Abstract
Glycosaminoglycans (GAGs) are heterogeneous, linear, highly charged, anionic polysaccharides consisting of repeating disaccharides units. GAGs have some biological significance in cancer progression (invasion and metastasis) and cell signaling. In different cancer types, GAGs undergo specific structural changes. In the present study, in depth investigation of changes in sulfation pattern and composition of GAGs, heparan sulfate (HS)/heparin (HP), chondroitin sulfate (CS)/dermatan sulfate and hyaluronan (HA) in normal renal tissue (NRT) and renal cell carcinoma tissue (RCCT) were evaluated. The statistical evaluation showed that alteration of the HS (HSNRT = 415.1 ± 115.3; HSRCCT = 277.5 ± 134.3), and CS (CSNRT = 35.3 ± 12.3; CSRCCT = 166.7 ± 108.8) amounts (in ng/mg dry tissue) were statistically significant (p < 0.05). Sulfation pattern in NRT and RCCT was evaluated to reveal disaccharide profiles. Statistical analyses showed that RCCT samples contain significantly increased amounts (in units of ng/mg dry tissue) of 4SCS (NRT = 25.7 ± 9.4; RCCT = 117.1 ± 73.9), SECS (NRT = 0.7 ± 0.3; RCCT = 4.7 ± 4.5), 6SCS (NRT = 6.1 ± 2.7; RCCT = 39.4 ± 34.7) and significantly decreased amounts (in units of ng/mg dry tissue) of NS6SHS (RCCT = 28.6 ± 6.5, RCCT = 10.2 ± 8.0), NS2SHS (RCCT = 44.2 ± 13.8; RCCT = 27.2 ± 15.0), NSHS (NRT = 68.4 ± 15.8; RCCT = 50.4 ± 21.2), 2S6SHS (NRT = 1.0 ± 0.4; RCCT = 0.4 ± 0.3), and 6SHS (NRT = 60.6 ± 17.5; RCCT = 24.9 ± 12.3). If these changes in GAGs are proven to be specific and sensitive, they may serve as potential biomarkers in RCC. Our findings are likely to help us to show the direction for further investigations to be able to bring different diagnostic and prognostic approaches in renal tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (hypernephroma) is an epithelial tumor that arises from the tubular structures of the kidney. It generally occurs in adults and accounts for 2–3 % of all adult malignancies, although the incidence varies substantially worldwide, between genders, races and geographic locations [1–3]. There is a wide spectrum of histological RCC subtypes, of which the most common three are clear cell RCC (70 %), papillary RCC (10–15 %) and chromophobe RCC (3–5 %) [4]. Nearly half of the patients are diagnosed incidentally; while others present with non-specific features, such as fatigue, weight loss or anemia [5]. The surgical removal of all or part of the kidney (nephrectomy) is the major treatment [6].
Glycosaminoglycans (GAGs) are heterogeneous, linear, highly charged, anionic polysaccharides consisting of repeating disaccharide units and can be divided into four classes: heparan sulfate (HS) (which includes heparin), chondroitin sulfate (CS) (which includes dermatan sulfate), keratan sulfate (KS) and hyaluronan (HA). These classes differ in the structure of their repeating disaccharides and also in their biological functions [7–9]. Repeating disaccharide units consist of either sulfated or non-sulfated monosaccharides. Their sulfation pattern is generated during biosynthesis through the action of several sulfotransferases, resulting monosulfated, disulfated, and trisulfated disaccharide units [10]. When HS and CS are enzymatically degraded using polysaccharide lyases, a number of structurally distinctive disaccharides with different sulfation pattern are obtained [11]. The most commonly found disaccharide units produced from mammalian cell GAGs are given in Table 1. The amounts and sulfation patterns of GAGs show variability in different tissues, organs and even in different part of the same organ [12, 13].
GAGs are widely found on cell surfaces, inside cells and within the extracellular matrix. GAGs, with the exception of hyaluronan, are biosynthesized as protein/GAG conjugates known as proteoglycans (PGs). The biological functions of PGs are principally determined by the structure of their GAG chains [8, 9, 14]. GAGs interact with hundreds of key ligands and receptors including growth factors, cytokines, chemokines, proteases, protease inhibitors, coagulant and anticoagulant proteins, complement proteins, lipoproteins, and lipolytic enzymes [9, 15, 16]. These interactions are critical in a variety of physiological and pathophysiological processes such as fertilization, embryonic development, immune response, inflammation, angiogenesis, tumorigenesis, and metastasis [16–19]. The structures of GAGs, especially their sulfation pattern, affect interaction with various ligands [20, 21].
