tsukazaki K., Nozawa S., Iwamori M.: Expression of glycolipids bearing Lewis phenotypes in tissues and cultured cells of human gynecological cancers. Jpn. J. Cancer Res. 93, 1129–1137 (2002)
Sugiyama T., Miyazawa M., Mikami M., Goto Y., Nishijima Y., Ikeda M., Hirasawa T., Muramatsu T., Takekoshi S., Iwamori M.: Enhanced expression of sulfatide, a sulfated glycolipid, in well-differentiated endometrial adenocarcinoma. Int. J. Gynecol. Cancer. 22, 1192–1197 (2012)
Kubushiro K., Tsukazaki K., Tanaka J., Takamatsu K., Kiguchi K., Mikami M., Nozawa S., Nagai Y., Iwamori M.: Human uterine endometrial adenocarcinoma: characteristic acquirement of synthetic potentials for II3SO3-LacCer and ganglio series sulfoglycosphingolipids after transfer of the cancer cells to culture. Cancer Res. 52, 803–809 (1992)
Chatani H., Tanaka M., Nagata T., Araki T., Kusunoki S.: Guillain-Barré syndrome-like-onset neurosarcoidosis positive for immunoglobulin G anti-N-acetylgalactosaminyl-GD1a antibody. J. Clin. Neurosci. 21, 170–172 (2014)
Ogawa G., Kaida K., Kuwahara M., Kimura F., Kamakura K., Kusunoki S.: An antibody to the GM1/GalNAc-GD1a complex correlates with development of pure motor Guillain-Barré syndrome with reversible conduction failure. J. Neuroimmunol. 254, 141–145 (2013)
Hoffman L.M., Brooks S.E., Stein M.R., Schneck L.: SV-40 transformation: effect on GM2 ganglioside in cultured cell lines. Biochim. Biophys. Acta. 1084, 94–100 (1991)
Iwamori M., Kaido T., Iwamori Y., Ohta Y., Tsukamoto K., Kozaki S.: Involvement of the C-terminal tail of Arthrobacter ureafaciens sialidase isoenzyme M in cleavage of the internal sialic acid of ganglioside GM1. J. Biochem. 138, 327–334 (2005)
Iwamori M., Nakasa M., Yamazaki K., Iwamori Y., Tanaka K., Aoki D., Adachi S., Nomura T.: Bacterial species-characteristic profiles of molecular species, and the antigenicity of phospholipids and glycolipids in symbiotic Lactobacillus, Staphylococcus and Streptococcus species. Glycoconj. J. 29, 199–209 (2012)
Perez Castro S., Iñarrea Fernández A., Lamas González M.J., Sarán Diez M.T., Cid Lama A., Alvarez Martín M.J., Pato Mosquera M., López-Miragaya I., Estévez N., Torres Piñón J., Oña Navarro M.: Human papillomavirus (HPV) E6/E7 mRNA as a triage test after detection of HPV 16 and HPV 18 DNA. J. Med. Virol. 85, 1063–1068 (2013)
Tiltman A.J.: The pathology of cervical tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 19, 485–500 (2005)
Kusunoki S., Chiba A., Kon K., Ando S., Arisawa K., Tate A., Kanazawa I.: N-acetylgalactosaminyl GD1a is a target molecule for serum antibody in Guillain-Barré syndrome. Ann. Neurol. 35, 570–576 (1994)
Kaida K., Sonoo M., Ogawa G., Kamakura K., Ueda-Sada M., Arita M., Motoyoshi K., Kusunoki S.: GM1/GalNAc-GD1a complex: a target for pure motor Guillain-Barre syndrome. Neurology. 71, 1683–1690 (2008)
Okawa S., Sugawara M., Takahashi S., Otani T., Hashimoto M., Kusunoki S., Ohnishi H.: Tolosa-hunt syndrome associated with cytomegalovirus infection. Intern. Med. 52, 1121–1124 (2013)
Kaida K., Kusunoki S., Kamakura K., Motoyoshi K., Kanazawa I.: Guillain-Barré syndrome with IgM antibody to the ganglioside GalNAc-GD1a. J. Neuroimmunol. 113, 260–267 (2001)
Houliston R.S., Yuki N., Hirama T., Khieu N.H., Brisson J.R., Gilbert M., Jarrell H.C.: Recognition characteristics of monoclonal antibodies that are cross-reactive with gangliosides and lipooligosaccharide from Campylobacter jejuni strains associated with Guillain-Barré and Fisher syndromes. Biochemistry. 46, 36–44 (2007)
Kaji R., Kusunoki S., Mizutani K., Oka N., Kojima Y., Kohara N., Kimura J.: Chronic motor axonal neuropathy associated with antibodies monospecific for N-acetylgalactosaminyl GD1a. Muscle Nerve. 23, 702–706 (2000)
Livingston P.O., Natoli E.J., Calves M.J., Stockert E., Oettgen H.F., Old L.J.: Vaccines containing purified GM2 ganglioside elicit GM2 antibodies in melanoma patients. Proc. Natl. Acad. Sci. U. S. A. 84, 2911–2915 (1987)
Ault K.A., Future II Study Group: Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 369, 1861–1868 (2007)
Flores E.R., Allen-Hoffmann B.L., Lee D., Sattler C.A., Lambert P.F.: Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology. 262, 344–354 (1999)
Mikami M., Harasawa M., Sugiyama T., Nishijima Y., Goto Y., Hirasawa T., Muramatsu T., Iwamori M.: Induction of the differentiation of cultured endometrial carcinoma cells by type I collagen: Relevance of sulfolipids. Oncol. Lett. 1, 113–117 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, K., Miyazawa, M., Mikami, M. et al. Enhanced expression of unique gangliosides with GM2-determinant in human uterine cervical carcinoma-derived cell lines. Glycoconj J 33, 745–754 (2016). https://doi.org/10.1007/s10719-016-9668-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9668-0