In a variety of diseases, such as cancer, rheumatoid arthritis, and infectious diseases, the functions of GAGs have been extensively studied to define their specific roles [22–24]. Studies related to cancer show the important roles of GAGs and PGs in cancer progression (invasion and metastasis) and cell signaling [15, 25]. Clinical studies in cancer point out changes in expression of some specific GAGs and PGs [26, 27]. Their expression and degradation are known to affect all stages of tumorigenesis [26]. In different cancer types, GAGs undergo specific structural changes [28–39].
The importance of GAGs in renal cell carcinoma (RCC) has been discussed in a recent study [40]. An increased amount of CS and a decreased amount of HS was reported in renal cell carcinoma tissue (RCCT). Also no significant changes in the urine excretion rates of CS, and HS were observed. However, there is no information on changes in GAG sulfation pattern, which is critical for revealing structural changes in GAGs in RCC.
The aim in this study is to investigate the sulfation pattern and composition of GAGs (HS, CS and HA) in normal renal tissue (NRT) and RCCT. The detailed structural characterization of GAGs is the first step towards understanding the biological functions of PGs and GAGs in RCC. This can help in the understanding the causes for the disease and for the elucidation of the metabolic pathways of RCC. Moreover, GAGs may serve as diagnostic and prognostic biomarkers for RCC. Increasing knowledge on the GAG functions in biological processes may also lead to suggest new GAG-based therapeutic approaches and GAG-based therapeutic targets.
Materials and methods
Biological samples and materials
Tissues for analysis were obtained from thirteen radical nephrectomy materials. Two samples were taken from each nephrectomy, one from tumor and one from non-neoplatic renal cortex distant from the tumor. The study was approved by the Local Research Ethics Committee.
Actinase E was from Kaken Biochemicals (Tokyo, Japan). Unsaturated disaccharides standards of CS (0S, ΔUA-GalNAc; 4S, ΔUA-GalNAc4S; 6S, ΔUA-GalNAc6S; 2S, ΔUA2S-GalNAc; 2S4S or SB, ΔUA2S-GalNAc4S; 2S6S or SD, ΔUA2S- GalNAc6S; 4S6S or SE, ΔUA-GalNAc4S6S; and TriS, ΔUA2S-GalNAc4S6S), unsaturated disaccharides standards of heparan sulfate (0S, ΔUA-GlcNAc; NS, ΔUA-GlcNS; 6S, ΔUA-GlcNAc6S; 2S, ΔUA2S-GlcNAc; 2SNS, ΔUA2S-GlcNS; NS6S, ΔUA-GlcNS6S; 2S6S, ΔUA2S-GlcNAc6S; and TriS, ΔUA2S-GlcNS6S) and unstaturated disaccharides standard of hyaluronan (ΔUA-GlcNAc) were obtained from Seikagaku (Japan) (Table 1). Recombinant Flavobacterial heparinum heparin lyase I, II and III were expressed in Linhardt laboratory using Escherichia coli strains, provided by Professor Jian Liu (University of North Carolina, College of Pharmacy, Chapel Hill, North Carolina). Chondroitin lyase ABC from Proteus vulgaris and chondroitin lyase ACII from Arthrobacter aurescens was from Seikagaku Corporation (Tokyo, Japan). AMAC (≥98.0 %) and sodium cyanoborohydride (≥95.0 %) was supplied from Sigma (St. Louis, MO, USA). All other chemicals were of reagent grade. Vivapure Q Mini H columns were from Sartorius Stedium Biotech (Bohemia, NY, USA). Amicon ultracentrifugal filters (YM-10; 1000 molecular weight cut-off) were from Millipore (Billerica, MA).
Isolation of GAGs from renal tissue samples
Tissue samples (RCCT and NRT) were homogenized and individually subjected to proteolysis at 55 °C with 10 % (w/v) of actinase E (20 mg/mL in HPLC grade water, Kaken Biochemicals, Tokyo, Japan). After proteolysis, particulates were removed from the resulting solutions by centrifugation at 12,000 × g. The supernatant was applied to Vivapure Q Mini H column (Bohemia, NY, USA) equilibrated with 200 μL of 8 M urea containing 2 % CHAPS (pH 8.3) under centrifugal force (700 × g). The columns were then washed with 200 μL of 8 M urea containing 2 % CHAPS at pH 8.3, followed by two washes with 200 μL of 200 mM NaCl. GAGs were released from the column by washing three-times with 450 μL of 16 % NaCl and then collected eluent was desalted using YM-10 spin column. Finally, the GAGs were lyophilized [9].
Breakdown of GAGs to disaccharide products
The recovered GAGs from renal tissue samples were depolymerized using the enzyme mixture of heparinase I, II, III, chondroitinase ABC and chondroitinase AC II at 37 °C for 10 h. The enzymatic products were then passed through the YM-10 spin columns then were freeze-dried for AMAC labeling reaction [9].
Derivatization of disaccharides with AMAC
The freeze-dried renal tissue samples were re-dissolved in 5 μL of 0.1 M AMAC in acetic acid/dimethyl sulfoxide (DMSO) (3:17, v/v) and left at room temperature for 30 min. After that, 5 μL of 1 M NaBH3CN was added to the reaction mixture and mixture was incubated at 45 °C for 4.5 h [9, 41]. Finally, AMAC-labeled disaccharides were diluted to various concentrations using DMSO:water (50:50; v/v), and then LC-MS analysis was performed.
Disaccharide analysis by UPLC-MS
UPLC-MS analyses were performed on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Inc. Wilmington, DE) equipped with a 6300 ion trap and a binary pump. The column used was a Poroshell 120 C18 column (2.1 × 150 mm, 2.7 μm, Agilent) at 45 °C. Eluent A was 80 mM ammonium acetate solution and eluent B was methanol. Solution A and 15 % solution B were flowed (150 μL/min) through the column for 5 min followed by a linear gradient from 15 to 30 % solution B from 5 to 30 min. The column effluent entered the electrospray ionization MS source for continuous detection by MS. The electrospray interface was set in negative ionization mode with a skimmer potential of −40.0 V, a capillary exit of −40.0 V and a source temperature of 350 °C, to obtain the maximum abundance of the ions in a full-scan spectrum (150–1200 Da). Nitrogen (8 L/min, 40 psi) was used as a drying and nebulizing gas [9, 42].
Statistical analysis
IBM SPSS for Windows Version 20.0 was used for the statistical analysis. The measurements were summarized as mean ± standard deviation, median, minimum and maximum values. Wilcoxon test was used to understand if there are any significant differences between the normal and neoplastic samples. Significance was taken to be p < 0.05 for Wilcoxon test. Results are visualized with the mean ± standard deviation graphs and the box-plots, where appropriate.
Results
RCCT and NRT samples were obtained from the patients who had undergone to the radical nephrectomy. In the sampling process, non-neoplastic kidney tissue away from the tumor (NRT) and renal cancer tissue (RCCT) came from the same specimen and these two tissue samples were compared. Known amount of the dried tissue samples (about 20–30 mg) were subject to Actinase E digestion freeing the GAGs from their protein backbone in their PG form. The GAGs, thus obtained, were prepared for disaccharide analysis by UPLC-MS (Fig. 1). The sum of disaccharide amounts was expressed as total HS, CS, and HA.
Extracted ion chromatograms (EICs) of AMAC-labeled disaccharides. a Analysis of AMAC-labeled disaccharides obtained on heparin lyase treatment of an NRT sample. The assigned peaks are used to establish the disaccharide composition of this sample. b Analysis of AMAC-labeled disaccharides obtained on heparin lyase treatment of an RCCT sample. The peak assignment is identical to that shown in panel a
Composition of GAGs in tissue samples
Majority of RCC originates in the renal cortex. In the present study, NRT samples were, thus, taken from the cortical region of the nephrectomy materials. Nevertheless we also obtained tissue samples from renal medulla in addition to cortex. The amount of the HS, CS and HA in medulla were found to be higher than their amount in cortex (Table 2) [43]. The predominant GAG in both regions was HS followed by CS, and/or HA (Table 2, Fig. 2a).
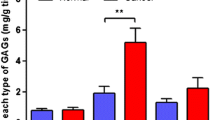
The content and types of GAGs present in analyzed kidney tissues. a Cortex and medulla; HS/HP:Heparan sulfate/Heparin, CS/DS:Chondroitin Sulfate/Dermatan Sulfate, HA: Hyaluronan. b RCCT and NRT. RCCT; Renal cell carcinoma tissue. NRT: Normal renal tissue. The amounts of GAGs were expressed as mean. The error bars were given with +/− Standard deviation
When RCCT and NRT were compared in terms of compositional and structural differences of GAGs between RCCT and NRT, the results showed that HS was the dominant GAG, followed by CS and HA in RCCT as in NRT (Fig. 2b, Table 2). It was observed that the amount of CS significantly increased, whereas the amount of HS decreased in cancer. There was no difference in the amount of HA between NRT and RCCT (p = 0.515 > 0.05). The major GAG in both tissues (NRT, RCCT) was HS, despite the elevated levels of CS in RCCT.
The statistics showed that the differences between cancer and normal tissue taking HS and CS amounts into account were significant (p < 0.05) (Fig. 3). In contrast, HA levels did not differ between NRT and RCCT (p = 0.515 > 0.05). The interspecimen variability in regards to the contained CS, HS and HA was higher in renal cancer than that observed in non-neoplastic renal tissue (Table 2).
Box-and-whiskers plot for HS/HP, CS/DS and HA differences between RCCT and NRT. RCCT: Renal cell carcinoma tissue. NRT: Normal renal tissue. HS/HP:Heparan sulfate/Heparin, CS/DS:Chondroitin Sulfate/Dermatan Sulfate, HA: Hyaluronan. The box-and-whisker plot is helpful to see the distribution of GAGs and disaccharide amount in the tissue samples and to compare these groups. In the plots, boxes represent the value of interquartile range (1st quartile-3rd quartile). The bold line at the center of boxes represents the median (2nd quartile). The vertical lines (whiskers) in the plot go down to the minimum and up to the maximum value
Disaccharide profile of tissue samples
NRT and RCCT were examined to determine their disaccharide composition and their HS, CS, and HA content. The results demonstrated that both NRT and RCCT contained 8 different HS disaccharides (0SHS, NSHS, 6SHS, 2SHS, NS6SHS, NS2SHS, 2S6SHS, Tri SHS), 7 different CS disaccharides (0SCS, 6SCS, 4SCS, SBCS, SDCS, SECS, TriSCS, but no 2SCS), and a single HA disaccharide (0SHA) with varying amounts. Their disaccharide compositions were shown in Fig. 4 and Table 3.
The unsulfated HS (0SHS) was the most abundant and the 2S6SHS was the least abundant disaccharide units of HS found in both normal and neoplastic tissues. Among the sulfated HS disaccharides, the NSHS that contains only one sulfo group was the most abundant one in both NRT and RCCT. The other sulfated HS disaccharides detected were 6SHS, NS2SHS, NS6SHS, 2SHS, and TriSHS, which are listed from most to least abundant.
When disaccharide profile of CS was examined, the 4SCS was the most abundant CS disaccharide in both tissue types, 6SCS was the second as being found at relatively high levels. The trisulfated disaccharide (TriSCS) was found to be the least abundant CS disaccharide present in the normal kidney and renal cancer. The 2SCS disaccharide was completely absent.
Statistical analysis showed that RCCT contained significantly increased amounts of 6SCS, 4SCS, SECS, (p6SCS = 0.028, p4SCS = 0.007, pSECS, = 0.007) and significantly decreased amounts of 6SHS, NS6SHS, NS2SHS, 2S6SHS (p6SHS = 0.005, pNS6SHS = 0.005, pNS2SHS, = 0.007, p2S6SH = 0.005) in comparison with non-neoplastic renal cortex (Figs. 5 and 6). Each CS and HS disaccharide content in NRT and RCCT showed variability from 32 % to 107 % and from 22.7 % to 140 %, respectively (Table 3).
Discussion
Tumors have multiple mechanisms that promote their growth, invasion and spread [26, 28]. GAGs, and their binding proteins play essential roles in these mechanisms. It is known that different types of cancer are associated with specific compositional and structural alteration of GAGs [28–39]. In this study, we perform in depth investigation in terms of sulfation pattern and compositional changes of GAGs (HS, CS and HA) in RCCT.
Our results show that the amount of CS is significantly increased (p < 0.05) in renal cell carcinoma (RCCT) when compared to normal renal tissue (NRT). Similar changes have been observed in different mesenchymal and epithelial neoplasms, such as hepatic carcinoma, prostate, pancreas, lung, breast, and gastric cancer [28–39, 44]. In the transformed fibroblasts and mammary carcinoma cells, elevated CS levels are related to neoplastic cell proliferation, adhesion and migration [21]. The proliferation and invasiveness of tumor cells are decreased in melanoma cells treated with CS-degrading enzymes [45]. The amount of CS is generally increased in tumor cells [45].
We have found the amount of HS decreased in RCCT in our study like that of other reported malignancies, including bladder cancer, hepatocellular carcinoma, and gastric cancer [30, 33, 46]. A reduction of HS reportedly promotes invasion and migration of tumor cells [47, 48]. A decreased HS in RCC is thought to be associated with increased activity of heparanase. In a recent study heparanase activity was evaluated and was found to increase be elevated in RCC [40]. Heparanase is an endohydrolase that cleaves glycosidic bonds in heparan sulfate. It has functions in normal cell processes, including the regulation of angiogenesis and tissue repair by releasing HS-bound growth factors and enzymes such as basic fibroblast growth factor (bFGF) and lipoprotein lipase. In tumor cells, heparanase promotes tumor growth, invasion and stimulates angiogenesis. Moreover, up-regulated heparanase is associated with metastatic potential of human tumor cells. Elevated levels of heparanase are also detected in the sera of cancer patients with metastatic tumors [49–53].
Sulfation patterns of HS and CS are important because of their interaction with many ligands and receptors [54] affecting their biological roles. Highly sulfated CS strongly interacts with various protein ligands and receptors, which are implicated in the growth and/or progression of tumors [29]. Specific binding resulting from certain sulfation patterns in CS, was also observed [45]. For example, CS-E (rich in SECS) binds to L- and P-selectin, and CS-A (rich in 4SCS), CS-B (having an iduronic acid (IdoA) residue and rich in 4SCS), CS-C (rich in 6SCS), CS-D (rich in SDCS) and CS-E (rich in SECS) bind to CD44 receptor [47].
Changes in the sulfation pattern of the HS are also crucial [33]. The interaction between bFGF and hepatocyte growth factor with HS depend on HS chains with 2-O-sulfo IdoA residues (rich in 2SHS, NS2SHS, 2S6SHS, and TriSHS) and 6-O-sulfo groups on the glycosamine residues (rich in 6SHS, NS6SHS,2S6SHS, and TriSHS), respectively [31]. It is noteworthy that GAGs interact with bFGF and vascular endothelial growth factor, which are given clinical importance as being potential tumor markers for RCC [55].
The malignant phenotypes in various cancer types show different sulfation patterns in GAGs [34]. Sulfation pattern of CS/DS in rectum, pancreas, colon, gastric carcinomas are changed and non-sulfated and 6-sulfated disaccharides in tumor are remarkably increased in comparison to normal counterparts that may contribute to the growth, proliferation and migration of cancer cells [28–32, 52]. Also the increased amount of 6S is thought to correlate with the aggressiveness of tumor cells [35].
In our RCC tissue samples, the amounts of 4SCS and SECS disaccharides were found significantly elevated when compared with normal tissue. Similarly, an increased amount of 4SCS has also been observed in human laryngeal cancer. Reported studies related to ovarian cancer have recently shown that these 4,6-disulfated chondroitin sulfate-rich regions (CS-E) reflect the adhesive properties of ovarian tumor cells [36]. CS-E is also reported to regulate cellular signaling, such as the Wnt/beta-catenin pathway [56, 57], which is responsible for critical pro-tumorigenic and pro-metastatic transformation in many human cancers [36, 58].
The sulfation pattern of HS in RCC was different from that in NRT in our study such that the amounts of the 6SHS, NS6SHS, NS2SHS, and 2S6SHS were significantly decreased.
In the current study, we interestingly have noted that the amount of HA did not differ between the RCC and NRT samples. In a normal cell, HA has some important functions such as keeping tissues hydrated, maintaining osmotic balance and supporting cartilage integrity. Also it interacts with cell surface receptors and modulates cell adhesion, migration and proliferation. In tumor tissues, HA promotes tumor metastasis by opening spaces up for tumor cells and supports tumor cell migration by interacting with cell surface HA receptors. The amount of HA is elevated in certain cancers, including colon, breast, prostate, bladder, lung, and Wilms’tumor [59–62].
In conclusion, this study demonstrates differences of GAGs in composition and sulfation patterns between RCCT and NRT. The documentation of these changes is an essential prerequisite to understand their biological functions in RCC. Future studies are necessary to discover the underlying mechanisms that are responsible for the altered GAG motifs, such as changes in the activity of biosynthetic enzymes (i.e., sufotransferases) or catabolic enzymes (i.e., sufatases and heparanase). In addition, for these alterations to be considered as biomarkers, their sensitivity and specificity need to be evaluated in a larger group of patients. In the future, studies like this may lead to the development of new diagnostic and prognostic approaches.
References
Znaor A., Lortet-Tieulent J., Laversanne M., Jemal A., Bray F.: International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 67, 519–530 (2015)
Rini B.I., Campbell S.C.: Renal cell carcinoma. Lancet. 73, 1119–1132 (2009)
Ljungberg B., Campbell S.C., Yong Cho H., Jacqmin D., Eun Lee J., Weikert S., Kiemeney L.A.: The epidemiology of renal cell carcinoma. Eur. Urol. 60, 615–621 (2011)
Lopez-Beltran A., Carrasco J.C., Cheng L., Scarpelli M., Kirkali Z., Montironi R.: Update on the classification of renal epithelial tumors in adults. Int. J. Urol. 16(5), 432–433 (2009)
Cohen H.T., McGovern F.J.: Renal-Cell Carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005)
Rini B.I., Rathmell W.K., Godley P.: Renal cell carcinoma. Curr. Opin. Oncol. 20(3), 300–306 (2008)
Ly M., Laremore T.N., Linhardt R.J.: Proteoglycomics: recent progress and future challenges. OMICS. 14(4), 389–399 (2010)
Hileman R.E., Fromm J.R., Weiler J.M., Linhardt R.J.: Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. BioEssays. 20, 156–167 (1998)
Ucakturk E., Cai C., Lingyun L., Guoyun L., Fuming Z., Linhardt R.J.: Capillary electrophoresis for total glycosaminoglycan analysis. Anal. Bioanal. Chem. 406, 4617–4626 (2014)
Avci F.Y., DeAngelis P.L., Liu J., Linhardt R.J.: Fronteirs in Modern Carbohydrate Chemistry. In: Demchenko A.V. (ed.) Enzymatic Synthesis Of Glycosaminoglycans: Improving On Nature, pp. 253–284, Washington, DC (2007)
Volpi N., Galeotti F., Yang B., Linhardt R.J.: Analysis of glycosaminoglycan-derived, precolumn, 2-aminoacridone–labeled disaccharides with LC-fluorescence and LC-MS detection. Nat. Protoc. 9, 541–558 (2014)
Shao C., Shi X., White M., Huang Y., Hartshorn K., Zaia J.: Comparative glycomics of leukocyte glycosaminoglycans. FEBS J. 280, 2447–2461 (2013)
Leymarie N., McComb M.E., Naimy H., Staples G.O., Zaia J.: Differential characterization and classification of tissue specific glycosaminoglycans by tandem mass spectrometry and statistical methods. Int. J. Mass Spectrom. 15(312), 144–154 (2012)
Linhardt R.J., Toida T.: Role of glycosaminoglycans in cellular communication. Acc. Chem. Res. 37, 431–438 (2004)
Sasisekharan R., Raman R., Prabhakar V.: Glycomics approach to structure-function relationships of glycosaminoglycans. Annu. Rev. Biomed. Eng. 8, 181–231 (2006)
Sanderson R.D., Yang Y., Suva L.R., Kelly T.: Heparan sulfate proteoglycans and heparanase—partners in osteolytic tumor growth and metastasis. Matrix Biol. 23, 341–352 (2004)
Capila I., Linhardt R.J.: Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 41, 391–412 (2002)
Kreuger J., Spillmann D.: Li, J-p, lindahl, U.: interactions between heparan sulfate and proteins: the concept of specificity. J. Cell Biophys. 174, 323–327 (2006)
Chi L., Wolff J.J., Laremore T.N., Restaino O.F., Xie J., Schiraldi C., Toida T., Amster I.J., Linhardt R.J.: Structural analysis of bikunin glycosaminoglycan. J. Am. Chem. Soc. 130, 2617–2625 (2008)
Shipp E.L., Hsieh-Wilson L.C.: Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem. Biol. 14, 195–208 (2007)
Malavaki C., Mizumoto S., Karamanos N., Sugahara K.: Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 49, 133–139 (2008)
Sabol J.K., Wei W., López-Hoyos M., Seob Y., Andaya A., Leary J.A.: Heparan sulfate differences in rheumatoid arthritis versus healthy sera. Matrix Biol. 40, 54–61 (2014)
Jinno A., Park P.W.: Role of glycosaminoglycans in infectious disease. Methods Mol. Biol. 1229, 567–585 (2015)
Lv H.Z., Yu G.L., Sun L.L., Zhang Z., Zhao X., Chai W.G.: Elevate level of glycosaminoglycans and altered sulfation pattern of chondroitin sulfate are associated with differentiation status and histological type of human primary hepatic carcinoma. Oncology. 72, 347–356 (2007)
Raman K., Kuberan B.: Chemical tumor biology of heparan sulfate proteoglycans. Curr. Chem. Biol. 4(1), 20–31 (2010)
Yip G.W., Smollich M., Götte M.: Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 5(9), 2139–2148 (2006)
Asimakopoulou A.P., Theocharis A.D., Tzanakakis G.N., Karamanos N.K.: The biological role of chondroitin sulfate in cancer and chondroitin-based anticancer agents. In Vivo. 22, 385–390 (2008)
Theocharis A.D.: Human colon adenocarcinoma is associated with specific post-translational modifications of versican and decorin. Biochim. Biophys. Acta. 1588, 165–172 (2002)
Skandalis S.S., Kletsas D., Kyriakopoulou D., Stavropoulos M., Theocharis D.A.: The greatly increased amounts of accumulated versican and decorin with specific post-translational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim. Biophys. Acta. 1760, 1217–1225 (2006)
Theocharis A.D., Vynios D.H., Papageorgakopoulou N., Skandalis S.S., Theocharis D.A.: Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int. J. Biochem. Cell Biol. 35, 376–390 (2003)
Molist A., Romarís M., Lindahl U., Villena J., Touab M., Bassols A.: Changes in glycosaminoglycan structure and composition of the main heparin sulphate proteoglycan from human colon carcinoma cells (perlecan) during cell differentiation. Eur. J. Biochem. 254, 371–378 (1998)
Theocharis A.D., Tsara M.E., Papageorgacopoulou N., Karavias D.D., Theocharis D.A.: Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim. Biophys. Acta. 1502, 201–206 (2000)
Tátrai P., Egedi K., Somorácz A., van Kuppevelt T.H., ten Dam G., Lyon M., Deakin J.A., Kiss A., Schaff Z., Kovalszky I.: Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J. Histochem. Cytochem. 58(5), 429–441 (2010)
Skandalis S.S., Stylianou M., Vynios D.H., Papageorgakopoulou N., Theocharis D.A.: The structural and compositional changes of glycosaminoglycans are closely associated with tissue type in human laryngeal cancer. Biochimie. 89, 1573–1580 (2007)
Kalathas D., Theocharis D.A., Bounias D., Kyriakopoulou D., Papageorgakopoulou N., Stavropoulos M.S., Vynios D.H.: Alterations of glycosaminoglycan disaccharide content and composition in colorectal cancer: structural and expressional studies. Oncol. Rep. 22, 369–375 (2009)
Oliveira-Ferrer L., Heßling A., Trillsch F., Mahner S., Milde-Langosch K.: Prognostic impact of chondroitin-4-sulfotransferase CHST11 in ovarian cancer. Tumor Biol. (2015). doi:10.1007/s13277-015-3652-3
Joo E.J., Weyers A., Li G., Gasimli L., Li L., Choi W.J., Lee K.B., Linhardt R.J.: Carbohydrate-containing molecules as potential biomarkers in colon cancer. OMICS J. Integr. Biol. 18(4), 231–241 (2014)
Weyers A., Yang B., Park J.H., Kim Y.S., Kim S.M., Lee S.E., Zhang F., Lee K.B., Linhardt R.J.: Microanalysis of stomach cancer glycosaminoglycans. Glycoconj. J. 30, 701–707 (2013)
Weyers A., Yang B., Yoon D.S., Park J.H., Zhang F., Lee K.B., Linhardt R.J.: A structural analysis of glycosaminoglycans from lethal and non-lethal breast cancer tissues: towards a novel class of theragnostics for personalized medicine in oncology. OMICS. 16, 79–89 (2012)
Teixeira L., Batista A., Matos L.L., Machado L.R., Suarez E.R., Theodoro T.R., Martins J.R.M., Nader H.B., Pompeo A.C.L., Pinhal M.A.S.: Heparanase expression and glycosaminoglycans profile in renal cell carcinoma. Int. J. Urol. 19, 1036–1040 (2012)
Kitagawa H., Kinoshita A., Sugahara K.: Microanalysis of glycosaminoglycan-derived disaccharides labeled with the fluorophore 2-aminoacridone by capillary electrophoresis and high-performance liquid chromatography. Anal. Biochem. 232, 114–121 (1995)
Yang B., Chang Y., Weyers A.M., Sterner E.R., Linhardt R.J.: Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography–mass spectrometry. J Chromatog A. 1225, 1291–1299 (2012)
Takeuchi J., Sobue M., Shamoto M., Yoshida M., Sato E., Leighton J.: Cell surface glycosaminoglycans of cell line MDCK derived from canine kidney. Cancer Res. 37, 1507–1512 (1977)
Asimakopoulou A.P., Theocharis A.D., Tzanakakis G.N., Karamanos N.K.: The biological role of chondroitin sulfate in cancer and chondroitin-based anticancer agents. In Vivo. 22, 385–390 (2008)
Ten Dam G.B., Westerlo E.M.A., Purushothaman A., Stan R.V., Bulten J., Sweep F.C.G.J.: Antibody GD3G7 selected against embryonic glycosaminoglycans defines chondroitin sulfate-E domains highly up-regulated in ovarian cancer and involved in vascular endothelial growth factor binding. Am J Pathol. 171(4), 1324–1333 (2007)
De Klerk D.P.: The glycosaminoglycans of human bladder cancers of varying grade and stage. J. Urol. 134(5), 978–981 (1985)
Afratis N., Gialeli C., Nikitovic D., Tsegenidis T., Karousou E., Theocharis A.D.: Pava ̃, M.S., tzanakakis, G.N., karamanos, N.K.: glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 279, 1177–1197 (2012)
Sanderson R.D.: Heparan sulfate proteoglycans in invasion and metastasis. Semin. Cell Dev. Biol. 12(2), 89–98 (2001)
Vlodavsky I., Goldshmidt O., Zcharia E., Atzmon R., Rangini-Guatta Z., Elkin M., Peretz T., Friedmann Y.: Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin. Cancer Biol. 12, 121–129 (2002)
Götte M., Yip G.W.: Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res. 66(21), 10233–10237 (2006)
Vlodavsky I., Friedmann Y., Elkin M., Aingorn H., Atzmon R., Ishai-Michaeli R., Bitan M., Pappo O., Peretz T., Michal I., Spector L., Pecker I.: Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 5(7), 793–802 (1999)
Takaoka M., Naomoto Y., Ohkawa T., Uetsuka H., Shirakawa Y., Uno F., Fujiwara T., Gunduz M., Nagatsuka H., Nakajima M., Tanaka N., Haisa M.: Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab. Investig. 83(5), 613–622 (2003)
Parish C.R., Freeman C., Hulett M.D.: Heparanase: a key enzyme involved in cell invasion. Biochim. Biophys. Acta. 1471, M99–M108 (2001)
Lai J.P., Chien J., Strome S.E., Staub J., Montoya D.P., Greene E.L., Smith D.I., Roberts L.R., Shridhar V.: HSulf-1 modulates HGF-mediated tumor cell invasion and signaling in head and neck squamous carcinoma. Oncogene. 23, 1439–1447 (2004)
Muramatsu T., Muramatsu H.: Glycosaminoglycan-binding cytokines as tumor markers. Proteomics. 8, 3350–3359 (2008)
Iida J., Wilhelmson K.L., Ng J., Lee P., Morrison C., Tam E., Overall C.M., McCarthy J.B.: Cell surface chondroitin sulfate glycosaminoglycan in melanoma: role in the activation of pro-MMP-2 (pro-gelatinase a). Biochem. J. 403, 553–563 (2007)
Willis C.M., Kluppel M.: Inhibition by chondroitin sulfate E can specify functional Wnt/beta-catenin signaling thresholds in NIH3T3 fibroblasts. J. Biol. Chem. 287, 37042–37056 (2012)
Arend R.C., Londono-Joshi A.I., Straughn Jr. J.M., Buchsbaum D.J.: The Wnt/beta-catenin pathway in ovarian cancer: a review. Gynecol. Oncol. 131, 772–779 (2013)
Lokeshwar V.B., Rubinowicz D., Schroeder G.L., Forgacsi E., Minnai J.D., Block N.L., Nadji M., Lokeshwar B.L.: Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 276(15), 11922–11932 (2001)
Lokeshwar V.B., Obek C., Soloway M.S., Block N.L.: Tumor-associated hyaluronic acid: a new sensitive and specific urine marker for bladder cancer. Cancer Res. 57, 773–777 (1997)
Posey J.T., Soloway M.S., Ekici S., Sofer M., Civantos F., Duncan R.C., Lokeshwar V.B.: Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res. 63, 2638–2644 (2003)
Franzmann E.J., Schroeder G.L., Goodwin W.J., Weed D.T., Fisher P., Lokeshwar V.B.: Expression of tumor markers hyaluronic acid and hyaluronidase (Hyal1) in head and neck tumors. Int. J. Cancer. 106, 438–445 (2003)
Acknowledgments
This research was funded by Hacettepe University Scientific Research Projects Coordination Unit (Project ID: 014 D06 301 001-641 to EU) and by the U.S. National Institutes of Health (HL62244 to RJL).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ucakturk, E., Akman, O., Sun, X. et al. Changes in composition and sulfation patterns of glycoaminoglycans in renal cell carcinoma. Glycoconj J 33, 103–112 (2016). https://doi.org/10.1007/s10719-015-9643-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-015-9643-1